Abstract
Vancomycin is frequently inappropriately prescribed, especially as empirical treatment. The aim of this study was to evaluate (i) the amount of inappropriate continued empirical vancomycin use as a proportion of total vancomycin use and (ii) the risk factors associated with inappropriate continued empirical vancomycin use. We reviewed the medical records of adult patients who had been prescribed at least one dose of parenterally administered vancomycin between January and June 2012, in a single tertiary care hospital. When empirically prescribed vancomycin treatment was continued after 96 h without documentation of beta-lactam-resistant Gram-positive microorganisms in clinical specimens with significance, the continuation was considered inappropriate, and the amount used thereafter was considered inappropriately used. We identified risk factors associated with inappropriate continued empirical vancomycin use by multiple logistic regression. During the study period, the amount of parenterally administered vancomycin prescribed was 34.2 defined daily doses (DDDs)/1,000 patient-days (1,084 prescriptions for 971 patients). The amount of inappropriate continued empirical vancomycin use was 8.5 DDDs/1,000 patient-days, which represented 24.9% of the total parenterally administered vancomycin used (8.5/34.2 DDDs/1,000 patient-days). By multivariate analyses, inappropriate continued empirical vancomycin use was independently associated with the absence of any documented etiological organism (adjusted odds ratio [aOR], 1.60 [95% confidence interval {CI}, 1.06 to 2.41]) and suspected central nervous system (CNS) infections (aHR, 2.33 [95% CI, 1.20 to 4.50]). Higher Charlson's comorbidity index scores were inversely associated with inappropriate continued empirical vancomycin use (aHR, 0.90 [95% CI, 0.85 to 0.97]). Inappropriate continued empirical vancomycin use represented 24.9% of the total amount of vancomycin prescribed, which indicates room for improvement.
INTRODUCTION
With the aim of reducing vancomycin resistance, the Centers for Disease Control and Prevention (CDC) developed criteria for the appropriate use of vancomycin almost 2 decades ago (1). Despite the presence of these well-established guidelines, it has been reported that the proportions of inappropriately used vancomycin range between 20% and 70% (2–7).
Determination of the appropriateness of vancomycin for specific treatment is unambiguous; it is considered appropriate for treating beta-lactam-resistant Gram-positive microorganisms, but it is inappropriate for beta-lactam-susceptible microorganisms unless the patient is severely allergic to beta-lactam antimicrobials (1). Determination of the appropriateness of vancomycin for the prevention of infectious diseases is also unambiguous, because only prophylaxis for major surgical procedures involving implantation of prosthetic materials or devices at institutions that have a high rate of infections caused by methicillin-resistant Staphylococcus aureus (MRSA) or methicillin-resistant Staphylococcus epidermidis (MRSE) is considered appropriate (1).
It is difficult to determine the appropriateness of empirical vancomycin use, especially in hospitals with a high prevalence of MRSA, because poor outcomes have been documented for patients with MRSA bacteremia for whom appropriate therapy was delayed (8). Although the Infectious Diseases Society of America (IDSA) recommends empirical vancomycin use in several circumstances for patients with neutropenia (9), continued empirical use of vancomycin for presumed infections in patients whose culture results are negative for beta-lactam-resistant Gram-positive microorganisms is considered inappropriate. In the guidelines of the CDC and the IDSA, it is strongly recommended that empirical vancomycin treatment be stopped when available culture results fail to reveal beta-lactam-resistant Gram-positive bacterial infections (1, 9); however, it is well known that empirical vancomycin use is inappropriately continued for a proportion of patients (2, 3, 10, 11).
In our institution, methicillin resistance was noted for 58.3% and 70.0% of clinical isolates of S. aureus and S. epidermidis, respectively (12). Because of the high prevalence of beta-lactam-resistant Gram-positive bacteria, vancomycin is frequently prescribed empirically, especially for critically ill patients. We conducted this study in a university-affiliated hospital in which MRSA and MRSE are prevalent, to evaluate (i) the amount of inappropriately continued empirical vancomycin use as a proportion of the total amount of vancomycin used and (ii) the risk factors associated with the inappropriate continuation of empirical vancomycin treatment.
MATERIALS AND METHODS
We conducted this retrospective study at Seoul National University Hospital, a 1,600-bed, university-affiliated hospital (Seoul, South Korea), between January and June 2012. We reviewed the medical records of patients who had been prescribed at least one dose of parenterally administered vancomycin during the study period. We considered antimicrobial treatment before knowledge of the culture results, including the antibiogram, to be empirical. We included only patients who were at least 18 years of age. Two prescriptions for the same patient that were separated by 8 days or more were considered independent uses (10).
