Abstract
Although antibiotics treat bacteremia in inhalational anthrax, pathogenesis is mainly driven by bacterial exotoxins. Raxibacumab, an IgG1 monoclonal antibody, binds the protective antigen (PA) of Bacillus anthracis, thus blocking toxin effects and leading to improved survival in the rabbit and monkey models of inhalational anthrax. To assess raxibacumab's added benefit over levofloxacin (LVX) alone, rabbits surviving to 84 h after a challenge with 200 times the median (50%) lethal dose of B. anthracis spores were randomized to receive 3 daily intragastric LVX doses of 50 mg/kg of body weight, with the first LVX dose administered just prior to administration of a single intravenous dose of placebo or 40 mg/kg raxibacumab. The percentages of animals alive at 28 days following the last LVX dose were compared between the 2 treatment groups using a two-sided likelihood-ratio chi-square test. The 82% survival rate for the LVX-raxibacumab combination was higher than the 65% survival rate for LVX alone (P = 0.0874). There were nearly 2-fold fewer deaths for the combination (7 deaths; n = 39) than for LVX alone (13 deaths; n = 37), and the survival time was prolonged for the combination (P = 0.1016). Toxin-neutralizing-activity titers were similar for both treatment groups, suggesting that survivors in both groups were able to mount a toxin-neutralizing immune response. Microscopic findings considered consistent with anthrax were present in animals that died or became moribund on study in both treatment groups, and there were no anthrax-related findings in animals that survived. Overall, raxibacumab provided a meaningful benefit over antibiotic alone when administered late in the disease course.
INTRODUCTION
Bacillus anthracis causes inhalational anthrax (1), and its endospores have been developed to be a highly lethal bioterrorism threat (2, 3). While antibiotics can be used to effectively treat bacteremia, human inhalational anthrax mortality ranges from 45 to 80% (1, 2, 4) mainly due to anthrax exotoxin-driven pathogenesis (1, 5–7).
The anthrax toxin is a tripartite toxin that contains enzymatic and binding moieties. Lethal factor (LF) and edema factor (EF) have enzymatic activities. Protective antigen (PA) is the gatekeeper moiety that binds to cell receptors and then binds and translocates LF and EF into the cell (2). Raxibacumab is a fully human IgG1 monoclonal antibody (MAb) that binds PA (8), thus blocking the binding of PA to its cell receptors, the binding of LF and EF, and the internalization of anthrax toxin (6, 9).
The clinical presentation of inhalational anthrax is similar in rabbits, monkeys, and humans (10–13). Using the rabbit and monkey models of inhalational anthrax, raxibacumab efficacy was demonstrated when it was used as an intervention at the onset of disease either as monotherapy (14) or with antibiotics (unpublished data). However, antibiotics are highly effective when administered at the onset of disease (15, 16), so it was not possible to determine the added benefit over antibiotic alone. This study assessed the added benefit when raxibacumab treatment, in addition to antibiotics, was initiated late in the course of disease in New Zealand White rabbits.
MATERIALS AND METHODS
The in-life portions of this good laboratory practice study were conducted at the Battelle Biomedical Research Center (West Jefferson, OH). The study was approved by Battelle's Institutional Animal Care and Use Committee (22). The study was conducted in accordance with the GlaxoSmithKline policy on the care, welfare, and treatment of laboratory animals. The quantitative assays for plasma raxibacumab concentrations, antiraxibacumab antibody (ADA) concentrations, and toxin-neutralizing activity (TNA) titers, as well as the statistical analysis and raxibacumab pharmacokinetic (PK) analysis, were performed at Human Genome Sciences, Inc. (HGS; Rockville, MD). The quantitative assays for plasma LVX concentrations were performed at Covance Bioanalytical Services, LLC (Indianapolis, IN). The determination of plasma PA concentrations and the PA kinetic analysis performed at HGS have been reported previously (17).
Test and control articles.
Raxibacumab was expressed in the NS0 mouse myeloma cell line and secreted into culture medium, from which it was purified by a series of chromatography and filtration steps. Raxibacumab (lot 71128; HGS) was supplied as a sterile liquid formulation. Storage was at 2 to 8°C.
Placebo (raxibacumab formulation buffer; lot 71043; HGS) was supplied as a sterile liquid formulation. Storage was at 2 to 8°C.
Commercially available levofloxacin (Levaquin; Janssen) oral solution (25 mg/ml; lot AEB2V00) was used. It is a multiuse self-preserving aqueous solution of LVX with a pH ranging from 5.0 to 6.0. LVX oral solution was administered as supplied, without dilution. It was stored at room temperature (between 15°C and 30°C).
Experimental animals.
