Abstract
N-acylated homoserine lactonases are known to inhibit the signaling molecules of the biofilm-forming pathogens. In this study, gold nanoparticles were coated with N-acylated homoserine lactonase proteins (AiiA AuNPs) purified from Bacillus licheniformis. The AiiA AuNPs were characterized by UV-visible spectra, Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), and X-ray diffraction (XRD). The synthesized AiiA AuNPs were found to be spherical in shape and 10 to 30 nm in size. Treatment with AiiA protein-coated AuNPs showed maximum reduction in exopolysaccharide production, metabolic activities, and cell surface hydrophobicity and potent antibiofilm activity against multidrug-resistant Proteus species compared to treatment with AiiA protein alone. AiiA AuNPs exhibited potent antibiofilm activity at 2 to 8 μM concentrations without being harmful to the macrophages. We conclude that at a specific dose, AuNPs coated with AiiA can kill bacteria without harming the host cells, thus representing a potential template for the design of novel antibiofilm and antibacterial protein drugs to decrease bacterial colonization and to overcome the problem of drug resistance. In summary, our data suggest that the combined effect of the lactonase and the gold nanoparticles of the AiiA AuNPs has promising antibiofilm activity against biofilm-forming and multidrug-resistant Proteus species.
INTRODUCTION
Biofilm formation by Proteus species has been reported as a source of catheter-associated urinary tract infection that gives rise to serious complications. Proteus vulgaris, Proteus mirabilis, and Proteus penneri are known to cause urinary tract infections in humans (1). Among the Proteus spp., P. vulgaris is capable of forming crystalline biofilms and generating alkaline urine within 24 h (2). Such biofilm-forming pathogenic bacteria are the major cause of many chronic and recurrent infections, such as periodontitis, endocarditis, chronic otitis, and urinary tract and wound infections (3). Moreover, bacteria that adhere to indwelling medical devices such as intravenous catheters, artificial joints, and cardiac pacemakers or to damaged tissue can cause persistent infections through biofilm formation (2, 4, 5). Mature biofilms of Proteus spp. develop on the surface of uroepithelial cells or catheters; organisms within the mushroom-shaped structure regulate a variety of cellular functions, such as glutamine synthesis, autoinducer 2 (AI-2), cyclic dipeptides, and putrescine synthesis (6–8). Catheter-associated urinary tract infections (CAUTIs) affect more than 400,000 patients per year in the United States (9). Antibiotics used to treat these biofilm-forming pathogens are not active against the recalcitrant biofilms; rather, they target their planktonic counterparts, which create selective pressure on the bacteria, which develop resistance to a particular drug (10). Disrupting the multicellular structure of a Proteus biofilm was proposed as one of the most promising strategies for increasing the sensitivity of pathogens to antibiotics and the host immune system (11, 12). Hence, effective therapeutic agents are required to control urinary tract infections caused by biofilm-forming Proteus spp. Recently, disruption of quorum sensing (QS) by quorum quenching has been suggested as an anti-infective strategy to control pathogenic bacteria through interference with the colonization processes, including biofilm formation and invasion of host tissues (13–15). N-Acyl homoserine lactonase enzymes that degrade acyl homoserine lactone (AHL) might have commercial value as a means to manipulate cell-to-cell signaling (16, 17).
N-Acyl homoserine lactonase plays a critical role in hydrolyzing the lactone bond within the acyl homoserine lactone moiety, thus changing the relative conformational structure of the signaling molecule; this prevents binding to the LuxR transcriptional regulator, resulting in quorum-sensing inhibitory activity against pathogens (18, 19). The first AHL-degrading enzyme was identified in Bacillus strain 240B1 (20). To date, more than 20 AHL-degrading enzymes have been identified in bacteria, fungi, and mammals (15, 21, 22). Many Bacillus AHL lactonase-like enzymes have been reported, which share approximately ∼90% sequence identity (22). These enzymes contain the conserved motif HXHXDH and a zinc binding motif and therefore are classified as metallic β-lactamases (23). Recent developments in the field of nanotechnology have helped to some extent to overcome problems with drug resistance and biofilm formation through synthesis of bioactive materials (24). Chemically synthesized nanoparticles (NPs) give rise to toxicity, thereby limiting their biomedical applications (25). Microbially mediated nanoparticles may also give rise to toxic substances such as endotoxin on the surface of the nanoparticles, which limits their use in the medical field (26). Therefore, the trend has shifted to the use of biologically synthesized nanoparticles. Gold can be stabilized with a wide variety of molecules, such as peptides, proteins, DNA, and polymers (27). The use of gold coating over magnetic nanoparticles to achieve both a biocomposite surface and high magnetic properties has drawn intense scientific and technological interest for potential applications in biomedical field (28). These materials exhibit unique thermal, mechanical, and biological properties (29, 30) compared to other free enzymes and proteins. The performance of bionanoparticles for the control of microbes is dependent on both the chemical properties of the gold nanoparticles and the bioactivity of the enzymes and proteins. Colloidal gold nanoparticles have flexible properties, such as plasmon resonance, optical properties, and the presence of a bioconjugation surface for molecular probes, compared to those of other inorganic nanoparticles (31). These properties have attracted attention with a view to develop a new approach to control biofilm-forming bacteria and will be used in the field of nanomedicine. In the present work, we have synthesized AuNPs coated with AiiA protein (AiiA AuNPs) and studied their AHL lactonase activity, antibiofilm activity against Proteus, and cytotoxic effects against macrophages. We found that 2 μM AiiA AuNPs demonstrated maximum degradation of 1.8 mg N-hexanoyl-l-homoserine lactone (C6-HSL) (liter−1 h−1) and inhibited the exopolysaccharide (EPS) production, hydrophobicity, metabolic activity, and biofilm formation of the isolated Proteus strains DPr1, DPr2, and DPr3 and P. vulgaris ATCC 49565. Furthermore, AiiA AuNPs did not show any significant cytotoxic effect against the macrophages tested at a 2 μM concentration. To our knowledge, this is the first study on AiiA AuNPs to determine their antibiofilm potential against Proteus.
