Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infections are becoming increasingly difficult to treat, owing to acquired antibiotic resistance. The emergence and spread of MRSA limit therapeutic options and require new therapeutic strategies, including novel MRSA-active antibiotics. Filamentous temperature-sensitive protein Z (FtsZ) is a highly conserved bacterial tubulin homologue that is essential for controlling the bacterial cell division process in different species of S. aureus. We conjugated a locked nucleic acid (LNA) that targeted ftsZ mRNA with the peptide (KFF)3K, to generate peptide-LNA (PLNA). The present study aimed to investigate whether PLNA could be used as a novel antibacterial agent. PLNA787, the most active agent synthesized, exhibited promising inhibitory effects on four pathogenic S. aureus strains in vitro. PLNA787 inhibited bacterial growth and resolved lethal Mu50 infections in epithelial cell cultures. PLNA787 also improved the survival rates of Mu50-infected mice and was associated with reductions of bacterial titers in several tissue types. The inhibitory effects on ftsZ mRNA and FtsZ protein expression and inhibition of the bacterial cell division process are considered to be the major mechanisms of PLNA. PLNA787 demonstrated activity against MRSA infections in vitro and in vivo. Our findings suggest that ftsZ mRNA is a promising new target for developing novel antisense antibiotics.
INTRODUCTION
Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA), is one of the most prominent pathogens known to pose a severe threat to human health (1). Statistical data from recent epidemiological studies indicate that the prevalence of MRSA is spreading globally. MRSA, particularly community-associated MRSA, has increased markedly in prevalence and continues to pose a significant public health challenge (2–4). β-Lactam antibiotics are most effective against infections caused by S. aureus. However, widespread use of β-lactam antibiotics has led to increasing prevalence of drug-resistant S. aureus; thus, the therapeutic options available for treating MRSA infections have become seriously limited, and MRSA infections are becoming increasingly difficult to treat. Indeed, MRSA has become resistant to the entire class of β-lactam antibiotics and to most available antibiotics (5–7). Although linezolid, daptomycin, and telavancin are new drugs to treat MRSA infections, the development of new and non-cross-resistant antibacterial agents with novel mechanisms of action against MRSA is still needed.
Antisense oligonucleotides are potential therapeutic agents for prevention of the translation of essential genes at the mRNA level with antisense nucleic acid analogues, such as phosphorothioate oligodeoxynucleotides (PS-ODNs), peptide-nucleic acids (PNAs), locked nucleic acids (LNAs), and phosphorodiamidate morpholino oligomers (8, 9). LNAs are nucleic acid analogues in which the ribose ring is locked by a 2′-O,4′-C-methylene bridge. LNA oligomers have high affinity for RNA or DNA targets, are quite stable against nucleases, and have low toxicity (10). Consequently, LNA oligomers have been studied as gene-silencing agents in many diseases. Moreover, LNAs have been shown to kill Escherichia coli by inhibiting RNase P RNA and the hepatitis C virus by targeting the internal ribosomal entry site (IRES) (11, 12). Antisense agents must bind to accessible regions of the targeted mRNA to form stable oligodeoxynucleotide (ODN)-RNA complexes. Many previous studies have shown that the translation start codon region of mRNA is the effective region for antisense inhibition (13–15). Recently, some studies have demonstrated that antisense agents targeting non-start codon regions have efficient antisense effects, such as PNA targeting of rpoD mRNA of MRSA and LNA targeting of the IRES of hepatitis C virus (12, 16). This implies that possible targeting sites are generally nucleotide sequences free of any double-stranded secondary structures, which can be theoretically forecasted by RNA structure software (17).
The filamentous temperature-sensitive protein Z (FtsZ) is an essential protein for bacterial cell division and viability. FtsZ self-assembles into polymers and then forms a Z-ring at the site of division, which serves as a scaffold to recruit other key protein constituents of the cell division machinery (18–20). Inhibiting ftsZ gene expression or disrupting FtsZ protein activity may prove to be a novel therapeutic strategy against bacterial infections, by disrupting bacterial growth patterns. One previous study indicated that a small-molecule inhibitor of FtsZ could treat infections caused by S. aureus. Recently, several other studies indicated that small-molecule inhibitors of FtsZ PC190723 also could treat infections caused by S. aureus (21). In addition, targeting the ftsZ gene of Pseudomonas aeruginosa with PNAs resulted in complete growth inhibition (22). Therefore, FtsZ has been highlighted as a promising therapeutic target for exploiting efficacious antibacterial agents active against drug-sensitive and drug-resistant bacterial strains. Although FtsZ is absent from eukaryotes and shows limited sequence homology with tubulin at the gene level (23), it may provide an appealing target for antisense antibacterial agents. However, little work has been performed to investigate whether antisense agents targeting the ftsZ gene can affect the growth of S. aureus, particularly MRSA. Furthermore, additional investigation is required to elucidate in vitro and in vivo effects and the underlying mechanisms of antisense agents against MRSA infection.
The objectives of the current study were to investigate the activity of an LNA targeting ftsZ mRNA in MRSA in vitro and in vivo and to study the mechanism of the LNA. In order to achieve this, we synthesized a peptide-LNA (PLNA) conjugate and observed its effects on a MRSA-induced cell infection and in a C57BL/6J mouse infection model. Moreover, we explored the mechanism of action of the PLNA with MRSA.
MATERIALS AND METHODS
Chemicals.
Antibiotics used in this study were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Culture media were purchased from Land Bridge Technology Co. (Beijing, China). All chemicals and solvents used in this study were of analytical grade.
Organisms.
Laboratory methicillin-sensitive S. aureus (MSSA) strain ATCC 29213 and MRSA strain WHO-2 were obtained from the Chinese National Center for Surveillance of Antimicrobial Resistance (Beijing, China). Mu50 was purchased from MicroBiologics (St. Cloud, MN). MRSA clinical strains MRSA01 to MRSA07 were obtained from the clinical laboratory of Xijing Hospital (Xi'an, China).