Specific pathogen-free (SPF) New Zealand White (NZW) rabbits [Oryctolagus cuniculus Hra:(NZW) SPF] weighing between 2.75 kg and 4.5 kg and surgically implanted with vascular access ports (VAPs) were supplied by Covance Research Products, Inc. (Denver, PA). Of the 210 rabbits (105 males and 105 females) supplied, 180 were required for the study, with the extra animals being available as replacements before spore challenge, if required. No rabbits were replaced after spore challenge. After a quarantine period (minimum of 7 days), rabbits that were in good health, that were free of malformations, and that exhibited no signs of clinical disease were randomly selected for the study. Rabbit age was not a criterion for placement on study. The animals were placed in individual cages and were given water and feed ad libitum. All rabbits were housed in compliance with the most recent United States Department of Agriculture (USDA) guidelines.
Challenge of animals with aerosolized B. anthracis spores.
B. anthracis (Ames strain) spores were aerosolized by a Collison nebulizer (BGI, Waltham, MA). Animals were placed individually in a plethysmography chamber inside a class III biosafety cabinet and challenged via the inhalation route with a target dose of 200 times the median (50%) lethal dose (LD50) of the aerosolized spores, which is equivalent to 2.1 × 107 spores on the basis of published LD50 data (13). Rabbits were not anesthetized prior to challenge.
To estimate the inhaled aerosol concentrations of B. anthracis, effluent aerosol streams were collected directly from an animal exposure port via an inline impinger (model 7541; Ace Glass Incorporated, Vineland, NJ). Serial dilutions of impinger samples were plated on Trypticase soy agar (TSA) plates, and the CFU were enumerated.
Experimental design.
This was a parallel-group, blinded, randomized, placebo-controlled study. Rabbits were exposed to 200 times the LD50 of anthrax spores on day 0. After 84 h, animals that were alive (n = 76) received treatment first with LVX oral solution administered by gastric intubation and second with an intravenous (i.v.) injection of raxibacumab or raxibacumab buffer. The appropriate dose of LVX was drawn up into a syringe just prior to each gavage. Animals were administered 3 doses of LVX (50 mg/kg of body weight): initially once at 84 ± 4 h of actual exposure time and then every 24 ± 1 h thereafter for another 2 days, if the animals survived to receive each treatment. Raxibacumab (40 mg/kg, 0.8 ml/kg) or raxibacumab buffer (0.8 ml/kg) was administered via the VAP or a marginal ear vein immediately after administration of the first LVX dose.
The primary endpoint of the study was survival 28 days after the last dose of LVX. Animals were observed until day 35 (i.e., at treatment at 84 h [3.5 days], followed by 3 days of LVX dosing, and 28 days after last dose of LVX) and euthanized.
Animal observations and temperature monitoring occurred approximately every 6 h between the time of spore challenge and 10 days postchallenge. Monitoring was twice daily on all other study days up to day 35; there was a single observation on day 35 prior to euthanasia. Death or euthanasia was recorded at the time observed, and a complete necropsy was performed. Blood samples were also taken to assess the bacteremia; plasma PA, raxibacumab, LVX, and ADA concentrations; and TNA titers.
Complete gross necropsies were conducted on all challenged rabbits that died on study to confirm that death was due to anthrax. Gross pathology, tissue preservation, and histopathology were conducted on all animals that were treated. The tissues evaluated included brain, bronchial and mediastinal lymph nodes, heart, kidney, liver, lungs, and spleen. The study pathologist was blinded and unaware of the treatment administered to each rabbit until after the slides had been read.
Assessment of bacteremia.
Bacteremia was measured immediately before spore challenge (baseline), at every 12 h after challenge up to the time of treatment, at 2 and 24 h after each LVX dose, and at 5, 6, 7, 14, 21, and 28 days after the first LVX dose. Cultures were performed as described by Migone et al. (see the supplemental material for reference 14). The limit of detection (LOD) of the assay was 25 CFU/ml of whole blood.
Additionally, quantitative bacteremia was assessed at 4 time points, at 24 h after the challenge time, immediately prior to treatment initiation, at 24 h after the first LVX dose (prior to the second dose), and at 2 days following the last LVX dose (study day 8), as follows: 100 μl of whole blood was plated in triplicate on TSA. In addition, a series (10−1 to 10−8) of 1:10 serial dilutions was performed by transferring 100 μl of whole blood or a previous dilution into 900 μl of phosphate-buffered saline. For each dilution prepared, 100 μl was plated in triplicate on TSA. Typically, the acceptable countable range for the enumeration of counts in samples is 25 to 250 CFU, with average counts falling outside this range reported only as being positive for bacteremia and with no quantifiable number being reported.
Levofloxacin assay.
Blood specimens were collected prior to spore challenge, immediately prior to each LVX dose, at 2 h after each LVX dose, and at 1 and 2 days after the third LVX dose. Blood was collected in chilled EDTA blood tubes that were inverted to mix the blood with the anticoagulant and placed on ice or cold packs. Within 1 h of collection, the plasma was harvested, filtered, and frozen at ≤−70°C.
Plasma samples were analyzed for LVX using a high-performance liquid chromatography/mass spectroscopy/mass spectroscopy (LC/MS/MS) assay. LVX and its internal standard, levofloxacin-d3, were extracted from a 50-μl aliquot of rabbit plasma using protein precipitation. The extracted samples were injected into a liquid chromatograph equipped with an Atlantis dC18 column (50 by 2.1 mm; particle size, 5 μm). The detection method used was mass spectroscopy/mass spectroscopy with positive-ion electrospray ionization. The calibration range for the assay was from 20 to 10,000 ng/ml.