MATERIALS AND METHODS
Bacterial growth conditions.
The clinical strains were isolated from specimens (urinary tract infection) from the Karaikudi Government Hospital, Tamil Nadu, India. Environmental strains were isolated from crustaceans of the Cuddalore coast, on the southeast coast of India, situated about 250 km south of Chennai (11°27′N, 79°47′ E). We selected only effective biofilm-forming and antibiotic-resistant environmental (DPr1 and DPr2) and clinical (DPr3 and DPr4) strains. The four selected strains were identified at the genus level based on 16S rRNA gene sequencing. P. vulgaris ATCC 49565 was used as the reference strain. Proteus strains were grown and maintained on tryptic soy agar (TSA) or tryptic soy broth (TSB) at 37°C. Chromobacterium violaceum CV026 was used for the AHL lactonase activity bioassay (32). The reporter strain Chromobacterium violaceum ATCC 12472 was used to determine quorum-sensing inhibitory activity, and strains were also cultured in Luria-Bertani (LB) broth at 33°C and stored at −80°C for the further studies.
Purification of AiiA protein from Bacillus licheniformis.
The aiiA gene of Bacillus licheniformis isolated from the crustacean Fenneropenaeus indicus was amplified by PCR using modified primers incorporating NdeI and EcoRI restriction sites (underlined) (5′ to 3′: AIF1, GGG AAT TCC ATA TGA CAG TAA AAA AGC TTT ATT TC; AIR1, CCG GAA TTC CGG CTA TAT ATA CTC CGG GAA CTC) with 40 cycles of 5 min at 95°C, 1 min at 50°C, and 2 min at 72°C and a final 10-min incubation at 72°C. The PCR-amplified DNA was purified using a Wizard SV gel and PCR clean-up system (Promega), digested with NdeI and EcoRI (NEB), ligated into the expression plasmid pET-32a (Novogene), and then used to transform Escherichia coli BL21 cells. Plasmid DNA was isolated from recombinant clones using a purification kit (Wizard SV Minipreps DNA; Promega, USA), and the insertion in one (pET-AiiA) was verified by sequencing by the dye termination method (Applied Biosystems, USA) using vector-specific primers and a Li-Cor 4200 DNA sequencer. AiiA protein was purified from IPTG (isopropyl-β-d-thiogalactopyranoside)-induced E. coli BL21 cultures using Ni-nitrilotriacetic acid (NTA) columns (Qiagen) following the method described by the manufacturer. Expression and isolation were monitored by SDS-PAGE, and the protein concentration was determined by the Bradford method (33). Purified AiiA protein showed a single 28-kDa band in the SDS-PAGE (data not shown). AiiA protein was lyophilized, stored at 4°C, and used for nanoparticle preparation.
AiiA AuNPs.
To prepare gold nanoparticles coated with AiiA protein (AiiA AuNPs), purified AiiA protein (2 mg) was incubated with a 0.5 mM concentration of HAuCl4 in a 150-ml Erlenmeyer flask and agitated at 37°C and 200 rpm. The pH of the final solution was adjusted to 5.0. The mixture was then incubated at 37°C until a colored solution was obtained. The reaction between AiiA protein and HAuCl4 was continued for 24 h to confirm that the intensity of the color was stable.
Characterization of AiiA AuNPs. (i) UV-visible spectroscopic analysis.
For spectroscopic analysis, 1 ml of AiiA AuNPs was withdrawn from the reaction mixture and absorbance was measured with a UV-visible spectrophotometer (UV-1800; Shimadzu, Japan) from 300 to 800 nm for 100 days.
(ii) TEM.
For transmission electron microscopy (TEM) analysis, a small volume of AiiA AuNPs was loaded on a carbon-coated copper grid, and the solvent was allowed to evaporate for 30 min. TEM measurements were performed on a JOEL 1200 EX TEM at an accelerating voltage of 80 kV. The crystal structure of the AiiA AuNPs was determined using the selected area electron diffraction (SAED) pattern.
(iii) XRD and FTIR analyses.
In order to determine their crystal nature, AiiA AuNPs were centrifuged, washed, and air dried. The air-dried powder was coated on a glass surface and then subjected to X-ray diffraction (XRD) analysis on a PAN analytical XRD analyzer (X'pert PRO) operating in the transmission mode at 40 kV and 30 mA with CuK radiation. Two milligram of the AiiA AuNPs was mixed with 200 mg KBr (FTIR grade) and pressed into a pellet. The AiiA AuNP pellet was placed in the sample holder, and Fourier transform infrared (FTIR) spectra were recorded with an FTIR spectrometer (Perkin-Elmer, Shelton, CT) at a resolution of 4 cm−1.