When empirically prescribed vancomycin treatment was continued beyond 96 h after initiation without the documentation of clinically significant beta-lactam-resistant Gram-positive microorganisms from culture specimens, in the absence of severe allergy to beta-lactam antimicrobials, we defined the continuation as inappropriate, and the amount of drug used thereafter was counted as inappropriately used. The clinical significance of culture isolates was assessed by 2 independent infectious disease specialists. We chose 96 h as the criterion for determining whether the empirical vancomycin treatment was prescribed continuously because by 96 h physicians were able to obtain the results of microbiological examinations carried out when the empirical vancomycin treatment was first prescribed. We did not determine the appropriateness of empirical vancomycin use for the first 96 h of treatment. The amount of vancomycin consumed was recorded as the total grams of drug, converted into defined daily doses (DDDs) per 1,000 patient-days, in accordance with World Health Organization recommendations (13).
We compared patients for whom vancomycin treatment was discontinued within 96 h in the absence of documentation of beta-lactam-resistant Gram-positive microorganisms in clinical specimens and those for whom vancomycin use was continued inappropriately beyond this time. We identified risk factors associated with the inappropriate continuation of empirical vancomycin treatment by multiple logistic regression. Patients who died within 72 h after the initiation of empirical vancomycin treatment were excluded from the analysis, because discontinuation of vancomycin use was presumably not intended by the physicians in those cases.
We collected variables including age, sex, duration of treatment, comorbid conditions, presence of neutropenia, azotemia, and/or any retained prostheses or invasive devices at the time of initiation of vancomycin treatment, suspected diagnosis for vancomycin use, intensive care unit (ICU) admission, admission department (medical or surgical), presentation with septic shock, consultations with infectious disease physicians (including the timing and frequency during vancomycin use), antibiotic treatment history within the previous month, presence of documented etiological organisms, 30-day mortality rate, length of stay after vancomycin initiation, and 90-day readmission rate. The severity of underlying conditions was assessed according to Charlson's comorbidity index score (14). For patients admitted to the ICU, acute physiology and chronic health evaluation II (APACHE II) and simplified acute physiology score II (SAPS II) scores were calculated (15, 16). The medical department included the departments of internal medicine, neurology, emergency medicine, and rehabilitation medicine. The departments of general surgery, orthopedic surgery, thoracic surgery, neurosurgery, otorhinolaryngology, urology, plastic surgery, and obstetrics and gynecology were classified as the surgical department.
Descriptive results for continuous variables were expressed as median values and interquartile ranges (IQRs) or as means and standard deviations (SDs), as appropriate. Differences in clinical characteristics were assessed by using the chi-square test and the Mann-Whitney test for categorical and continuous variables, respectively. A conditional backward stepwise logistic regression model was adopted to adjust for confounding variables and to identify factors associated with inappropriate continued use of vancomycin. Among the variables representing the severity of the patients' condition, only Charlson's comorbidity index scores were included in the multivariate analysis. We considered factors to be statistically significant when two-tailed P values were <0.05. Data analyses were performed using SPSS software (version 21.0; SPSS Inc., Chicago, IL). This study was approved by the institutional review board of Seoul National University Hospital.
RESULTS
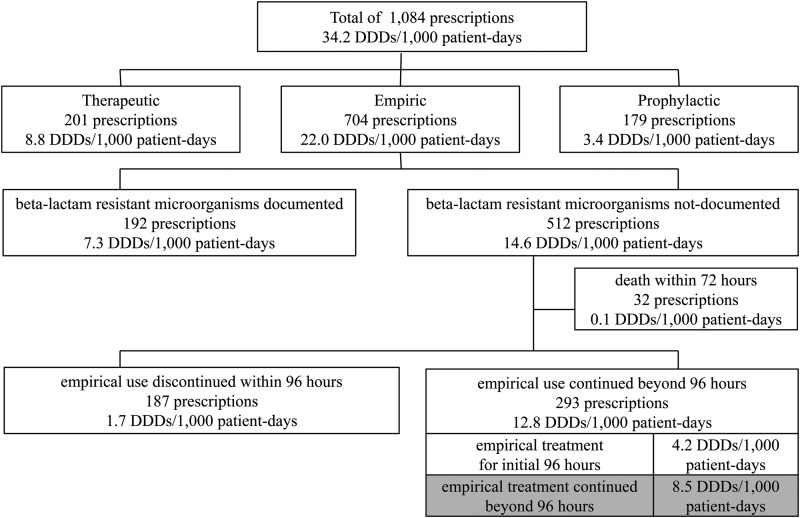
During the study period, a total of 1,084 prescriptions of one or more doses of parenterally administered vancomycin were given to 971 patients. Among the 1,084 prescriptions, 18.5% (201/1,084 prescriptions) were for specific treatment of documented infections, 16.5% (179/1,084 prescriptions) were prophylactic, and 65.0% (704/1,084 prescriptions) were empirical.