Specific-pathogen-free male C57BL/6J mice, 6 to 8 weeks of age and weighing 18 to 20 g, were used in this study. The experimental and animal care procedures were approved by the Animal Care and Use Committee of Fourth Military Medical University.
PLNA synthesis.
The sequence of the most active LNA used in this study, LNA787, was tGaCtcGccaCCagtaataTT (in which the LNA bases are represented by uppercase characters). The sequence of LNA787 is complementary to nucleotides 787 to 808 in the coding region of ftsZ mRNA in MRSA. The control scrambled LNA sequence was gTTttgGatcGtCttCGC (in which uppercase characters represent the LNA bases). Free LNA and the conjugate of the cell-penetrating peptide (CPP) (KFF)3K and the LNA with a Cys-succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate-C6 (Cys-SMCC-C6) linker, i.e., CPP-conjugated LNA (PLNA), were synthesized and purified by Bio-Synthesis (Lewisville, TX). The sequence of PLNA787 used in this study was KFFKFFKFFK-Cys-SMCC-C6-tGaCtcGccaCCagtaataTT (in which uppercase characters represent the LNA bases).
Bacterial susceptibility testing.
MIC values were determined by the standard 2-fold broth dilution tube method, according to the guidelines of the Clinical and Laboratory Standards Institute. PLNA787, LNA787, (KFF)3K, scrambled PLNA, oxacillin (OXA), ampicillin (AMP), and ceftazidime (CAZ) MIC values were determined for strains Mu50 and WHO-2 and clinical isolates MRSA01 to MRSA07, using duplicate samples. Quality control was ensured by testing the control strain, ATCC 29213, in every experiment. Independent experiments were performed in triplicate.
Bacterial growth assay.
Bacterial strains ATCC 29213, Mu50, MRSA01, and MRSA02 were cultured until optical density values at 630 nm reached 0.5 to 0.6. Cells were subsequently diluted to 1.5 × 108 CFU/ml. PLNA787 was added to cell cultures at final concentrations of 0.78, 1.56, 3.13, and 6.25 μM. An equal volume of diluent was used as a control. The mixed cultures were incubated at 35°C, with agitation at 210 rpm, for 6 h. Cultures were diluted in a suitable gradient, and 50-μl aliquots of diluted cells were spread on Mueller-Hinton (MH) agar. Plates were incubated for 24 h at 35°C. The numbers of colonies were counted, and the total CFU per sample was determined by correcting the colony count by the dilution factor.
To determine the growth curves for ATCC 29213, Mu50, MRSA01, and MRSA02 in the broth, cells were diluted and treated with equal volumes of diluents and PLNA787 at final concentrations of 0.78, 1.56, 3.13, and 6.25 μM. Cultures were monitored by measuring the optical density at 630 nm, using a microplate reader (Bio-Rad Laboratories, Tokyo, Japan), at 2-h intervals for 18 h.
Epithelial cell infection assay.
Gastric mucosal epithelial cells were plated in 96-well culture dishes at a density of 1.5 × 104 cells/ml. A volume of 200 μl Dulbecco's modified Eagle's medium (DMEM) (HyClone, Logan, UT) supplemented with 10% fetal bovine serum was added to each well. Cells were then incubated at 37°C for 48 h. An overnight Mu50 culture was diluted with DMEM with 10% fetal bovine serum and transferred to wells containing epithelial cells at a final concentration of 1 × 107 CFU/ml. PLNA787 (3.13, 6.25, and 12.5 μM) and an equal volume of diluent were immediately added to cell cultures, and the cultures were incubated at 37°C for 24 h. Ten-microliter samples of culture supernatants were removed, diluted to the appropriate concentration, and plated on MH agar, to measure viable bacterial cells, at 3, 6, 12, and 24 h. At the 24-h time point, cells were observed and images were captured with an inverted light microscope, under ×100 magnification.
C57BL/6J mouse infection assay.
Infection of C57BL/6J mice was induced by intraperitoneal administration of 1 × 106 CFU/ml Mu50 in 0.4 ml MH broth supplemented with 5% mucin. After bacterial challenge, mice were randomly treated with 20 mg/kg AMP (twice daily for 2 days), 3 mg/kg PLNA787 in a single dose, or the same amount of diluents. Control mice did not receive intraperitoneal inoculations with Mu50. To assess bacterial clearance, 5 mice in each group were sacrificed after bacterial challenge for 24 h, blood, liver, spleen, lung, and kidney samples were harvested aseptically, weighed, and homogenized in sterile saline solution, and bacterial counts were determined by appropriate dilution and plating. Colony counts were expressed as CFU/g tissue. The survival of 10 mice in each group was monitored every 6 h for 7 days after infection, and the cumulative survival rate was determined.
RNA isolation and real-time PCR assay.
To determine whether PLNA787 could inhibit the expression of ftsZ mRNA, Mu50 cultures were treated with diluents or PLNA787 at 0.39, 0.78, 1.56, 3.13, or 6.25 μM for 18 h. Total RNA was extracted from bacterial cultures using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. cDNA was prepared with random primers using a PrimeScript RT reagent kit (TaKaRa Biotechnology Co., Dalian, China) and was analyzed with SYBR Premix Ex Taq (TaKaRa Biotechnology Co., Dalian, China), using gene-specific oligonucleotide primers, in a thermal cycler, with an initial denaturation step at 95°C for 2 min and then 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. Primers for the ftsZ and 16S rRNA genes were as follows: ftsZ, 5′-AGAGGCTGCTGATATTGTCCA-3′ and 5′-TTGTTCCGAATCCAGTGCTAC-3′ (173-bp product); 16S, 5′-GCCGTAAACGATGAGTGCTAA-3′ and 5′-CGAATTAAACCACATGCTCCA-3′ (156-bp product). Relative expression of ftsZ was calculated by the 2−ΔΔCt method, with normalization to 16S rRNA levels. Amplified products were visualized on agarose gels, and images were captured with an AlphaImager 2200 digital imaging system (AlphaInnotech, San Leandro, CA).