Raxibacumab assay.
Blood specimens were collected in EDTA blood tubes prior to spore challenge, immediately prior to the first LVX dose, and at 5 min, 2 and 8 h, and 1, 2, 4, 7, 14, 21, and 28 days after the raxibacumab dose. Plasma was harvested, filtered, and frozen at ≤−70°C.
Plasma samples were analyzed for raxibacumab using an electrochemiluminescence (ECL)-based assay (see the supplemental material for reference 14). The lower limit of quantitation was 720 ng/ml of raxibacumab in 100% rabbit plasma.
Antiraxibacumab antibody assay.
Blood specimens were collected in EDTA blood tubes prior to spore challenge and at 28 days after the raxibacumab dose. Plasma was harvested, filtered, and frozen at ≤−70°C.
Plasma samples were assessed for ADA activity as described by Migone et al. (see the supplementary material for reference 14). The LOD was 0.5 μg/ml of ADA in the absence of drug and 10 μg/ml in the presence of up to 150 μg/ml raxibacumab.
Toxin-neutralizing activity assay.
For the toxin-neutralizing activity assay, blood specimens were collected in EDTA blood tubes prior to spore challenge and at 28 days after the raxibacumab dose. Plasma was harvested, filtered, and frozen at ≤−70°C.
TNA titers in plasma samples were determined using a cell killing assay based on that described by Hering et al. (18). The LOD was a titer of 52 at a minimum (initial) 1:50 sample dilution.
MICs.
The MICs of LVX were determined using a broth microdilution method to assess the antimicrobial susceptibility of the B. anthracis challenge material and B. anthracis-positive cultures of terminal blood samples collected from animals that received at least one LVX dose and succumbed to disease prior to day 35. If a terminal sample was not available, the last positive blood culture was used. Briefly, control and test plates were prepared using Mueller-Hinton broth (MHB). LVX was added to the appropriate wells, and samples were serially diluted across the entire plate. The control plate included Staphylococcus aureus as a quality control organism and B. anthracis challenge material as the standard control organism. The plates were covered and incubated at 37°C for 16 to 20 h. The wells were observed for signs of bacterial growth. The MIC was determined as the lowest concentration of LVX that completely inhibited the growth of B. anthracis. This method was not validated and used simply to assess gross changes in LVX susceptibility.
Pharmacokinetic analyses.
PK analyses for all raxibacumab-dosed rabbits were conducted using population analysis techniques with NONMEM software. Since raxibacumab PKs in rabbits are consistent with a 2-compartment model with first-order elimination from the central compartment (19), that model was applied to the results obtained in this study. Body weight, sex, age, size of spore challenge, duration of spore challenge, survival time, survival status, time to first bacteremia by culture, bacteremia outcome at each collection time, and immunogenicity status were evaluated as potential covariates for the PK parameters. PK parameter estimates for individual animals were derived from the final model.
Plasma LVX concentration-time profiles for each rabbit were analyzed individually. The maximum plasma LVX concentration after each dose (Cmax,n) was defined as the concentration measured 2 h after the nth dose, while the minimum plasma drug concentration after the nth dose (Cmin,n) was defined as the concentration measured just prior to the subsequent dose or, for the third dose, at 24 h after that dose.
Statistical analysis.
The primary efficacy endpoint was 28-day survival, defined as the proportion of animals that survived to 28 days after the last LVX dose. On the basis of previous experience (14), about 35% of animals would be expected to survive until 84 h after spore challenge. It was estimated that 32 animals would be treated in each of the 2 active-treatment groups. This study design was prospectively estimated to have an approximately 52% power to detect a 25% absolute improvement in 28-day survival for the raxibacumab-LVX combination group over that for the LVX group, assuming 40% 28-day survival in the LVX group. The study was not powered to demonstrate a statistically significant difference at a medically important change in mortality, which would be less than the 25% improvement used for the sample size calculation.
The primary efficacy analysis was performed on the intention-to-treat (ITT) population (rabbits that survived to 84 h and were eligible to receive treatment) using a two-sided likelihood-ratio chi-square test. All statistical tests were two-sided and performed at a significance level of 0.05, unless otherwise specified.
To further explore efficacy, subgroup analyses of the primary efficacy endpoint were performed using a two-sided likelihood-ratio chi-square test in subgroups of rabbits confirmed to have been toxemic (i.e., to have had detectable serum PA levels), bacteremic, toxemic and bacteremic, or toxemic and/or bacteremic at or before the time of treatment initiation.
As an exploratory efficacy analysis, the log-rank test was used to compare the survival time from the time of initiation of spore challenge between the groups in the ITT population. In addition, the survival time from the time of treatment initiation was compared using the same method.