Biofilm assay.
The biofilm inhibitory concentration (BIC) of AiiA AuNPs was determined as the lowest concentration that produced visible disruption to biofilm formation. Examination of the BIC of AiiA AuNPs on biofilm formation was performed in 24-well polystyrene plates (34). Briefly, overnight cultures of Proteus at 106 CFU/ml were inoculated with 1 ml of fresh Zobell marine broth (ZMB) in the presence of AuCl2 (2 μM), AiiA protein alone (120 U ml−1), and AiiA AuNPs (0.5 to 4 μM). The plates were incubated for 24 h at 37°C. After incubation, the cultures were discarded, and the wells were gently rinsed twice with deionized water and allowed to air dry before staining with crystal violet (CV). The wells were stained with 210 μl of 0.1% (wt/vol) CV for 10 min before being rinsed twice with deionized water and air dried. CV was then eluted with 210 μl dimethyl sulfoxide, and the A595 was measured using a Bio-Rad enzyme-linked immunosorbent assay (ELISA) reader.
AHL lactonase activity bioassay.
The AHL lactonase activity of the bacteria treated with AuCl2 (2 μM), AiiA protein alone (120 U ml−1), and AiiA AuNPs (2 μM) was determined in a C6-HSL well diffusion bioassay using C. violaceum CV026 as the reporter strain (31). A lawn of C. violaceum CV026 was prepared by mixing 3 ml of overnight culture on LB agar plates, and then wells of 5 mm in diameter were made in the agar plates. An AHL lactonase reaction mixture containing 10 μl sample plus 190 μl of solution containing 24 nM C6-HSL in 50 mM phosphate buffer (pH 8.0) was prepared and incubated at 25°C for 45 min before the reaction was terminated by the addition of 50 μl 10% (wt/vol) SDS. The reaction mixture was then transferred into the wells, and the radius of the C. violaceum CV026 nonpigmented zone was used to determine the residual C6-HSL levels after 2 days of incubation. The AHL lactonase activity of the bacteria treated with AuCl2 (2 μM), AiiA protein alone (120 U ml−1), and AiiA AuNPs (2 μM) is reported here as the degradation rate of AHL (mg liter−1 h−1) or as the units of AHL-degrading activity mg−1 protein, where 1 U is defined as the amount of enzyme required to hydrolyze 1 nM C6-HSL per minute under the conditions described here. For the standard curve, y = 24 nM × e0.42 × x/106 and r2 = 0.996 (y, in nmol, is the amount of C6-HSL, and x, in mm, is the distance of diffusion of C6-HSL).
Quantification of CSH by MATH assay.
The effect of the AiiA protein and AiiA AuNPs on cell surface hydrophobicity (CSH) of Proteus was measured by the microbial adhesion to hydrocarbon (MATH) assay. Bacteria were grown in tryptic soy broth at 37°C for 18 to 24 h, harvested by centrifugation at 6,000 rpm for 5 min at 25°C, washed with and resuspended in sterile distilled water, and adjusted to an optical density (OD) at 600 nm of 0.6 ± 0.04. The AiiA AuNPs/AiiA protein and toluene (1 ml) were added to 2 ml of the adjusted cell suspension (A600 = 0.6 ± 0.04) in a test tube. After vortexing for 1 min, the cell suspensions were incubated at room temperature overnight, and the OD of the aqueous phase was measured (A600). The hydrophobicity index (HI) of Proteus was calculated as HI = 100(E ×100/E0) (35). The results were expressed as the proportion of the cells which were excluded from the aqueous phase, determined by the equation, where E0 is the initial optical density of the cell suspension and E is the optical density of the aqueous phase after its separation from the toluene phase.
Quantification of EPS.
The level of exopolysaccharide (EPS) produced by Proteus was determined by carbohydrate assay. Sterile catheter pieces were immersed in a Proteus culture containing 2 μM AiiA AuNPs or TSA broth (control) in 24-well polystyrene plates and were incubated for 24 h. The sterile catheters were vortexed with 0.9% NaCl solution. Cell suspensions were transferred to test tubes to which an equal volume of 5% phenol and 5 volumes of sulfuric acid containing 0.2% hydrazine sulfate were added. The mixture was incubated in dark for 1 h and centrifuged at 10,000 × g for 10 min, and the optical density of the supernatant was measured at 490 nm (36). The percentage of EPS was calculated as follows: EPS production (%) = [(control OD − test OD)/control OD] × 100.
Assessment of biofilm metabolic activity using XTT reduction assay.
Metabolic activities of cells during biofilm formation was assessed using the XTT [2,3-bis(2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reduction assay, which measures the reduction of a tetrazolium salt by metabolically active cells to a colored water-soluble formazan derivative which can be quantified colorimetrically. AiiA AuNPs (2 μM) and AiiA protein (120 U ml−1) were added to the Proteus biofilms in a 96-well polystyrene tissue culture plate containing ZMB and 2% (wt/vol) glucose, and then the plates were incubated as described above for the biofilm assay. Following incubation, the biofilms were washed five times with phosphate-buffered saline (PBS), and then 100 μl PBS and 12 μl XTT-menadione solution (12.5:1, vol/vol) were added to the wells. The plates were then incubated for 3 h in the dark at 37°C. Following incubation, 100 μl of the solution was transferred to fresh wells, and the change in the color of the solution was measured at 450 nm spectrophotometrically (36).