Beta-lactam-resistant Gram-positive microorganisms were documented for 192 of the 704 empirical prescriptions and were not documented for the other 512 prescriptions. In the latter cases, 32 patients (with 32 prescriptions) died within 72 h. We analyzed the remaining 480 prescriptions to identify risk factors associated with inappropriate continuation of empirical vancomycin treatment.
Vancomycin use was discontinued within 96 h in 39.0% of these prescriptions (187/480 prescriptions), but the drug was used continuously for ≥96 h in 61.0% (293/480 prescriptions) (Fig. 1). During the study period, the total amount of parenterally administered vancomycin prescribed was 34.2 DDDs/1,000 patient-days. The amounts consumed for specific treatment, prophylaxis, and empirical treatment were 8.8 DDDs/1,000 patient-days (25.7%), 3.4 DDDs/1,000 patient-days (10.0%), and 22.0 DDDs/1,000 patient-days (64.3%), respectively. The amount of inappropriately continued empirical vancomycin treatment was 8.5 DDDs/1,000 patient-days (Fig. 1), which represented 24.9% (8.5/34.2 DDDs/1,000 patient-days) of the total amount of vancomycin used.
FIG 1.
Numbers of prescriptions and amounts of parenterally administered vancomycin prescribed, according to indication. Inappropriate continued empirical vancomycin use is marked in gray.
The demographic and clinical characteristics of patients for whom empirical vancomycin treatment was prescribed but beta-lactam-resistant Gram-positive microorganisms with significance were not documented in clinical specimens are listed in Table 1. The median age of the patients was 61 years, and 59.6% were men. Patients with retained prostheses or devices accounted for nearly 50% of the subjects. The most frequent clinical reason for initiation of vancomycin treatment was pneumonia (90 prescriptions [18.8%]), followed by intraabdominal infections (81 prescriptions [16.9%]) and central nervous system (CNS) infections (70 prescriptions [14.6%]). Microbiological examinations to identify etiological organisms were performed for 462 prescriptions (96.3%), and the proportions with examinations did not differ between the two groups. Etiological organisms were more frequently identified in the group for which vancomycin use was appropriately discontinued (P = 0.009). For 41 prescriptions (8.5%), the patients had received other antibiotics in the 30 days preceding the vancomycin prescription. An infectious disease specialist was consulted at least once for 186 prescriptions (40.0%). The mean time until the first consultation was 1.44 days, and 37 patients received consultations more than once. The time until consultation was significantly shorter for patients who adequately discontinued empirical vancomycin therapy (P = 0.005).
TABLE 1.
Demographic and clinical characteristics of patients for whom empirical vancomycin treatment was prescribed and beta-lactam-resistant Gram-positive microorganisms were not documented in clinical specimens with significance
| Characteristics | Total | Treatment discontinued appropriately within 96 h | Treatment continued inappropriately beyond 96 h | P |
|---|---|---|---|---|
| No. of prescriptions | 480 | 187 | 293 | |
| Age (median [IQR]) (yr) | 61 (50–71) | 62 (53–72) | 60 (48–70) | 0.282 |
| Male (no. [%]) | 285 (59.6) | 106 (56.7) | 179 (61.1) | 0.338 |
| Comorbid conditions (no. [%]) | ||||
| Diabetic mellitus | 108 (20.3) | 47 (22.6) | 61 (18.9) | 0.270 |
| Chronic liver disease | 98 (20.4) | 48 (25.7) | 50 (17.1) | 0.023 |