An analysis of variance (ANOVA) was used to determine if there were differences in the multiple of the LD50 of B. anthracis spores administered between treatment groups. Age and body weight were evaluated as continuous variables and are presented using the mean, standard deviation (SD), median, and range. An ANOVA was used to determine treatment imbalances at the baseline for age and body weight, and the likelihood-ratio test was used to determine treatment imbalances at the baseline for sex.
Unpaired t tests were used to compare LVX Cmax,n and Cmin,n values between the treatment groups.
RESULTS
Study population and spore challenge.
Baseline characteristics of sex, age, body weight, and toxemic status for the 180 challenged rabbits are summarized in Table S1 in the supplemental material. No significant differences in demographic or baseline characteristics were observed between the two treatment groups.
The mean spore challenge for the raxibacumab-LVX group was 14% higher than that for the LVX-alone group (P = 0.0291; Table 1). A post hoc sensitivity analysis of the primary efficacy endpoint was performed.
TABLE 1.
Extent of anthrax exposure following a target B. anthracis spore challenge of 200 times the LD50 in New Zealand White rabbits in which treatment was initiated at 84 h postchallenge
| Group | Multiple of LD50 |
||
|---|---|---|---|
| Mean ± SD | Median | Range | |
| Spore-challenged rabbits (n = 180) | 187.5 ± 45.1 | 183 | 83.0–348.0 |
| Rabbits that died before treatmenta (n = 104) | 188.6 ± 43.5 | 183 | 86.0–305.0 |
| Rabbits that survived until treatment withb: | |||
| LVX + placebo (n = 37) | 173.8 ± 43.1 | 162 | 83.0–277.0 |
| LVX + raxibacumab (n = 39) | 197.4 ± 49.1 | 198 | 105.0–348.0 |
Rabbits that were challenged and died before treatment.
Rabbits that survived until the 84-h postchallenge treatment time. Rabbits were administered daily 50-mg/kg LVX doses intragastrically for 3 days. Immediately after the first LVX dose, rabbits were administered a single intravenous injection of placebo or 40 mg/kg raxibacumab. P was 0.0291 for the difference between the two treatment groups and was determined by a one-way ANOVA.
Bacteremia.
Bacteremia results are summarized in Table S2 in the supplemental material. Of the 76 treated animals, 75 (98.6%) were bacteremic prior to treatment. For both groups, >27% of the animals were abacteremic by 2 h after the first LVX dose. By 24 h after treatment initiation, >94% were abacteremic, and all animals had negative blood cultures by 24 h after their second LVX dose. Of the treated animals that died, 57% in the combination group and 85% in the antibiotic-alone group were abacteremic at death.
Efficacy.
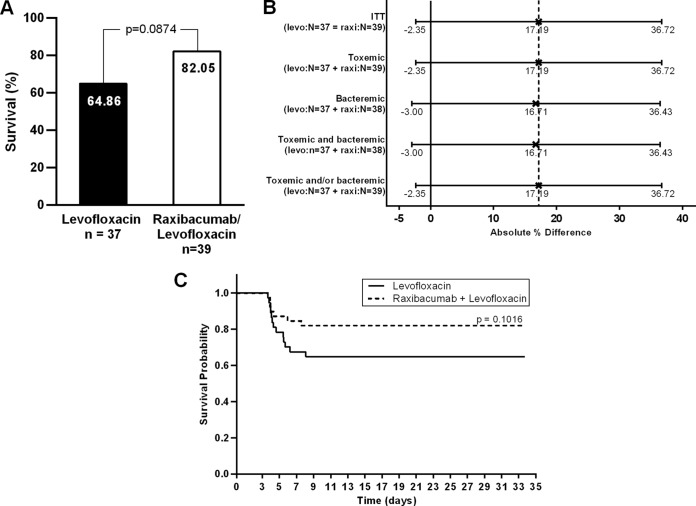
The survival rate for rabbits treated with the raxibacumab-LVX combination (32/39 rabbits, 82.05%) was numerically higher than that for rabbits treated with LVX alone (24/37 rabbits, 64.86%), but the difference did not achieve statistical significance (P = 0.0874) (Fig. 1A). The 17.19% difference in survival rates (95% confidence interval [CI], −2.35% to 36.72%) represents a nearly 2-fold reduction in the number of deaths (7 versus 13 rabbits in the raxibacumab-LVX and LVX groups, respectively), with an odds ratio (OR) of 2.48 (95% CI, 0.86 to 7.15) favoring the combination.
FIG 1.
Survival outcomes in New Zealand White rabbits after challenge with B. anthracis spores at ∼200 times the LD50 and treatment with LVX (50 mg/kg daily for 3 days) plus a single i.v. dose of placebo or 40 mg/kg raxibacumab administered at 84 h postchallenge. (A) Primary endpoint analysis. The P value is based on a two-sided likelihood-ratio chi-square test. (B) Absolute improvement in day 28 survival by subgroup and 95% CI for the raxibacumab-LVX combination group compared with the LVX-alone group. X, point estimate; solid vertical line, 0% benefit; dashed vertical line, point estimate of the survival benefit in the overall (ITT) population. The 95% CIs were calculated by normal approximation. levo, levofloxacin; raxi, raxibacumab. (C) Survival time from the time of spore challenge. The P value was obtained from a log-rank test comparing the survival times between the raxibacumab-LVX combination group and the LVX-alone group.