Western blot assay.
To determine whether PLNA787 could inhibit the expression of FtsZ protein, Mu50 cultures were treated with diluents or PLNA787 at 0.39, 0.78, 1.56, 3.13, or 6.25 μM for 18 h. PLNA787-treated bacterial cells and untreated controls were lysed using buffer (Dingguo Biotech Co., Beijing, China) containing lysozyme (100 mg/ml) and phenylmethylsulfonyl fluoride (1 mM). Protein concentrations were quantified using the bicinchoninic acid method (Pierce Biotechnology, Inc., Rockford, IL), and the total protein contents were equalized between samples. Total proteins were subsequently separated on sodium dodecyl sulfate-polyacrylamide gels (12%) and were transferred to polyvinylidene difluoride membranes (Millipore Corp., Billerica, MA) for Western blotting. Bacterial FtsZ polyclonal antibody (Acris Antibodies, Inc., San Diego, CA) was used at a dilution of 1:800 for overnight blotting at 4°C, and chemiluminescence signals were detected using an enhanced chemiluminescence kit after incubation with anti-rabbit IgG secondary antibody (1:5,000; Abcam, Cambridge, MA). Densitometric analysis of Western blots was conducted using ChemiDoc XRS (Bio-Rad, Hercules, CA), and the relative band intensities were compared to controls using Quantity One version 4.1.0.
Scanning and transmission electron microscopy.
Mu50 cells (1.0 × 108 CFU/ml) cultured with PLNA787 (0.78, 1.56, or 3.13 μM) at 120 rpm for 6 h were harvested and washed. Specimens were observed with a scanning electron microscope (Hitachi S-3400N) or a transmission electron microscope (JEM-2000EX; JEOL), and images were captured for analysis.
Statistical analysis.
Data are expressed as mean ± standard deviation (SD) and were analyzed with one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls post hoc test. Kaplan-Meier analysis was used to assess survival curves. Differences were considered statistically significant at P < 0.05.
RESULTS
In vitro antibacterial activity of anti-ftsZ PLNA787.
To evaluate the antibacterial activity of PLNA787 in vitro, MSSA strain ATCC 29213 and nine MRSA strains, including 2 laboratory strains (Mu50 and WHO-2) and 7 clinical isolates (MRSA01 to MRSA07) were used. PLNA787 exhibited antibacterial effects against ATCC 29213 and nine MRSA strains, with most MIC values ranging from 1.56 to 12.5 μM. In contrast, OXA, AMP, CAZ, and levofloxacin (LVX) exerted weak antibacterial effects on the nine MRSA strains. The MIC values for OXA, AMP, and CAZ were lower for the susceptible strain ATCC 29213 (0.5 to 8 μg/ml) but higher (to different degrees) for the nine MRSA strains. Free LNA787, free (KFF)3K, and scrambled PLNA did not exhibit antibacterial activity against ATCC 29213, Mu50, WHO-2, and MRSA01 to MRSA07, since MIC values were greater than 50 μM (Table 1).
TABLE 1.
MICs of anti-ftsZ PLNA and antibiotics in MSSA and MRSA isolates in MH broth cultures
| PLNA or antibiotic | MIC of strain:a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 29213 | Mu50 | WHO-2 | MRSA01 | MRSA02 | MRSA03 | MRSA04 | MRSA05 | MRSA06 | MRSA07 | |
| PLNA787 (μM) | 3.13 | 1.56 | 12.5 | 6.25 | 3.13 | 6.25 | 12.5 | 12.5 | 6.25 | 3.13 |
| Free LNA787 (μM) | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Free (KFF)3K (μM) | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| Scrambled PLNA (μM) | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| OXA (μg/ml) | 0.5 (S) | 256 (R) | 1,024 (R) | 1,024 (R) | 256 (R) | 512 (R) | 1,024 (R) | 1,024 (R) | 256 (R) | 128 (R) |
| AMP (μg/ml) | 1 (S) | 256 (R) | 1,024 (R) | 1,024 (R) | 256 (R) | 512 (R) | 1,024 (R) | 1,024 (R) | 256 (R) | 128 (R) |
| CAZ (μg/ml) | 8 (S) | 512 (R) | 256 (R) | 128 (R) | 256 (R) | 16 (I) | 8 (S) | 512 (R) | 128 (R) | 64 (R) |
| LVX (μg/ml) | 0.5 (S) | 128 (R) | 128 (R) | 256 (R) | 32 (R) | 2 (I) | 16 (R) | 256 (R) | 128 (R) | 64 (R) |
Data are derived from 3 independent experiments. OXA, oxacillin; AMP, ampicillin; CAZ, ceftazidime; LVX, levofloxacin; MH, Mueller-Hinton; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PLNA, peptide-locked nucleic acid; R, resistant; I, intermediate; S, susceptible.