Microscopic observation of Proteus biofilm inhibition by AiiA AuNPs.
For visualization of biofilms by light microscopy, Proteus biofilms were allowed to grow on a sterile catheter (1 by 1 cm) immersed in AiiA AuNPs (2 μM) in a 24-well polystyrene plate and incubated for 24 h at 30°C. Catheter pieces were stained with crystal violet as described above and examined using a Nikon microscope (Eclipse Ti 100×) at a magnification of ×40. Independently, another set of catheter pieces with biofilms were washed with PBS stained with acridine orange (0.1%), and the biofilm formation was quantified using a confocal laser scanning microscope (CLSM) (Carl Zeiss LSM 710) with a 488-nm argon laser, a BP 500-640 band pass emission filter, and Zen 2009 software (Carl Zeiss, Germany). CLSM biofilm observations were assessed using COMSAT software (37).
Cytotoxic effect of AiiA AuNPs on macrophages.
To determine the cytotoxic activity of the gold nanoparticles coated with AiiA protein (AiiA AuNPs) on macrophages, RAW 264.7 cells (1 × 104 cells/ml) in Dulbecco modified Eagle medium (DMEM) were grown in a 96-well plate at 37°C with 5% CO2 for 24 h, followed by treatment of cells with different concentrations of AiiA AuNPs for another 24 h. To determine the cell viability, MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] at a concentration of 2 to 8.0 μM was added to the wells and incubated for 4 h at 37°C with 5% CO2 in the dark. In metabolically active cells, MTT was reduced to an insoluble dark purple formazan. The formazan crystals were dissolved in dissolving buffer (11 g SDS in 50 ml of 0.02 M HCl and 50 ml isopropanol). The absorbance was read at 570 nm in an ELISA reader (Biotek, Germany) and compared with that of untreated cells, and the percentage of viable cells was calculated.
Statistical analysis.
All assays were repeated at least three times, and all statistical analyses were performed using SPSS. Values are expressed as mean ± standard deviation (SD). Mean values were compared using one-way ANOVA.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the clinical strains used in this study are as follows: Proteus sp. strain DPr1, HQ009354; P. vulgaris Dpr2, HQ116441; Proteus sp. strain Dpr3, HQ640435; and P. vulgaris Dpr4, HQ640434.
RESULTS
Characterization of AiiA AuNPs.
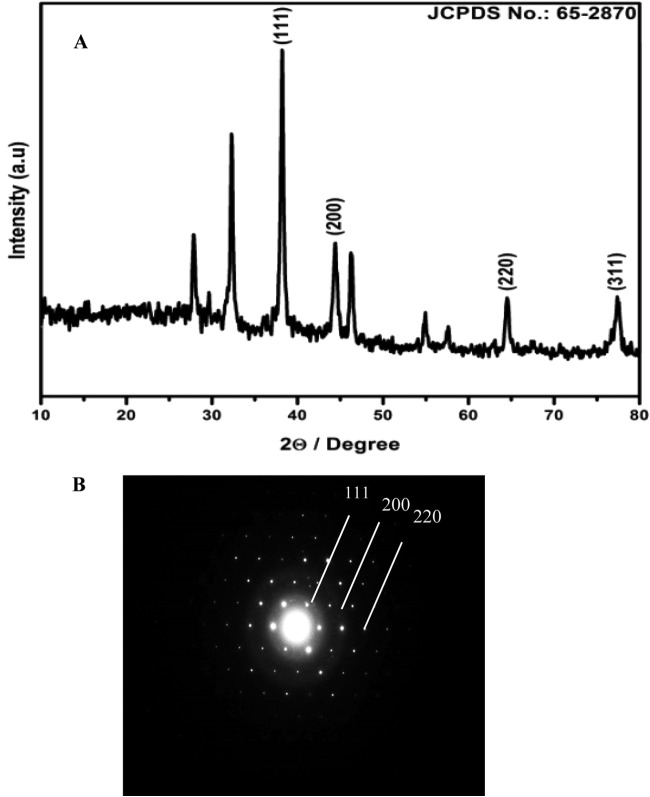
The reduction of aqueous HAuCl4 ions during the reaction with AiiA protein was monitored by UV-visible spectroscopy. A strong resonance at 550 nm was seen due to the excitation of surface plasmon vibrations in the gold nanoparticles (Fig. 1A). A change of the colorless solution to purple (Fig. 1B and C) confirmed the synthesis of gold nanoparticles coated with AiiA protein (AiiA AuNPs). The stability of the AiiA AuNPs was determined by measuring the absorption spectrum at intervals of 24 h for 100 days. No significant changes in the absorbance were observed during the storage, indicating that the AuNPs did not agglomerate and were stable during this period (data not shown). TEM analysis showed that AiiA AuNPs were spherical and monodispersed, and the average particle diameter was about 10 to 30 nm. (Fig. 1D). The crystalline nature of AiiA-coated gold nanoparticles was determined by XRD. The characteristic XRD peaks at 2θ of 38.2°, 44.3°, 64.6°, and 77.6° are indexed to the 111, 200, 220, and 311 crystallographic planes of the gold nanoparticles coated with AiiA protein, respectively (Fig. 2A). The high crystallinity of AiiA AuNPs is evident from bright circular spots in the SAED pattern (Fig. 2B). Fourier transform infrared (FTIR) analysis was performed in order to identify the possible biomolecules responsible for the reduction of the gold ions and capping of the bioreduced gold nanoparticles. FTIR spectra obtained from the AiiA AuNPs showed the occurrence of three bands at 3,439 cm−1, 2,924 cm−1, and 2,853 cm−1, which were assigned to the stretching vibrations of primary, secondary, and tertiary amines, respectively. The band at 1,592 cm–1 has been identified as an amide II band, which arose due to carbonyl stretch and —N—H— stretch vibrations in the amide linkages of the proteins. The bands at 1,400 cm−1 and 1,043 cm−1 demonstrate C—C and C—N stretching, respectively. The band at 1,743 cm−1 corresponds to the carbonyl stretching vibration in the C=O region. The presence of amide linkages between amino acid residues in the polypeptides showed a signature in the infrared region of the electromagnetic spectrum (Fig. 3). Major functional groups such as C=O and N—H are involved in the metal interaction with Au, which leads to the conjugate reduction of synthesized NPs, indicating the change in the secondary structure spectra. The physical characterization revealed that the synthesized AiiA AuNPs were monodispersed, crystalline, and capping AiiA protein.