| Chronic lung disease | 27 (5.6) | 9 (4.8) | 18 (6.1) | 0.685 |
| Congestive heart failure or myocardial infarction | 45 (9.4) | 22 (11.8) | 23 (7.8) | 0.151 |
| Cerebrovascular disease | 56 (11.7) | 18 (6.1) | 38 (7.9) | 0.266 |
| Solid malignancy | 192 (40.0) | 79 (42.2) | 113 (38.6) | 0.422 |
| Hematological malignancy | 92 (19.2) | 33 (17.6) | 59 (20.1) | 0.499 |
| Connective tissue disease | 24 (5.0) | 13 (7.0) | 11 (3.8) | 0.135 |
| Azotemia | 117 (24.4) | 61 (32.6) | 56 (19.1) | 0.001 |
| Neutropenia | 81 (16.9) | 29 (15.5) | 52 (17.7) | 0.523 |
| Prostheses or devices (no. [%]) | ||||
| Any prostheses or devices | 234 (48.8) | 102 (54.5) | 132 (45.1) | 0.042 |
| Central venous catheters | 158 (32.9) | 70 (37.4) | 88 (30.0) | 0.093 |
| Cardiac devices | 22 (4.6) | 11 (5.9) | 11 (3.8) | 0.277 |
| Bone and joint devices | 33 (6.9) | 13 (7.0) | 20 (6.8) | 0.958 |
| Cerebrospinal fluid space devices | 17 (3.5) | 4 (2.1) | 13 (4.4) | 0.215 |
| Artificial vascular grafts | 9 (1.9) | 6 (3.2) | 3 (1.0) | 0.085 |
| Other prostheses or devicesa | 10 (2.1) | 4 (2.1) | 6 (2.0) | |
| Suspected site of infection (no. [%]) | ||||
| Pneumonia | 90 (18.8) | 34 (18.2) | 56 (19.1) | 0.799 |
| Intraabdominal infection | 81 (16.9) | 42 (22.5) | 39 (13.3) | 0.009 |
| CNS infection | 70 (14.6) | 13 (7.0) | 57 (19.5) | <0.001 |
| Skin and soft tissue infection | 54 (11.3) | 17 (9.1) | 37 (12.6) | 0.232 |
| Cardiovascular infection | 39 (8.1) | 17 (9.1) | 22 (7.5) | 0.536 |
| Catheter-related infection | 29 (6.0) | 11 (5.9) | 18 (6.1) | 0.907 |
| Bone and joint infection | 27 (5.6) | 11 (5.9) | 16 (5.5) | 0.845 |
| Urinary tract infection | 5 (1.0) | 3 (1.6) | 2 (0.7) | 0.382 |
| Other infectionb | 17 (3.5) | 5 (2.7) | 12 (4.1) | 0.411 |
| Unknown | 68 (14.2) | 34 (18.2) | 34 (11.6) | 0.044 |
| Admission department (no. [%]) | 0.009 | |||
| Medical | 345 (71.9) | 147 (78.6) | 198 (67.6) | |
| Surgical | 135 (28.1) | 40 (21.4) | 95 (32.4) | |
| Severity measures | ||||
| Charlson's comorbidity index (median [IQR]) | 3 (2–6) | 4 (2–6) | 2 (2–5) | <0.001 |
| ICU admission (no. [%]) | 98 (20.4) | 39 (20.9) | 59 (20.1) | 0.849 |
| APACHE II score (median [IQR]) | 28 (19–32) | 28 (20–31) | 28 (17.25–33) | 0.586 |
| SAPS II score (median [IQR]) | 39 (29–50) | 39 (30–48) | 38 (27.50–52) | 0.872 |
| Septic shock (no. [%]) | 23 (4.8) | 16 (8.6) | 7 (2.4) | 0.002 |
| Microbiological testing before vancomycin prescription (no. [%]) | 462 (96.3) | 180 (96.3) | 282 (96.2) | 0.995 |
| Absence of documented etiological organism (no. [%]) | 343 (71.5) | 121 (64.7) | 222 (75.8) | 0.009 |
| Prior antibiotic therapy within 30 days (no. [%]) | 41 (8.5) | 13 (7.0) | 28 (9.6) | 0.319 |
| Infectious disease specialist consultations | ||||
| No. (%) with consultations | 186 (40.0) | 76 (42.0) | 110 (38.7) | 0.483 |
| Time until consultation (mean ± SD) (days) | 1.44 ± 1.547 | 1.00 ± 1.113 | 1.75 ± 1.727 | 0.005 |
| Frequency of consultations (mean ± SD) (no. during treatment period) | 1.21 ± 0.434 | 1.08 ± 0.271 | 1.30 ± 0.499 | 0.182 |
| Admission duration after vancomycin prescription (median [IQR]) (days) | 19.48 (10.61–33.72) | 14.52 (5.76–31.35) | 21.56 (13.47–36.66) | <0.001 |
| Death (no. [%]) | 69 (14.4) | 31 (16.6) | 38 (13.0)) | 0.272 |
| Readmission within 90 days (no. [%]) | 131 (27.3) | 54 (28.9) | 77 (26.3) | 0.533 |
Six continuous ambulatory peritoneal dialysis catheters, 1 epidural pain control device, 2 nasal prostheses, and 1 ureteral stent.