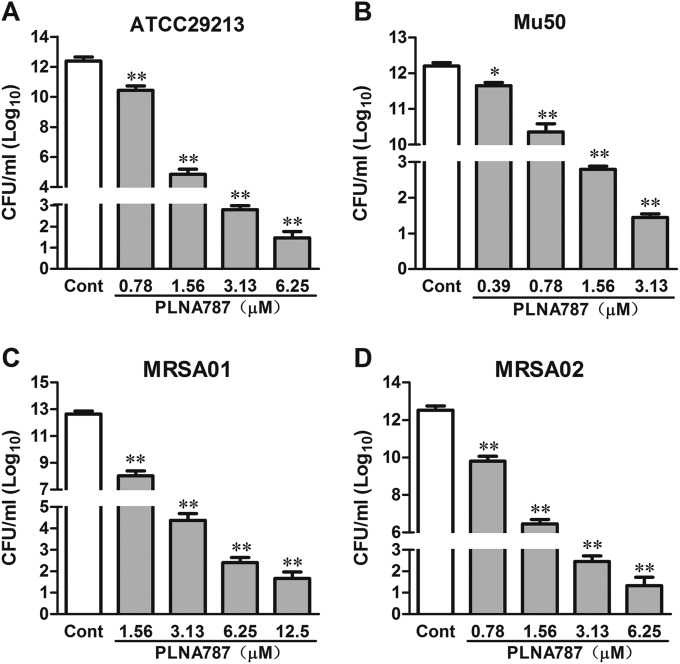
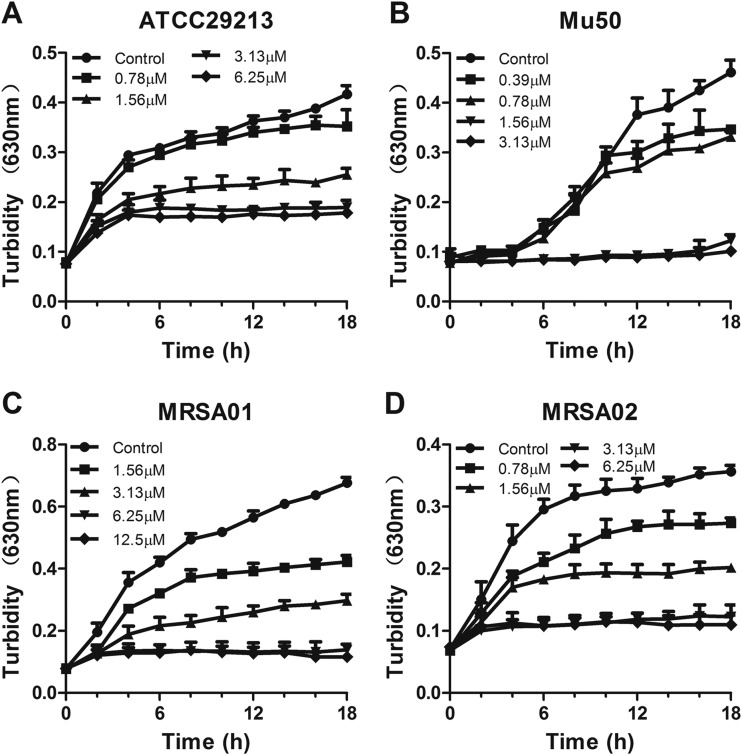
Next, we observed the growth inhibitory effect of PLNA787 on ATCC 29213, Mu50, MRSA01, and MRSA02 by assessing bacterial growth and viability. Compared with the control group, PLNA787 significantly reduced the numbers of these 4 bacterial strains on MH agar, in a concentration-dependent manner (Fig. 1). In addition, PLNA787 inhibited growth of the 4 pathogens in a time- and concentration-dependent manner. Representative results are shown in Fig. 2 and reveal that PLNA787 exhibited almost complete growth inhibition of ATCC 29213 and MRSA02 at 3.13 μM. Furthermore, PLNA787 showed potent growth inhibition of Mu50 and MRSA01 at 1.56 and 6.25 μM, respectively. The results of the cell growth assays were in accordance with cell viability assays using PLNA787 described above, suggesting that PLNA787 exhibited potent antibacterial activity with MSSA and MRSA strains.
FIG 1.
Effects of anti-ftsZ PLNA787 on the growth of MSSA or MRSA on MH agar. (A) CFU of ATCC 29213 in the presence of 0.78, 1.56, 3.13, or 6.25 μM PLNA787. (B) CFU of Mu50 in the presence of 0.39, 0.78, 1.56, or 3.13 μM PLNA787. (C) CFU of MRSA01 in the presence of 1.56, 3.13, 6.25, or 12.5 μM PLNA787. (D) CFU of MRSA02 in the presence of 0.78, 1.56, 3.13, or 6.25 μM PLNA787. The indicated concentrations of PLNA787, or an equal volume of phosphate-buffered saline as a control (Cont), were added to cell cultures containing 1.5 × 108 CFU/ml. Aliquots of each culture were collected at 6 h, diluted, and inoculated on solid agar for 24 h. CFU values were calculated from the numbers of colonies growing on the plates. Data represent the mean ± SD (n = 10). *, P < 0.05; **, P < 0.01, versus control.
FIG 2.
In vitro inhibitory effects of anti-ftsZ PLNA787 on the growth of ATCC 29213 (A), Mu50 (B), MRSA01 (C), and MRSA02 (D). Cells were cultured in liquid culture medium and treated with different concentrations of PLNA787 or phosphate-buffered saline as a control. The growth of strains was evaluated spectrophotometrically at 630 nm. Data represent the mean ± SD (n = 10).
Protective effects of anti-ftsZ PLNA787 on eukaryotic cells infected with Mu50.
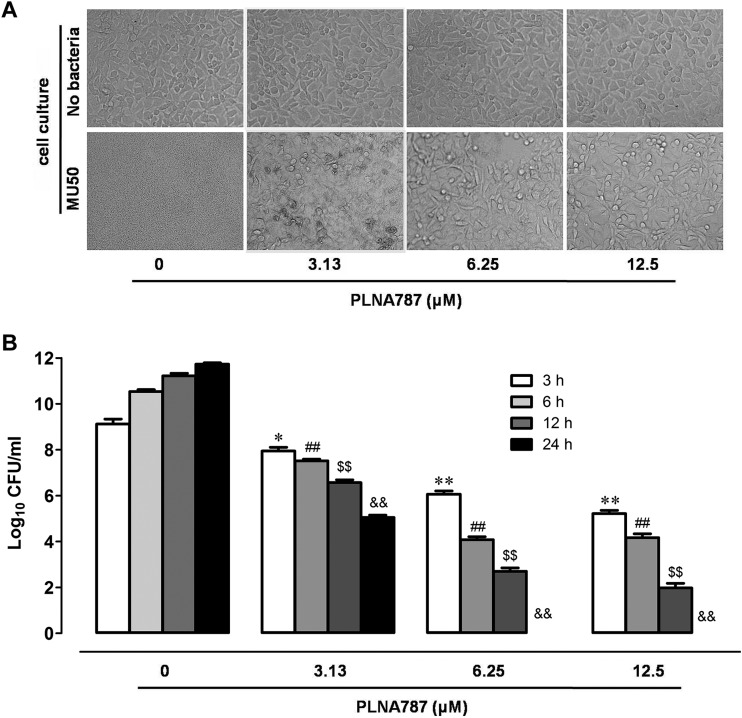
To examine the antibacterial potential of PLNA787 with human cells, we added different concentrations of PLNA787 to monolayers of human gastric mucosa epithelial cells infected with invasive Mu50. At 24 h postinfection, no cells in the control group survived, irrespective of treatment. Few gastric cells were visible, but there was an abundance of bacteria. In the PLNA787-treated group, however, PLNA787 protected cells from damage; an abundance of gastric cells and few bacteria were observed. No change in cell morphology was observed, and the cells remained healthy after treatment with the highest concentration of PLNA787 (12.5 μM) (Fig. 3A). In the control group, Mu50 multiplied 2-fold in 24 h in the presence of medium alone. At 3 h after infection, 3.13, 6.25, and 12.5 μM PLNA787 added to infected cells markedly reduced bacterial CFU. Further reductions in CFU were observed with time. PLNA787 at concentrations of 6.25 and 12.5 μM caused complete elimination of CFU, compared with infected cells in the absence of PLNA (Fig. 3B). The results indicated that PLNA787 has bactericidal capability against Mu50 in this cell infection model.