The sensitivity of the primary efficacy endpoint to the spore challenge difference between the groups was tested by comparing survival rates using a logistic regression model adjusted for baseline spore levels. The likelihood of survival was greater for the combination group (P = 0.0495). The adjusted OR was 3.12 (95% CI, 1.002 to 9.715).
One rabbit in the combination group was not bacteremic at any time while on study but did have detectable plasma PA. This animal survived to the end of the study. A sensitivity analysis that excluded data for this animal was performed: the survival rate difference was 16.72% (81.58% for raxibacumab-LVX and 64.86% for LVX alone).
Primary endpoint analyses were also performed for the subgroups by toxemia and/or bacteremia status prior to treatment (Fig. 1B). The survival benefit in the subgroups was consistent with that observed in the overall population.
The log-rank test was used to compare the survival times from the initiation of spore challenge between the groups (Fig. 1C). The probability of survival was numerically greater for the rabbits treated with the combination, but the difference was not statistically significant (P = 0.1016). The survival times from the time of treatment initiation were compared using the same method, and the survival time was also numerically greater for the rabbits treated with the combination, but the difference was not statistically significant (P = 0.1046) (see Fig. S1 in the supplemental material).
In a post hoc sensitivity analysis of survival times from the time of initiation of spore challenge between the treatments using a proportional hazard model, adjusted for baseline spore levels, the risk of dying was reduced by 59% (P = 0.0633) for the rabbits treated with the combination. The adjusted hazard ratio (HR) for the risk of dying was 0.41 (95% CI, 0.16 to 1.05) for rabbits treated with the combination versus rabbits treated with antibiotic alone.
Antiraxibacumab antibodies.
The immunogenicity outcome was unknown for the 7 raxibacumab-treated rabbits that died. Among the 32 surviving raxibacumab-treated rabbits, 23 (72%) were positive for ADAs (see Table S3 in the supplemental material).
Toxin-neutralizing antibodies.
All treated rabbits had nondetectable TNA titers prior to spore challenge. The mean ± SD TNA titers at 28 days after treatment were similar at 5,150 ± 3,495 for rabbits treated with LVX alone and 4,741 ± 3,005 for rabbits treated with raxibacumab-LVX, indicating that survivors in both groups mounted a toxin-neutralizing immune response (see Table S4 in the supplemental material).
MICs.
The MIC results confirmed that bacteria cultured from the blood of challenged animals were inhibited by concentrations of LVX similar to those that inhibited the bacteria in the challenge material (data not shown).
Levofloxacin pharmacokinetics.
There was some accumulation of LVX in the plasma of both groups, with trough levels for the second and third doses ranging from 344 to 796 ng/ml (Table 2). There were no significant differences in peak or trough levels between treatments (P ≥ 0.1972, unpaired t test), indicating that LVX PKs are unaltered by raxibacumab.
TABLE 2.
LVX PKs in rabbits administered 3 daily intragastric 50-mg/kg LVX doses alone or in combination with a single i.v. 40-mg/kg raxibacumab dose
| Parametera | LVX alone |
LVX + raxibacumab |
P valueb | ||
|---|---|---|---|---|---|
| No. of rabbits | Mean ± SD concn (ng/ml) | No. of rabbits | Mean ± SD concn (ng/ml) | ||
| Cmax,1 | 32 | 6,179 ± 2,921 | 36 | 5,454 ± 2,326 | 0.2661 |
| Cmin,1 | 25 | 424 ± 439 | 32 | 379 ± 214 | 0.6433 |
| Cmax,2 | 26 | 7,487 ± 4,105 | 33 | 7,205 ± 2,347 | 0.7561 |
| Cmin,2 | 25 | 471 ± 455 | 33 | 344 ± 178 | 0.1972 |
| Cmax,3 | 26 | 7,042 ± 3,962 | 34 | 7,772 ± 4,187 | 0.4928 |
| Cmin,3 | 25 | 796 ± 890 | 33 | 512 ± 904 | 0.2380 |
Cmax,n, maximum plasma LVX concentration after the nth dose, defined as the concentration measured 2 h after the dose; Cmin,n, minimum plasma LVX concentration after the nth dose, defined as the concentration measured just prior to the subsequent dose or at 24 h after the third dose.
From an unpaired t test.
Raxibacumab pharmacokinetics.
For ADA-positive rabbits, plasma raxibacumab concentrations became nondetectable at between 14 and 21 days postdosing, whereas ADA-negative rabbits maintained measurable concentrations up to 28 days postdosing (Fig. 2). Although mean raxibacumab concentrations were lower for nonsurvivors than for survivors, the SDs overlapped substantially between those subgroups.
FIG 2.