One epiglottitis case, 6 mediastinitis cases, 5 ocular infections, 2 perianal infections, 1 postauricular abscess, 1 submandibular abscess, and 1 skull base osteomyelitis case.
By univariate analysis, patients with an underlying liver disease or azotemia or any prostheses or devices tended to appropriately discontinue empirical vancomycin therapy. Patients with suspected CNS infections (70 prescriptions [14.6%]) tended to continue empirical vancomycin treatment inappropriately, whereas those with suspected intraabdominal infections (81 prescriptions [16.9%]) tended to discontinue empirical vancomycin treatment. Patients who had higher Charlson's comorbidity index scores or who were prescriptions to medical departments were more likely to appropriately discontinue vancomycin treatment. Patients with documented etiological organisms (137 prescriptions [28.5%]) tended to appropriately discontinue vancomycin therapy. By multivariate analysis, inappropriate continuation of empirical vancomycin treatment was independently associated with the absence of a documented etiological organism (adjusted odds ratio [aOR], 1.60 [95% confidence interval {CI}, 1.06 to 2.41]) and a suspected CNS infection (aOR, 2.33 [95% CI, 1.20 to 4.50]). Higher Charlson's comorbidity index scores were inversely associated with inappropriate vancomycin use (aOR, 0.90 [95% CI, 0.85 to 0.97]) (Table 2).
TABLE 2.
Summary of logistic regression analyses of factors associated with inappropriate continued use of empirical vancomycin beyond 96 h
| Factor | Univariate analysisa |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Age (per 10-yr increase) | 0.92 (0.81–1.03) | 0.14 | ||
| Gender | ||||
| Female | 1.00 | |||
| Male | 1.20 (0.83–1.74) | 0.34 | ||
| Comorbidities | ||||
| None | 1.00 | |||
| Diabetic mellitus | 0.78 (0.51–1.21) | 0.27 | ||
| Chronic liver disease | 0.60 (0.38–0.93) | 0.02 | ||
| Chronic lung disease | 1.30 (0.57–2.95) | 0.54 | ||
| Congestive heart failure or myocardial infarction | 0.64 (0.35–1.18) | 0.15 | ||
| Cerebrovascular disease | 1.40 (0.77–2.53) | 0.27 | ||
| Solid malignancy | 0.86 (0.59–1.25) | 0.42 | ||
| Hematological malignancy | 1.18 (0.73–1.89) | 0.50 | ||
| Any malignancyb | 0.98 (0.68–1.42) | 0.90 | ||
| Connective tissue disease | 0.52 (0.23–1.19) | 0.12 | ||
| Azotemia | 0.49 (0.32–0.74) | <0.001 | ||
| Neutropenia | 1.18 (0.72–1.93) | 0.52 | ||
| Any prostheses or devices | 0.68 (0.47–0.99) | 0.04 | 0.68 (0.46–1.01) | 0.053 |
| Suspected site of infection | ||||
| Pneumonia | 1.06 (0.66–1.71) | 0.80 | ||
| Intraabdominal infection | 0.53 (0.33–0.86) | 0.01 | ||
| CNS infection | 3.23 (1.72–6.09) | <0.001 | 2.33 (1.20–4.50) | 0.012 |
| Skin and soft tissue infection | 1.45 (0.79–2.65) | 0.23 | ||
| Cardiovascular infection | 0.81 (0.42–1.57) | 0.54 | ||
| Catheter-related infection | 1.05 (0.48–2.27) | 0.91 | ||
| Bone and joint infection | 0.92 (0.42–2.04) | 0.85 | ||
| Urinary tract infection | 0.42 (0.07–2.55) | 0.38 | ||
| Unknown | 0.59 (0.35–0.99) | 0.41 | ||
| Admission department | 0.04 | |||
| Medical department | 1.00 | |||
| Surgical department | 1.76 (1.15–2.70) | 0.01 | ||
| Ward | ||||
| General ward | 1.00 | |||
| ICU | 0.96 (0.61–1.51) | 0.85 | ||
| Severity of the infection | ||||
| None | 1.00 | |||
| Septic shock | 0.26 (0.11–0.65) | <0.001 | ||
| Severity scores | ||||
| Charlson's score | 0.89 (0.84–0.95) | <0.001 | 0.90 (0.85–0.97) | 0.004 |
| APACHE II score | 1.01 (0.97–1.05) | 0.70 | ||
| SAPS II score | 1.00 (0.97–1.02) | 0.82 | ||
| Prior antibiotic therapy within 30 days | ||||
| None | 1.00 | |||
| Prior antibiotic therapy | 1.41 (0.71–2.81) | 0.32 | ||
| Infectious disease specialist consultation | ||||
| Consultation with specialist | 1.00 | |||
| No consultation with specialist | 1.14 (0.78–1.66) | 0.50 | ||
| Microbiological testing | ||||
| None | 1.00 | |||
| Cultures performed | 1.00 (0.38–2.62) | 1.00 | ||
| Results of microbiological tests | ||||
| Documented etiological organism | 1.00 | |||
| Undocumented | 1.65 (1.11–2.46) | 0.01 | 1.60 (1.06–2.41) | 0.027 |
OR, odds ratio; aOR, adjusted odds ratio.