FIG 1.
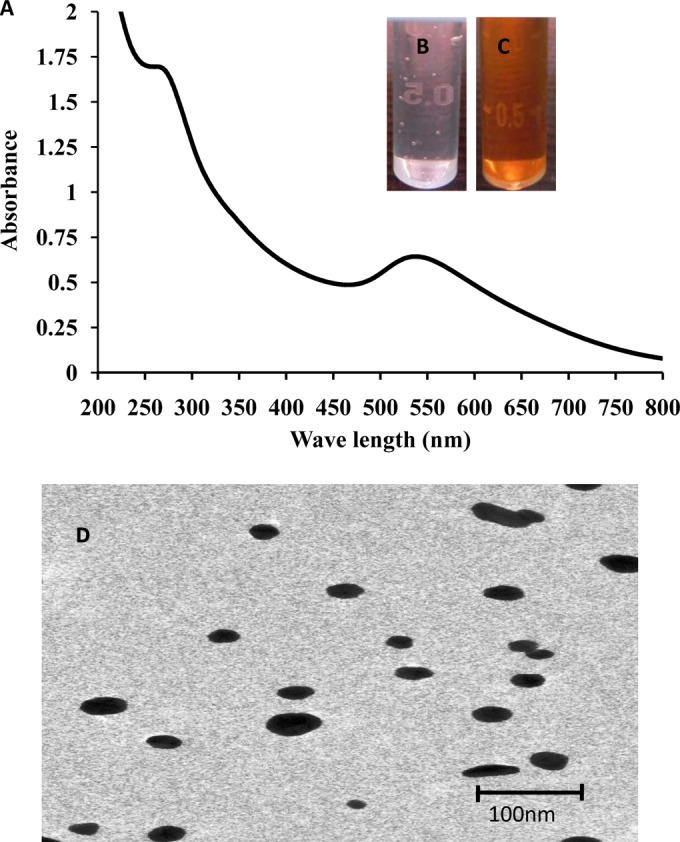
(A) UV-visible spectral analysis of AiiA AuNPs. (B) Before reaction. (C) After reaction for 6 h. (D) Transmission electron microscopy images of AiiA-coated gold nanoparticles with a particle diameter of about 10 to 30 nm.
FIG 2.
(A) X-ray diffraction patterns of AiiA AuNPs. (B) SAED pattern of AiiA AuNPs.
FIG 3.
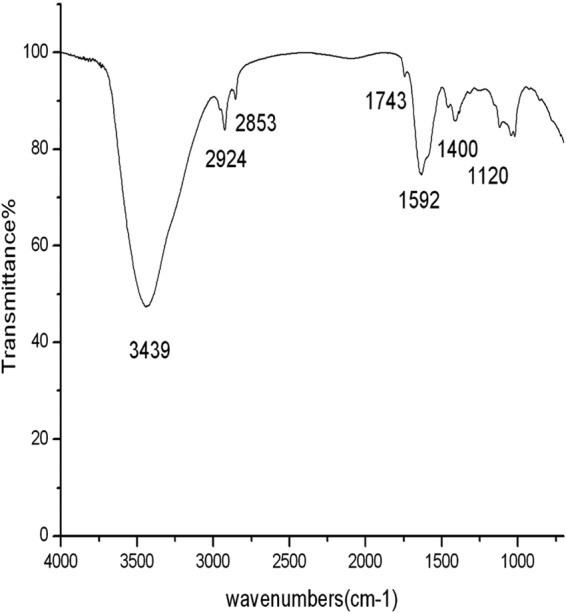
FTIR spectrum of AiiA AuNPs.
Biofilm inhibition and AHL lactonase assays.