FIG 3.
Protective effects of anti-ftsZ PLNA787 on epithelial cells infected with Mu50. Epithelial cells were infected with 1 × 107 CFU/ml Mu50 and immediately treated with different concentrations of PLNA787. Mu50 CFU were counted from cell culture supernatants at 3, 6, 12, and 24 h, and cultures were examined by light microscopy at 24 h. (A) Light micrographs of epithelial cell cultures. Magnification, ×100. (B) CFU of Mu50 in culture supernatants treated with different concentrations of PLNA787. The 24-h counts for 6.25 and 12.5 μM PLNA were 0 CFU/ml. Data represent the mean ± SD (n = 5). *, P < 0.05, versus 3-h group treated with 0 μM PLNA; **, P < 0.01, versus 3-h group treated with 0 μM PLNA; ##, P < 0.01, versus 6-h group treated with 0 μM PLNA; $$, P < 0.01, versus 12-h group treated with 0 μM PLNA; &&, P < 0.01, versus 24-h group treated with 0 μM PLNA.
In vivo protective effects of anti-ftsZ PLNA787.
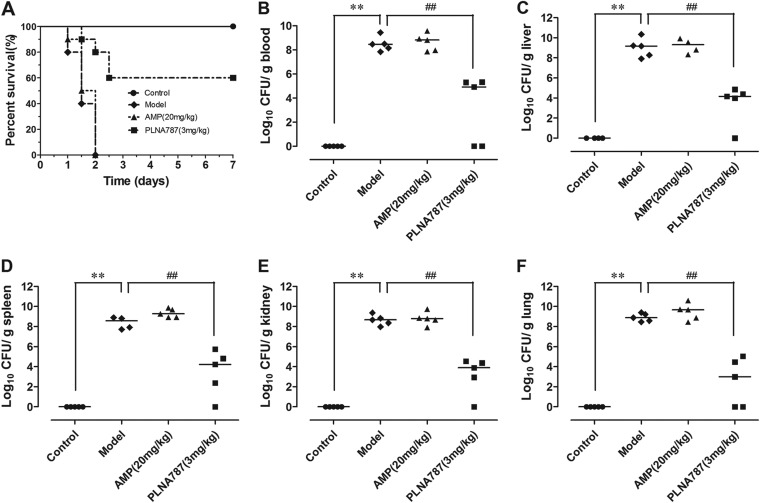
Since we found that PLNA787 exhibited stronger antibacterial activity in vitro and in a cell infection model, we next investigated whether PLNA787 could provide survival benefits in vivo, by performing animal infection experiments using Mu50. All mice in the Mu50-infected group and in the Mu50-infected and AMP-treated group (20 mg/kg AMP) died within 2 days. The survival rate was significantly increased in the Mu50-infected and PLNA787-treated group, and the rate increased from 0% to 60.0% in the Mu50-infected group treated with 3 mg/kg PLNA787 (Fig. 4A). Furthermore, rescue was associated with reductions in bacterial titers in tissues (such as blood, liver, spleen, lung, and kidney) of mice inoculated with Mu50. On average, approximate decreases of 4 log units of CFU were observed in blood, liver, spleen, lung, and kidney of mice that survived the lethal Mu50 infection. In contrast, no reductions in bacterial titers were found in septic mice treated with 20 mg/kg AMP (Fig. 4B to F). The results demonstrated that PLNA787 could rescue septic mice infected with Mu50 by inhibiting the growth of Mu50 in vivo.
FIG 4.
In vivo antibacterial activity of anti-ftsZ PLNA787 in a mouse model of Mu50-induced sepsis. C57BL/6J mice were inoculated with Mu50 (1 × 106 CFU). After 30 min, C57BL/6J mice received a single dose of anti-ftsZ PLNA787 at 3 mg/kg, AMP at 20 mg/kg (twice daily for 2 days), or the equivalent quantity of diluents as in the AMP group (Model). Control mice did not receive Mu50 inoculation. (A) Survival of C57BL/6J mice treated with anti-ftsZ PLNA787 (3 mg/kg), AMP (20 mg/kg), or diluents. Data represent the mean ± SD (n = 10). (B to F) Colonization of Mu50 inocula in blood (B), liver (C), spleen (D), kidney (E), and lung (F) cultures of C57BL/6J mice treated with anti-ftsZ PLNA787. Data represent the mean ± SD (n = 5). **, P < 0.01, versus control group; ##, P < 0.01, versus model group (infection group).