Includes both solid malignancies and hematological malignancies.
Thirty-day mortality rates and 90-day readmission rates were not significantly different for patients for whom empirical vancomycin treatment was discontinued appropriately versus continued inappropriately after 96 h. Patients for whom empirical vancomycin treatment was continued inappropriately were admitted for longer times than were those for whom empirical vancomycin use was appropriately discontinued (P < 0.001).
DISCUSSION
In this study, we found that the amount of inappropriately continued empirical vancomycin treatment represented 24.9% (8.5/34.2 DDDs/1,000 patient-days) of the total amount of prescribed vancomycin; there is thus room for improvement. The increased use of vancomycin has been associated with the development of drug resistance in Enterococcus species and S. aureus. In a meta-analysis, vancomycin use was associated with a 2.7-fold increased risk of vancomycin-resistant Enterococcus (VRE) acquisition (17). An association between increased use of vancomycin and the emergence of vancomycin-intermediate-resistant S. aureus and vancomycin-resistant S. aureus has also been reported (18).
The CDC has developed guidelines for the appropriate use of vancomycin, in order to reduce vancomycin resistance (1). However, the CDC guidelines offer little guidance regarding the empirical use of vancomycin, particularly with respect to institutions in which MRSA is endemic. The prevalence of MRSA was lower when the guidelines were developed, but now it is as high as 30 to 50% in many countries (19). Because of the association between delayed administration of adequate antibiotic therapy and poor prognoses for patients with MRSA bacteremia, the empirical use of vancomycin for suspected Gram-positive bacterial infections has been considered adequate by some researchers (10, 20). The initial empirical use of antibiotics with activity against all likely pathogens for patients with sepsis, severe sepsis, or septic shock is recommended by the Surviving Sepsis Campaign (21).
Antibiotic de-escalation is strongly recommended to minimize adverse events and the emergence of resistant microorganisms (22). Although there has been no randomized controlled trial, de-escalation after the causative pathogen has been identified is recommended (23). However, empirical vancomycin administration is de-escalated for less than 50% of candidates (4, 24). Failure to follow the recommendation on de-escalation may reflect a lack of confidence in laboratory results, the possibility of unidentified pathogens, or the perceived absence of any need for de-escalation.
Factors associated with the inappropriate use of vancomycin have been evaluated in several studies (2, 10, 11, 25). However, risk factors for inappropriate continuation of empirical vancomycin treatment are less frequently studied. A study by Junior et al., which analyzed inappropriate continuation of vancomycin therapy at 72 h after initiation, found that vancomycin use was continued due to critical clinical conditions, without the documentation of Gram-positive organisms, in over 50% of cases and related factors were age of less than 60 years, non-ICU admission, and the absence of neutropenia (2). In our study, the absence of documented etiological organisms, suspected CNS infection, and lower severity of comorbid conditions were independent risk factors for inappropriate continuation of empirical vancomycin treatment.
Physicians tend to discontinue empirical vancomycin treatment when the etiological organism is identified and is susceptible to other antibiotics. However, they are reluctant to do so when the etiological organism is not identified (23). De-escalation of empirical treatment with broad-spectrum antibiotics is recommended when the causative pathogen has been identified (21). However, there are no specific recommendations for de-escalation when the causative pathogen has not been detected, and further research is warranted to investigate the benefits of de-escalation in such situations. We noted in this study that physicians tended to continue empirical vancomycin use inappropriately for patients with suspected CNS infections, especially postneurosurgical patients, who accounted for 67.1%. Physicians may be reluctant to discontinue vancomycin therapy considering the potential sequelae of CNS infections if they are insufficiently managed.