The effect of AiiA AuNPs on Proteus biofilm formation was determined using a crystal violet (CV) staining method. AiiA AuNPs were found to significantly inhibit the biofilm formation by Proteus compared to AiiA alone. A 2 μM concentration of AiiA AuNPs was determined as the biofilm inhibitory concentration, and therefore, all subsequent experiments were performed with this concentration. Treatment for 24 h resulted in a decrease of more than 85% of the biofilm formation at a 2 μM concentration of AiiA AuNPs, whereas AiiA protein and AuCl2 alone resulted in approximately 40% and 60% decreases in biofilm formation, respectively (Fig. 4). The AHL lactonase assay showed degradation rates of 0.3 ± 0.1, 0.9 ± 0.3, and 1.8 ± 0.12 mg liter−1 h−1 C6-HSL with AuCl2, AiiA protein, and AiiA AuNP treatment, respectively (data not shown).
FIG 4.
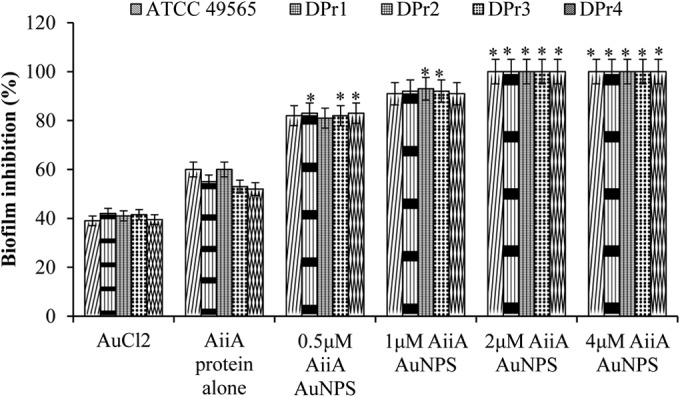
Effect of AiiA AuNPs on Proteus biofilm formation. Different concentration of AiiA AuNPs ranging from 0.5 μM to 4 μM were used along with the control AuCl2 (2 μM) and AiiA protein (120 U ml−1). *, P < 0.05 compared with the untreated sample.
Hydrophobicity and metabolism assays.
Bacterial adhesion to hydrocarbons is a major determinant of biofilm formation. AiiA AuNPs reduced the hydrophobicity indices of the Proteus strains compared with those of untreated and AiiA protein-treated Proteus. The reduction in the hydrophobicity index caused by AiiA AuNPs indicates reduction in accumulation of bacteria, which leads to the inhibition of biofilm formation compared to that of untreated samples (Fig. 5). Further, XTT assay also confirmed that the AiiA AuNPs indeed reduced the metabolic activities of the Proteus biofilms for all the tested Proteus strains compared to results with samples treated with AiiA protein alone and untreated samples (Fig. 6).
FIG 5.
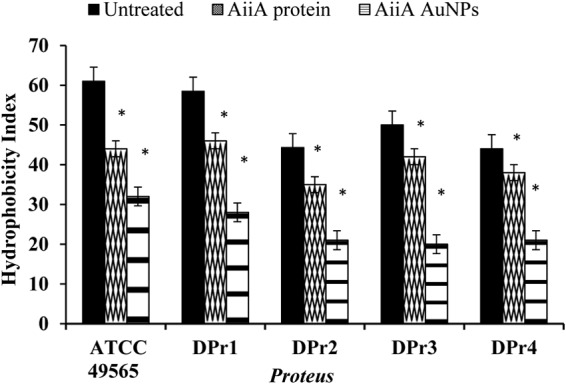
Effects of AiiA protein and AiiA AuNPs on the cell surface hydrophobicity index of Proteus. The reduction of the hydrophobicity index in AiiA AuNP-treated bacteria leads to the inhibition of biofilm formation in all the tested Proteus strains. *, P < 0.05 compared to the untreated sample.
FIG 6.
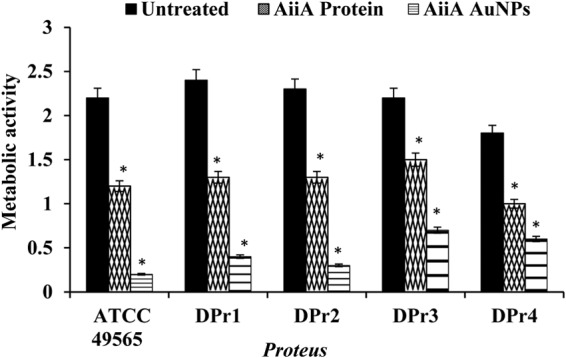
Effects of AiiA protein and AiiA AuNPs on the metabolic activity of Proteus biofilm. The AiiA AuNPs significantly reduced the metabolic activity of all the tested Proteus strains. *, P < 0.05 compared to the untreated sample.
Estimation of EPS production.
The analysis of EPS synthesis by Proteus showed that the AiiA Au NPs significantly reduced the synthesis of EPS compared to that by the untreated sample (Fig. 7). The reduction in exopolysaccharide (EPS) synthesis was found not only to inhibit the biofilm formation but also to remove the preformed biofilms effectively, although this effect was found to be dependent on the specificity of the EPS matrix composition of biofilms.
FIG 7.