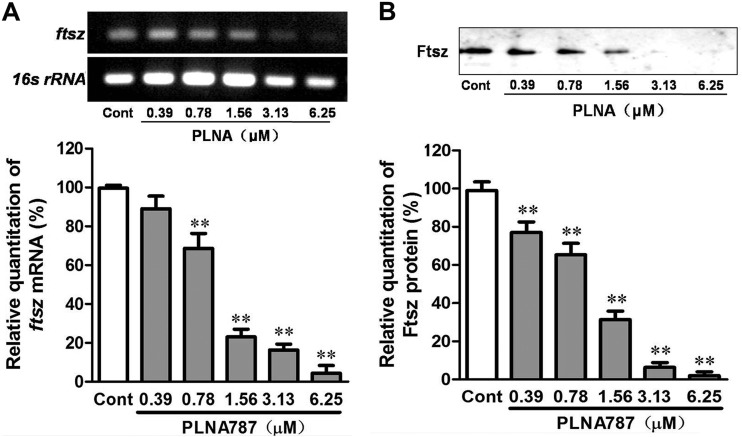
Effects of anti-ftsZ PLNA787 on ftsZ mRNA and protein expression.
We found that PLNA787 exhibited stronger antibacterial activity in vitro and in vivo, and we proceeded to determine whether the antibacterial effects of PLNA787 were caused by decreases in specific ftsZ gene transcripts through an antisense mechanism, with subsequent decreased expression of FtsZ protein. Different concentrations of PLNA787 were applied to Mu50 pathogen cultures, and the expression of ftsZ mRNA and its translation product was tested by real-time PCR and Western blotting. PLNA787 greatly inhibited the expression of ftsZ mRNA, in a concentration-dependent manner, in treated cultures, compared with untreated cultures (Fig. 5A). Additionally, to ascertain whether the antisense inhibition of ftsZ at the mRNA level could lead to decreased expression of its protein product, the expression of FtsZ protein was tested. The results demonstrated that FtsZ protein expression was significantly reduced following inhibition of ftsZ mRNA, in a concentration-dependent manner (Fig. 5B). Thus, anti-ftsZ PLNA787 could inhibit the expression of ftsZ mRNA and subsequently reduce the production of FtsZ protein.
FIG 5.
Anti-ftsZ PLNA787 inhibition of the expression of ftsZ mRNA and FtsZ protein. Mu50 cultures were treated with different concentrations of anti-ftsZ PLNA787 for 18 h before total RNA and protein were isolated. (A) ftsZ mRNA levels in Mu50 determined by real-time PCR assays. (B) FtsZ protein levels in Mu50 analyzed by Western blotting and quantified by densitometric normalization to values for the untreated control group (Cont). Data represent the mean ± SD (n = 3). **, P < 0.01, versus control group.
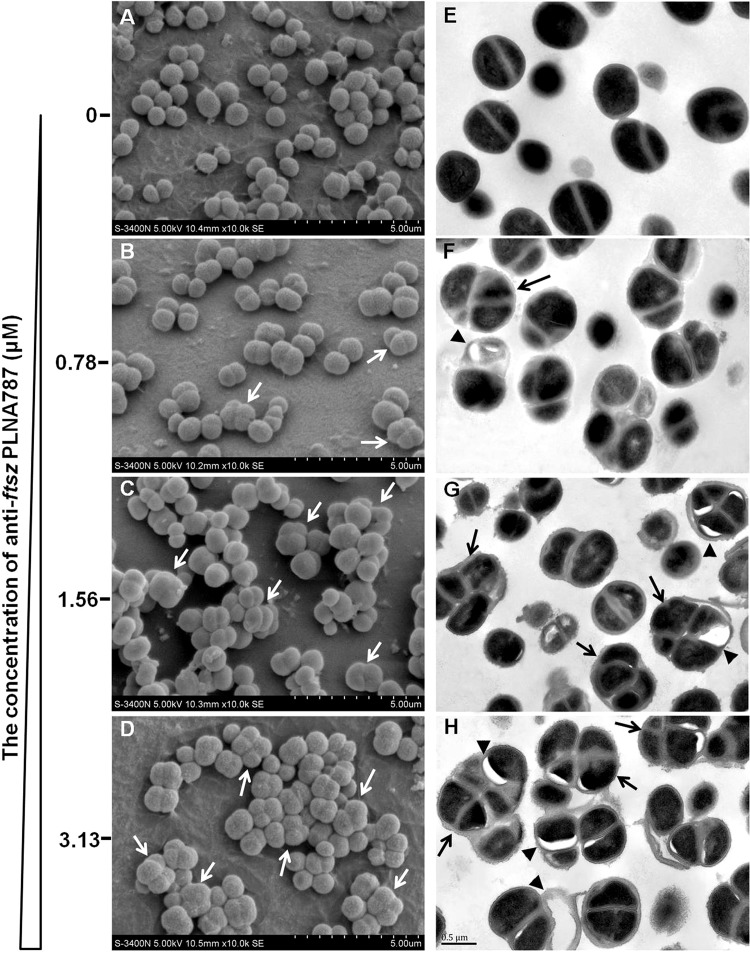
Effects of anti-ftsZ PLNA787 on Mu50 cell division.
We found that PLNA787 could inhibit the expression of ftsZ mRNA and FtsZ protein, which plays an important role in cell division. Therefore, we conducted scanning electron microscopy (SEM) and transmission electron microscopy (TEM) studies aimed at verifying whether PLNA787 could affect the cell division process. SEM images indicated the spherical form and relatively uniform length of Mu50 (Fig. 6A). However, SEM images revealed dramatic changes in bacterial morphology in the PLNA787-treated group. The bacteria exhibited an enlarged phenotype because of their inability to complete the cell division process; diploid and tetraploid bacteria were detected (Fig. 6B to D, white arrows).
FIG 6.
Morphology of Mu50, investigated by SEM and TEM. Mu50 cultures were treated with different concentrations of anti-ftsZ PLNA787 for 6 h. (A to D) SEM images of Mu50, revealing diploid and tetraploid bacteria (white arrows). (E to H) TEM images, revealing polyploid bacteria (black arrows) and visible bleb-like gaps appearing between the cell membrane and the cytoplasm (black arrowheads).