Interestingly, patients with higher Charlson's scores tended to have vancomycin use discontinued more appropriately than did patients with lower scores. The severity of infection, represented by septic shock, showed the same tendency, which is in contrast to the report that inappropriate empirical vancomycin prescriptions were more frequent for patients who were not admitted to the ICU, compared with those who were admitted to the ICU (2). Although we are unable to account for this, we think there is a possibility that patients with more-severe infections are likely to be more thoroughly evaluated to seek definitive therapy, rather than continuing empirical vancomycin treatment in the absence of any definite benefit.
It is difficult to dissuade physicians from prescribing vancomycin empirically for critically ill patients in a hospital with a high prevalence of MRSA or MRSE. Some interventions to increase the appropriateness of continued empirical vancomycin use at 72 to 96 h would be sensible and helpful in reducing the amount of vancomycin prescribed, considering that inappropriately continued vancomycin use makes up one-quarter of the vancomycin prescribed. The safety of vancomycin discontinuation in the absence of documented MRSA has been suggested by multiple studies (26, 27), and Hamilton et al. showed that a continuation form at 72 h was effective in reducing inappropriately continued vancomycin use (24). In cases of suspected ventilator-associated pneumonia, antibiotic discontinuation strategies based on clinical criteria were successful in safely reducing antibiotic treatment duration (28, 29). However, only studies of a quasi-experimental design, providing low-quality evidence, have been reported to date, and further studies with high quality are needed.
Our study has several limitations. First, we focused only on evaluating the appropriateness of continued empirical vancomycin use, and we did not determine the overall appropriateness of vancomycin treatment. We did not determine the appropriateness of the empirical vancomycin use for the first 96 h after the initiation of treatment because that could be ambiguous in hospitals in which MRSA is prevalent. Second, the 96-hour window might have reduced the proportion of inappropriately prescribed vancomycin, in comparison with the 72-hour window in preceding studies. Third, due to the retrospective nature of this study, there might have been unidentified confounding factors for the inappropriate continued use of vancomycin. In conclusion, inappropriate continued empirical vancomycin use represented 24.9% of the total amount of vancomycin used and was independently associated with the absence of documented etiological organisms, suspected CNS infections, and lower severity of comorbid conditions.
ACKNOWLEDGMENTS
This study was supported by grant 2013-2425 from the Clinical Research Institute, Seoul National University Hospital.
We do not have any commercial or other connections that might create any conflicts of interest.
REFERENCES
- 1.Hospital Infection Control Practices Advisory Committee. 1995. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Am J Infect Control 23:87–94. doi: 10.1016/0196-6553(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 2.Junior MS, Correa L, Marra AR, Camargo LF, Pereira CA. 2007. Analysis of vancomycin use and associated risk factors in a university teaching hospital: a prospective cohort study. BMC Infect Dis 7:88. doi: 10.1186/1471-2334-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo DO, Ribeiro E. 2009. Vancomycin use in a Brazilian teaching hospital: comparison with the Hospital Infection Control Practices Advisory Committee Guidelines (HICPAC). Braz J Infect Dis 13:161–164. doi: 10.1590/S1413-86702009000300001. [DOI] [PubMed] [Google Scholar]
- 4.Alfandari S, Levent T, Descamps D, Hendricx S, Bonenfant C, Taines V, Cattoen C, Arimane O, Grandbastien B. 2010. Evaluation of glycopeptide use in nine French hospitals. Med Mal Infect 40:232–237. doi: 10.1016/j.medmal.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Dib JG, Al-Tawfiq JA, Al Abdulmohsin S, Mohammed K, Jenden PD. 2009. Improvement in vancomycin utilization in adults in a Saudi Arabian medical center using the Hospital Infection Control Practices Advisory Committee guidelines and simple educational activity. J Infect Public Health 2:141–146. doi: 10.1016/j.jiph.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Vazin A, Japoni A, Shahbazi S, Davarpanah MA. 2012. Vancomycin utilization evaluation at hematology-oncology ward of a teaching hospital in Iran. Iran J Pharm Res 11:163–170. [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder GM, Patel PR, Kallen AJ, Strom JA, Tucker JK, D'Agata EM. 2013. Antimicrobial use in outpatient hemodialysis units. Infect Control Hosp Epidemiol 34:349–357. doi: 10.1086/669869. [DOI] [PubMed] [Google Scholar]