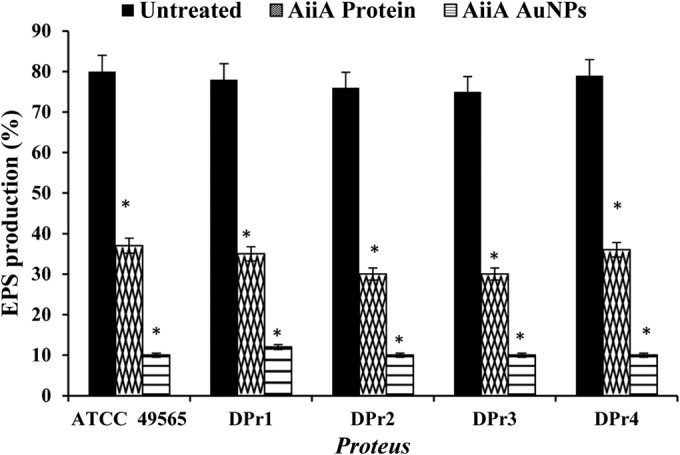
Effects of AiiA protein and AiiA AuNPs on Proteus EPS production. The reduction of EPS by AiiA AuNPs indicates inhibition of biofilm formation and also effective removal of preexisting biofilm. *, P < 0.05 compared with the untreated sample.
Microscopic observation of biofilm inhibition.
The light microscopic studies showed a well-developed biofilm formation by Proteus, whereas bacteria treated with AiiA AuNPs (2 μM) showed poor biofilm formation compared to that of the control (Fig. 8A). CLSM images showed the strong adhering ability of Proteus, which led to the development of dense biofilm formation on the catheter pieces, while treatment with AiiA AuNPs showed disintegrated and labile biofilm architecture formed by Proteus (Fig. 8B). Moreover, treatment with AiiA AuNPs also affected the thickness (μm) of the biovolume (μm3), and the average thickness (μm) was reduced in the biofilms formed by Proteus, as evidenced through COMSTAT analysis.
FIG 8.
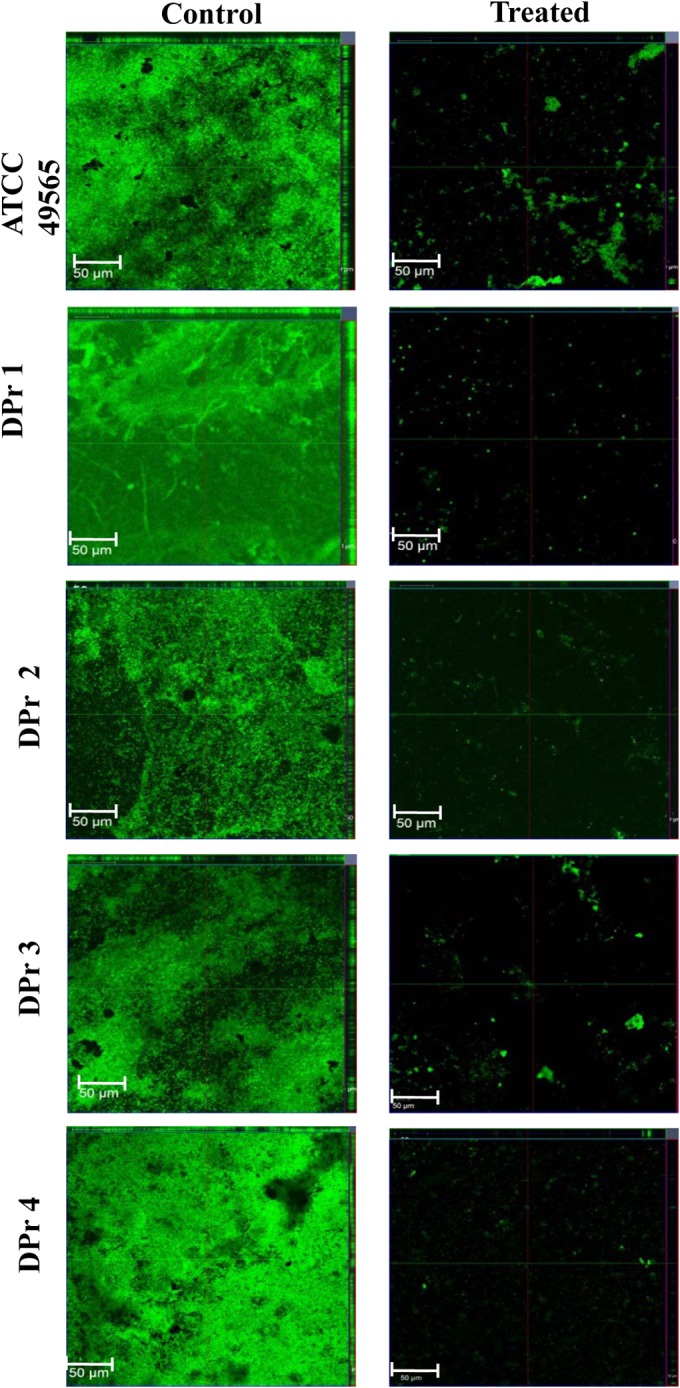
Confocal laser scanning microscopy visualization of the effect of AiiA AuNPs, revealing antibiofilm activity against Proteus strains at the BIC of 2 μM.
Cytotoxic effect of AiiA AuNPs on macrophages.