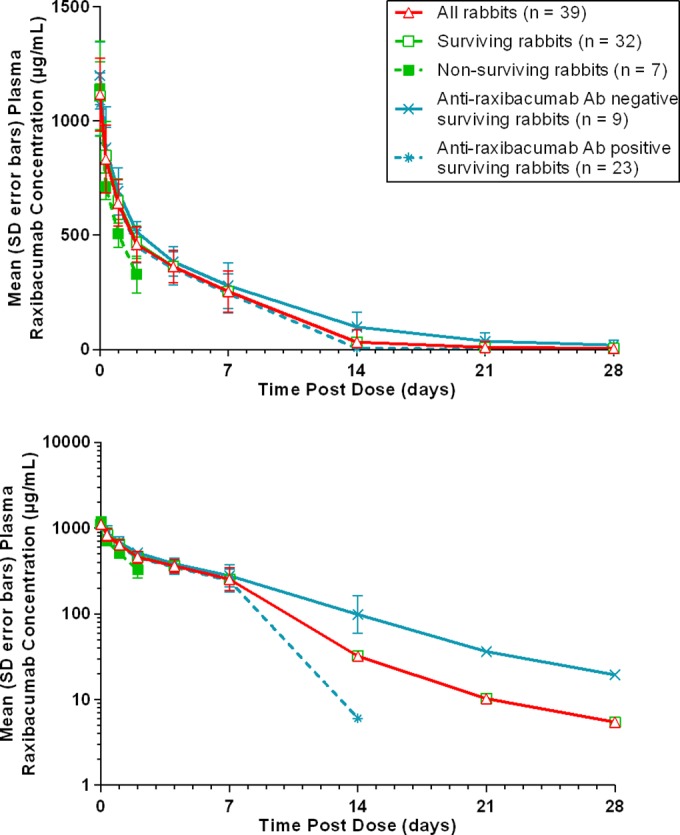
Plasma raxibacumab concentration-time profiles in rabbits administered 3 daily intragastric 50-mg/kg LVX doses in combination with a single i.v. 40-mg/kg raxibacumab dose. (Top) Linear scale; (bottom) semilogarithmic scale.
Raxibacumab concentrations were well described by a 2-compartment open model with first-order elimination from the central compartment (Table 3; see also Fig. S2 in the supplemental material). Body weight was the only significant covariate, accounting for the variability in clearance (CL).
TABLE 3.
Raxibacumab PKs in rabbits administered 3 daily intragastric 50-mg/kg LVX doses alone or in combination with a single i.v. 40-mg/kg raxibacumab dosea
| Parameter | Mean | CV | Mean ± SD valueb |
|---|---|---|---|
| Primary parameters | |||
| V1 (ml) | 112.17 (1.9)c | 10.8 (26.6) | |
| CL (ml/day) | 28.12 (5.5) | 27.5 (31.0) | |
| V2 (ml) | 60.22 (7.5) | 20.6 (87.8) | |
| CLD2 (ml/day) | 73.62 (10.6) | 27.6 (61.9) | |
| Secondary parameters | |||
| Cmax (μg/ml) | 1,114 ± 138 | ||
| AUC0–∞ (μg · day/ml) | 4,590 ± 1,106 | ||
| t1/2α (days) | 0.36 ± 0.05 | ||
| t1/2β (days) | 4.67 ± 1.10 | ||
| MRT (days) | 6.40 ± 1.63 | ||
| Vss (ml/kg) | 56.00 ± 5.74 |
The residual variability was the coefficient of variation of 6.2 (relative standard error [RSE], 51.7) for the proportional error component and SD of 45.5 μg/ml (RSE, 41.2 μg/ml) for the additive error component. The effect of weight on CL, in which weight was normalized to the median body weight for the LVX-raxibacumab group (3.12 kg), was calculated as CL × (weight/3.12)1.743 (RSE, 42.1), with CL for rabbits with weights of 2.75, 3.12, and 3.55 kg being 22.56, 28.12, and 35.21 ml/day, respectively. CV, coefficient of variation; V1, volume of distribution for the central compartment; CL, clearance; V2, volume of distribution for the peripheral compartment; CLD2, intercompartmental clearance; Cmax, maximum plasma drug concentration; AUC0–∞, area under the plasma drug concentration-time curve from time zero to infinite time; t1/2α, elimination half-life for the 1st phase; t1/2β, elimination half-life for the 2nd (terminal) phase; MRT, mean residence time; Vss, volume of distribution at steady state.
Based on the post hoc estimates for the individual rabbits.
Values in parentheses represent the relative standard error of the estimate (in percent).
Clinical observations, necropsy, and histopathology findings.
Abnormal clinical signs were generally first documented at approximately 48 to 72 h postchallenge. The most common abnormal clinical signs were lethargy, inappetence, stool abnormalities, and respiratory abnormalities. For the LVX-placebo-treated animals succumbing to disease, 62% (8/13) were found dead while on study, while 71% (5/7) of the LVX-raxibacumab-treated animals that died were found dead while on study. In both treatment groups, most animals that survived returned to normal by 10 to 11 days postchallenge.