TEM images revealed that Mu50 cells treated with different concentrations of PLNA787 exhibited an enlarged phenotype, compared with the untreated group, and the number of polyploid bacteria was markedly increased (Fig. 6E to H, black arrows). Bacterial membrane integrity was seriously damaged and the cytoplasm had contracted considerably, with visible bleb-like gaps appearing between the cell membrane and the cytoplasm (Fig. 6E to H, black arrowheads). The dramatic morphological changes observed in the bacteria were consistent with the antibacterial activity observed in the previous assays, implying that the antibacterial activity of PLNA787 may correlate with antisense inhibition of ftsZ mRNA, disruption of FtsZ function, and effects on the cell division process in live bacteria.
DISCUSSION
Infections caused by multidrug-resistant pathogens have become one of the most serious public health issues worldwide. Among them, the frequency of MRSA infections continues to grow in hospital-associated settings and within the community. MRSA-inducing infections present an increasing burden on health care resources, as well as increasing morbidity and mortality rates. Therefore, MRSA presents a formidable challenge for exploring new therapeutic strategies and antibiotics with novel mechanisms of action, distinct from those of existing antibiotics. Data from our research and the work of others have proven that antisense oligonucleotides provide a revolutionary approach to treating infections due to multidrug-resistant pathogens (13, 24, 25).
LNA is an antisense oligonucleotide with many desirable properties, including high affinity, RNase H activation, improved stability, and minimal toxicity. Introduction of 2 or more LNA moieties can offer efficient protection against nucleases, improved stability, and prolonged half-life in serum, due to its stability in resisting nucleases (26). Although RNase H cannot be activated by all-LNA moieties, it can be activated by LNA-DNA mixmers (26, 27). LNA-DNA mixmers cleave RNA-mRNA duplexes through the activity of RNase H, a type of RNA (double-stranded RNA)-specific RNase (28). Compared to other antisense oligonucleotides, LNA-DNA mixmers exhibit potent antisense effects and low toxicity (10, 26, 27). Therefore, in order to achieve excellent biological properties, we designed LNA-DNA mixmers containing seven LNA moieties in our study.
Although efficient cell delivery of antisense oligonucleotides is a key issue for their potential therapeutic use, LNA is able to enter cells without any means of delivery and downregulates the expression of target genes in multiple mammalian cell lines (29). However, the findings from our study showed that free LNA787 did not inhibit bacterial growth in ATCC 29213 and the nine MRSA strains during bacterial susceptibility tests. These results indicated that LNA was unable to efficiently penetrate the membranes of S. aureus cells, due to the rigid bacterial cell walls. Therefore, high-performance delivery of LNA is mandatory. (KFF)3K conjugated with PNA or phosphorodiamidate morpholino oligomer dramatically increased cell permeability and uptake of antisense oligonucleotides in a series of bacteria, including S. aureus, E. coli, and Salmonella enterica (16, 24, 30). To date, few studies have been performed to improve the permeability of LNA into bacteria through conjugation with the peptide (KFF)3K. In the present study, we synthesized the conjugated PLNA with (KFF)3K and LNA using the Cys-SMCC-C6 linker.
PLNA787 exhibited potent antibiotic activity in vitro against susceptible and resistant S. aureus strains, as revealed by bacterial susceptibility testing and bacterial growth assays. Free LNA787, free (KFF)3K, and scrambled PLNA did not affect S. aureus growth in bacterial susceptibility tests, which indicated that PLNA787 displayed antisense growth inhibition with sequence specificity. In contrast, PLNA787 showed therapeutic capabilities in Mu50-infected cultures of human gastric mucosa epithelial cells. Concurrently, PLNA787 failed to markedly influence the viability and morphology of epithelial cells, indicating that PLNA787 was not toxic toward eukaryotic cells at the concentrations tested. Moreover, similar results were obtained in Mu50–induced sepsis, a mouse infection model. PLNA787 cured sepsis in mice and significantly improved the survival rates by reducing the bacterial titers in the blood and tissue of septic mice infected with Mu50.
Although it was confirmed that PLNA787 exhibited potent growth inhibition of S. aureus in vitro and in vivo, the underlying mechanisms remain unclear. We investigated whether the biological activity of PLNA787 is dependent on changes in the expression and function of FtsZ. First, data from real-time PCR experiments revealed that PLNA787 induced decreased expression of ftsZ mRNA, in a concentration-dependent manner. Second, FtsZ protein expression was subsequently decreased because of inhibition of specific transcripts of the ftsZ gene and unsuccessful translation of transcripts through the antisense mechanism. Finally, SEM and TEM images verified loss of function of the FtsZ protein; as a consequence, the MRSA cell division process was inhibited and a diploid, tetraploid, or polyploid phenotype ensued.
This study has confirmed that PLNA787 is highly active against S. aureus, including both sensitive and resistant strains. Favorable in vivo protective effects were observed in infected eukaryotic cells and in mice with septicemia induced by MRSA. Thus, ftsZ provides a potential target for antisense LNAs, and PLNA may provide a novel strategy for treating MRSA infections.
ACKNOWLEDGMENT
This research was supported by the National Natural Science Foundation of China (grant 30901813).