- 8.Marchaim D, Kaye KS, Fowler VG, Anderson DJ, Chawla V, Golan Y, Karchmer AW, Carmeli Y. 2010. Case-control study to identify factors associated with mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect 16:747–752. doi: 10.1111/j.1469-0691.2009.02934.x. [DOI] [PubMed] [Google Scholar]
- 9.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 10.Roustit M, Francois P, Sellier E, Roch N, Vittoz JP, Foroni L, Stahl JP, Pavese P. 2010. Evaluation of glycopeptide prescription and therapeutic drug monitoring at a university hospital. Scand J Infect Dis 42:177–184. doi: 10.3109/00365540903413614. [DOI] [PubMed] [Google Scholar]
- 11.Logsdon BA, Lee KR, Luedtke G, Barrett FF. 1997. Evaluation of vancomycin use in a pediatric teaching hospital based on CDC criteria. Infect Control Hosp Epidemiol 18:780–782. doi: 10.2307/30141327. [DOI] [PubMed] [Google Scholar]
- 12.Kim NH, Hwang JH, Song KH, Choe PG, Park WB, Kim ES, Park SW, Kim HB, Kim NJ, Oh MD, Kim EC. 2012. Changes in antimicrobial susceptibility of blood isolates in a university hospital in South Korea, 1998-2010. Infect Chemother 44:275–281. doi: 10.3947/ic.2012.44.4.275. [DOI] [Google Scholar]
- 13.Maxwell M, Heaney D, Howie JG, Noble S. 1993. General practice fundholding: observations on prescribing patterns and costs using the defined daily dose method. BMJ 307:1190–1194. doi: 10.1136/bmj.307.6913.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall JR, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, Mercier P, Thomas R, Villers D. 1984. A simplified acute physiology score for ICU patients. Crit Care Med 12:975–977. doi: 10.1097/00003246-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Carmeli Y, Samore MH, Huskins C. 1999. The association between antecedent vancomycin treatment and hospital-acquired vancomycin-resistant enterococci: a meta-analysis. Arch Intern Med 159:2461–2468. doi: 10.1001/archinte.159.20.2461. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari HK, Sen MR. 2006. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis 6:156. doi: 10.1186/1471-2334-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 20.Wright SW, Wrenn KD. 1998. Appropriateness of vancomycin use in the emergency department. Ann Emerg Med 32:531–536. doi: 10.1016/S0196-0644(98)70030-7. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 22.Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 23.Silva BN, Andriolo RB, Atallah AN, Salomao R. 2013. De-escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev 3:CD007934. doi: 10.1002/14651858.CD007934.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton CD, Drew R, Janning SW, Latour JK, Hayward S. 2000. Excessive use of vancomycin: a successful intervention strategy at an academic medical center. Infect Control Hosp Epidemiol 21:42–45. doi: 10.1086/501703. [DOI] [PubMed] [Google Scholar]
- 25.Evans ME, Kortas KJ. 1996. Vancomycin use in a university medical center: comparison with Hospital Infection Control Practices Advisory Committee guidelines. Infect Control Hosp Epidemiol 17:356–359. doi: 10.2307/30141133. [DOI] [PubMed] [Google Scholar]
- 26.Boyce JM, Pop OF, Abreu-Lanfranco O, Hung WY, Fisher A, Karjoo A, Thompson B, Protopapas Z. 2013. A trial of discontinuation of empiric vancomycin therapy in patients with suspected methicillin-resistant Staphylococcus aureus health care-associated pneumonia. Antimicrob Agents Chemother 57:1163–1168. doi: 10.1128/AAC.01965-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacFadden DR, Elligsen M, Robicsek A, Ricciuto DR, Daneman N. 2013. Utility of prior screening for methicillin-resistant Staphylococcus aureus in predicting resistance of S. aureus infections. CMAJ 185:E725–E730. doi: 10.1503/cmaj.130364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micek ST, Ward S, Fraser VJ, Kollef MH. 2004. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest 125:1791–1799. doi: 10.1378/chest.125.5.1791. [DOI] [PubMed] [Google Scholar]
- 29.Kollef MH, Kollef KE. 2005. Antibiotic utilization and outcomes for patients with clinically suspected ventilator-associated pneumonia and negative quantitative BAL culture results. Chest 128:2706–2713. doi: 10.1378/chest.128.4.2706. [DOI] [PubMed] [Google Scholar]