Complete necropsies were performed on all animals. Gross lesions in nonsurvivors included discoloration or foci in the brain, lungs, and large intestines; enlargement of mediastinal lymph nodes; fluid in multiple body cavities, mesentery, thymus, and skin; and cystic structures in the lung. These were considered typical of anthrax (13) and correlated histologically with hemorrhage, edema, and acute inflammation. The only gross lesion present in any surviving animal—a renal cyst—was an incidental finding unrelated to anthrax or treatment. Microscopic findings (see Table S5 in the supplemental material) were considered consistent with anthrax (13) and were present in nearly all nonsurvivors. The findings included acute suppurative inflammation, necrosis, hemorrhage, and edema. There were brain findings (meningeal and parenchymal hemorrhage, meningeal vascular necrosis, and/or parenchymal necrosis; see Fig. S3 and S4 in the supplemental material) for 2 animals in the LVX-alone group. Three LVX-treated animals and 1 combination-treated animal had large rod-shaped bacteria in the brain, lung, bronchial and mediastinal lymph nodes, and/or spleen. The remaining treated animals lacked visible bacteria in any organs, although all were bacteremic prior to death and/or had inflammation and hemorrhage typical of anthrax. One rabbit in the LVX-alone group that died had no gross or microscopic findings associated with B. anthracis infection, and there was no apparent cause of death.
DISCUSSION
Raxibacumab has been shown to provide a statistically significant improvement in survival over placebo in B. anthracis-infected animals (14). Studies in spore-challenged rabbits and monkeys of concomitant administration of raxibacumab and antibiotics showed that raxibacumab did not alter the efficacy or PKs of the antimicrobials (unpublished data). This was not surprising, as antibiotics and raxibacumab have different mechanisms of action and different elimination pathways. However, the design of those studies did not allow the added benefit of raxibacumab over antibiotic alone to be evaluated. Thus, the objective of this study was to demonstrate the added benefit of raxibacumab. Other goals of this study were to target a survival rate with antibiotic alone similar to that observed during the anthrax attacks in humans in 2001 (4), while attaining antimicrobial exposure equivalent to that in humans.
Treatment at 84 h after spore challenge resulted in a survival rate of 65% for rabbits administered LVX alone, which approximated the 55% survival rate for humans in the 2001 attack (4). However, this required that 180 rabbits be subjected to spore challenge, with only the 42% remaining alive at 84 h after spore exposure to be treated. The 65% survival rate for rabbits that were administered LVX alone was achieved with LVX exposures intermediate to the reported peaks and troughs achieved following multiple oral doses in humans (20) (see Table S6 in the supplemental material).
Although the 17% difference in survival rate between the groups did not reach statistical significance (P = 0.0874), raxibacumab treatment reduced the percentage of deaths nearly in half (7/39 [18%] for the raxibacumab-LVX group versus 13/37 [35%] for the LVX-alone group). This difference is meaningful and demonstrates a survival rate in the antimicrobial-plus-raxibacumab arm that is higher than the survival rate in the antimicrobial-alone arm.
This study was not powered to produce a statistically significant (P < 0.05) result with an absolute difference in the survival rate of 17%. A trial with an 80% power to detect the observed 17% difference as statistically significant would require 116 animals per group to be treated (232 animals). With only 42% of the animals estimated to be alive for treatment at 84 h, the total sample size of spore-challenged animals would be 552, and of that total, 320 animals would die before the treatment time. A study of that size is not ethical and may not be feasible due to the limited availability of biosafety level 3 containment facilities in which this type of study must be performed.
In all surviving animals, bacteremia was essentially cleared within 24 h of the first LVX administration, with or without raxibacumab, while PA persisted for 1 to 3 days after bacteremia resolved, suggesting that toxin continues to circulate even after the bacteria have been killed. The persistence of toxemia after eradication of bacteremia provides the basis for the added benefit observed with the raxibacumab-LVX combination over LVX alone.
This study also confirmed that raxibacumab does not interfere with the ability of the animals to mount an immune response to PA, as evidenced by the development of TNA in all of the surviving animals in the raxibacumab-LVX treatment group.
While raxibacumab is immunogenic in rabbits (see Table S3 in the supplemental material), it is not in cynomolgus monkeys or humans (8, 14). Despite the incidence of ADAs in raxibacumab-treated rabbits, survival was numerically superior for rabbits treated with raxibacumab-LVX versus rabbits treated with LVX alone.
The lack of an impact of immunogenicity as a PK covariate is likely related to the observation that in ADA-positive animals, plasma raxibacumab concentrations became undetectable at between 14 and 21 days postdosing; that is, those animals did not contribute data over the entire duration of the elimination phase of the profile. For raxibacumab, the volume of distribution for the central compartment (V1; 112 ml) was similar to the plasma volume (21). The steady-state volume of distribution was 56% greater than V1, suggesting that raxibacumab is initially restricted to the plasma volume but subsequently distributes to tissues. CL ranged from 22.56 ml/day to 35.21 ml/day across the 2.75- to 3.55-kg weight range and is much less than the glomerular filtration rate (21), indicating that, as expected for a monoclonal antibody, there is virtually no renal clearance of raxibacumab.