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Johnson AP. 2011. Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother 66(Suppl 4):iv43–iv48. doi: 10.1093/jac/dkr076. [DOI] [PubMed] [Google Scholar]
- 3.Tattevin P, Schwartz BS, Graber CJ, Volinski J, Bhukhen A, Mai TT, Vo NH, Dang DN, Phan TH, Basuino L, Perdreau-Remington F, Chambers HF, Diep BA. 2012. Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 55:781–788. doi: 10.1093/cid/cis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadler JL, Petit S, Mandour M, Cartter ML. 2012. Trends in invasive infection with methicillin-resistant Staphylococcus aureus, Connecticut, USA, 2001-2010. Emerg Infect Dis 18:917–924. doi: 10.3201/eid1806.120182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RM, Price JR, Batty EM, Didelot X, Wyllie D, Golubchik T, Crook DW, Paul J, Peto TE, Wilson DJ, Cule M, Ip CL, Day NP, Moore CE, Bowden R, Llewelyn MJ. 2014. Healthcare-associated outbreak of meticillin-resistant Staphylococcus aureus bacteraemia: role of a cryptic variant of an epidemic clone. J Hosp Infect 86:83–89. doi: 10.1016/j.jhin.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miu-ling W, Kwok-ming P, Yuen-kong W, Shuk-kwan C, Lai-key K, Sik-on P. 2014. An outbreak of community-associated methicillin-resistant Staphylococcus aureus infection in a boarding school in Hong Kong Special Administrative Region (China). Western Pac Surveill Response J 5:1–6. doi: 10.5365/wpsar.2013.4.4.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandora TJ, Goldmann DA. 2012. Preventing lethal hospital outbreaks of antibiotic-resistant bacteria. N Engl J Med 367:2168–2170. doi: 10.1056/NEJMp1212370. [DOI] [PubMed] [Google Scholar]
- 8.Singh SB, Phillips JW, Wang J. 2007. Highly sensitive target-based whole-cell antibacterial discovery strategy by antisense RNA silencing. Curr Opin Drug Discov Devel 10:160–166. [PubMed] [Google Scholar]
- 9.Wright GD. 2009. Making sense of antisense in antibiotic drug discovery. Cell Host Microbe 6:197–198. doi: 10.1016/j.chom.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Doessing H, Vester B. 2011. Locked and unlocked nucleosides in functional nucleic acids. Molecules 16:4511–4526. doi: 10.3390/molecules16064511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruegelsiepe H, Brandt O, Hartmann RK. 2006. Antisense inhibition of RNase P: mechanistic aspects and application to live bacteria. J Biol Chem 281:30613–30620. doi: 10.1074/jbc.M603346200. [DOI] [PubMed] [Google Scholar]
- 12.Nulf CJ, Corey D. 2004. Intracellular inhibition of hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation by peptide nucleic acids (PNAs) and locked nucleic acids (LNAs). Nucleic Acids Res 32:3792–3798. doi: 10.1093/nar/gkh706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg DE, Marshall-Batty KR, Brinster LR, Zarember KA, Shaw PA, Mellbye BL, Iversen PL, Holland SM, Geller BL. 2010. Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J Infect Dis 201:1822–1830. doi: 10.1086/652807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilley LD, Mellbye BL, Puckett SE, Iversen PL, Geller BL. 2007. Antisense peptide-phosphorodiamidate morpholino oligomer conjugate: dose-response in mice infected with Escherichia coli. J Antimicrob Chemother 59:66–73. doi: 10.1093/jac/dkl444. [DOI] [PubMed] [Google Scholar]
- 15.Tan XX, Actor JK, Chen Y. 2005. Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection. Antimicrob Agents Chemother 49:3203–3207. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai H, Sang G, You Y, Xue X, Zhou Y, Hou Z, Meng J, Luo X. 2012. Targeting RNA polymerase primary σ70 as a therapeutic strategy against methicillin-resistant Staphylococcus aureus by antisense peptide nucleic acid. PLoS One 7:e29886. doi: 10.1371/journal.pone.0029886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Lawrence CE. 2003. A statistical sampling algorithm for RNA secondary structure prediction. Nucleic Acids Res 31:7280–7301. doi: 10.1093/nar/gkg938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi EF, Lutkenhaus J. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 19.Adams DW, Errington J. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 20.Erickson HP, Anderson DE, Osawa M. 2010. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev 74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR, Baker PJ, Barynin VV, Rice DW, Sedelnikova SE, Heal JR, Sheridan JM, Aiwale ST, Chauhan PK, Srivastava A, Taneja A, Collins I, Errington J, Czaplewski LG. 2008. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 321:1673–1675. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- 22.Ghosal A, Nielsen PE. 2012. Potent antibacterial antisense peptide-peptide nucleic acid conjugates against Pseudomonas aeruginosa. Nucleic Acid Ther 22:323–334. doi: 10.1089/nat.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogales E, Wolf SG, Downing KH. 1998. Structure of the αβ tubulin dimer by electron crystallography. Nature 391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 24.Bai H, You Y, Yan H, Meng J, Xue X, Hou Z, Zhou Y, Ma X, Sang G, Luo X. 2012. Antisense inhibition of gene expression and growth in Gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials 33:659–667. doi: 10.1016/j.biomaterials.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Meng J, Wang H, Hou Z, Chen T, Fu J, Ma X, He G, Xue X, Jia M, Luo X. 2009. Novel anion liposome-encapsulated antisense oligonucleotide restores susceptibility of methicillin-resistant Staphylococcus aureus and rescues mice from lethal sepsis by targeting mecA. Antimicrob Agents Chemother 53:2871–2878. doi: 10.1128/AAC.01542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, Broberger C, Porreca F, Lai J, Ren K, Ossipov M, Koshkin A, Jakobsen N, Skouv J, Oerum H, Jacobsen MH, Wengel J. 2000. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci U S A 97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braasch DA, Liu Y, Corey DR. 2002. Antisense inhibition of gene expression in cells by oligonucleotides incorporating locked nucleic acids: effect of mRNA target sequence and chimera design. Nucleic Acids Res 30:5160–5167. doi: 10.1093/nar/gkf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houseley J, Tollervey D. 2009. The many pathways of RNA degradation. Cell 136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Qu Z, Kim S, Shi V, Liao B, Kraft P, Bandaru R, Wu Y, Greenberger LM, Horak ID. 2011. Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther 18:326–333. doi: 10.1038/gt.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller BL, Deere JD, Stein DA, Kroeker AD, Moulton HM, Iversen PL. 2003. Inhibition of gene expression in Escherichia coli by antisense phosphorodiamidate morpholino oligomers. Antimicrob Agents Chemother 47:3233–3239. doi: 10.1128/AAC.47.10.3233-3239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]