In conclusion, this study achieved a survival rate (65%) with full-dose antimicrobial treatment that was consistent with the survival rate of 55% observed in human subjects in 2001. When raxibacumab was administered with the antimicrobial drug, it resulted in a mortality rate lower than that achieved with the antimicrobial alone (18% versus 35%, respectively). These data suggest that raxibacumab administered with an antimicrobial drug, even late in the course of the disease, when over half of the animals have already succumbed, confers a survival benefit over antimicrobial therapy alone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heather Mayfield and Eric Benson of Battelle Biomedical Research Institute, as well as John Muth, Michele Fiscella, Maggie Lewis, Jeff Carrell, Cecil Chen, Larry Lo, Stephen Ullrich, Ling Xu, and Chris Ward of Human Genome Sciences, Inc., for their support. We also thank Melanie McCort-Tipton, Lisa Buchholz, Angie Underberg, Dylan Frantz, and Anita Wyeth at Covance for their LVX bioanalytical assay support.
This work was performed in support of a contract with the U.S. Department of Health and Human Services administered by the Biomedical Advanced Research and Development Authority to produce raxibacumab for the Strategic National Stockpile as a BioShield product.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04606-14.
REFERENCES
- 1.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 2.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholmn MT, Parker G, Perl TM, Russell PK, Tonat K. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 3.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh W-J, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yewskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, , National Anthrax Epidemiologic Investigation Team . 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh W-J, Malecki JM, Gerberding JL, Hughes JM, Perkins BA, Members of the Anthrax Bioterrorism Investigation Team . 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N Engl J Med 341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 6.Ascenzi P, Visca P, Ippolito G, Spallarossa A, Bolognesi M, Montecucco C. 2002. Anthrax toxin: a tripartite lethal combination. FEBS Lett 531:384–388. doi: 10.1016/S0014-5793(02)03609-8. [DOI] [PubMed] [Google Scholar]
- 7.Artenstein AW, Opal SM. 2012. Novel approaches to the treatment of systemic anthrax. Clin Infect Dis 54:1148–1161. doi: 10.1093/cid/cis017. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian GM, Cronin PW, Poley G, Weinstein A, Stoughton S, Zhong J, Ou Y, Zmuda JF, Osborn BL, Freimuth WW. 2005. A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Infect Dis 41:12–20. doi: 10.1086/430708. [DOI] [PubMed] [Google Scholar]
- 9.Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol 20:597–601. doi: 10.1038/nbt0602-597. [DOI] [PubMed] [Google Scholar]
- 10.Fritz DL, Jaax NK, Lawrence WB, Davis KJ, Pitt ML, Ezzell JW, Friedlander AM. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab Invest 73:691–702. [PubMed] [Google Scholar]
- 11.Twenhafel NA, Leffel E, Pitt ML. 2007. Pathology of inhalational anthrax infection in the African green monkey. Vet Pathol 44:716–721. doi: 10.1354/vp.44-5-716. [DOI] [PubMed] [Google Scholar]
- 12.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab Invest 83:1201–1209. doi: 10.1097/01.LAB.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 13.Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch Pathol Lab Med 122:982–992. [PubMed] [Google Scholar]
- 14.Migone T-S, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer S. 2009. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med 361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander AM, Welkos SL, Pitt MLM, Ezzell JW, Worsham PL, Rose KJ, Ivins BE, Lowe JR, Howe GB, Mikesell P, Lawrence WB. 1993. Postexposure prophylaxis against experimental inhalational anthrax. J Infect Dis 167:1239–1243. doi: 10.1093/infdis/167.5.1239. [DOI] [PubMed] [Google Scholar]
- 16.Yee SB, Hatkin JM, Dyer DN, Orr SA, Pitt MLM. 2010. Aerosolized Bacillus anthracis infection in New Zealand white rabbits: natural history and intravenous LVX treatment. Comp Med 60:461–468. [PMC free article] [PubMed] [Google Scholar]
- 17.Corey A, Migone T-S, Bolmer S, Fiscella M, Ward C, Chen C, Meister G. 2013. Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/raxibacumab-treated New Zealand white rabbits. Toxins (Basel) 5:120–138. doi: 10.3390/toxins5010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hering D, Thompson W, Hewetson J, Little S, Norris S, Pace-Templeton J. 2004. Validation of the anthrax lethal toxin neutralization assay. Biologicals 32:17–27. doi: 10.1016/j.biologicals.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Corey AE. 2013. Protective antigen kinetics and raxibacumab pharmacokinetics explain survival in the rabbit model of inhalational anthrax, abstr T3185 Abstr Annu Meet Am Assoc Pharm Scientists. [Google Scholar]
- 20.Janssen Pharmaceuticals Inc. August 2013. Levaquin U S label. Janssen Pharmaceuticals Inc, Titusville, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020634s065,020635s071,021721s032lbl.pdf. [Google Scholar]
- 21.Davies B, Morris T. 1993. Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.