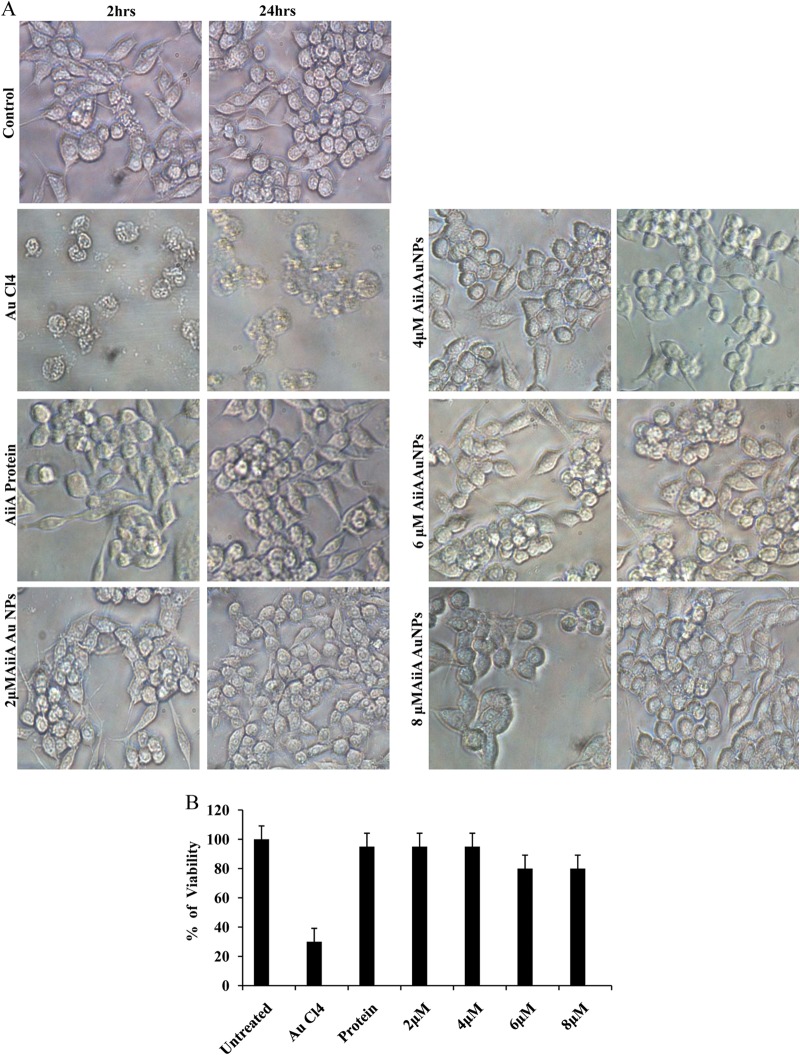
The cytotoxic effect of gold nanoparticles coated with AiiA protein (AiiA AuNPs) on macrophages was determined by MTT assay, which relies on the fact that metabolically active cells reduce MTT to purple formazan. Hence, the intensity of dye at 570 nm is directly proportional to the number of viable cells. AiiA AuNPs exerted no significant cytotoxic effect at concentrations of 2.0 to 8.0 μM (Fig. 9A), which was found to inhibit the biofilm formation by the Proteus strains tested in this study, indicating that the AiiA AuNPs display antibiofilm activity without being harmful to macrophages. Treatment with a higher dose (8 μM AuNPs) resulted in an approximately 20% reduction in cell viability. The cell morphology of RAW264.7 macrophages was not affected after treatment with different concentrations of AiiA AuNPs for 24 h. Cells grown with medium, AiiA protein, and AuCl4 were used as controls (Fig. 9B).
FIG 9.
(A) Cell morphology of RAW264.7 macrophages after treatment with different concentrations of AiiA AuNPs for 24 h. Cells grown with medium, AiiA protein, and AuCl4 were used as controls. (B) Cytotoxic activity of AiiA AuNPs on RAW264.7 mouse macrophages. Macrophages were treated with different concentrations of AiiA AuNPs for 24 h. Macrophages treated with AiiA protein and AuCl4 were used as controls. Cells viability was determined by MTT assay. Experiments were performed in triplicates; means ± SDs are shown.
DISCUSSION
In the present study, gold nanoparticles coated with AiiA protein (AiiA AuNPs) were purified from Bacillus licheniformis isolated from the crustacean F. indicus. The AiiA AuNPs were found to be spherical, monodispersed, and stable for longer periods of time. Ahmad et al. (38) reported that the stability of gold nanoparticles could be due to a capping agent, which may be proteins released by the bacteria. The FTIR spectrum of AiiA-AuNP interaction revealed conformational changes in the secondary structure of the purified AiiA protein. It is well known that proteins can bind to gold nanoparticles through either free amine groups or cysteine residues present in the proteins (39). In recent years, biofilm formation and antimicrobial resistance have been major challenges faced by the scientific community not only with clinical but also with environmental strains. In the present study, AiiA AuNPs were used to control multidrug-resistant (MDR) as well as potentially biofilm-forming clinical and environmental Proteus strains. Based on C6-HSL degradation tests, AiiA AuNPs were able to degrade C6-HSL significantly, to almost undetectable levels, within 2 h. C6-HSL was used as a test compound because it is produced by most of the biofilm-forming pathogens (40, 41). Impeding the bacterial adhesion at an early stage can significantly decrease the threat of further biofilm development. In the present study, the results confirmed that AiiA AuNPs prominently inhibited Proteus biofilm formation at its early stage at a 2 μM concentration as determined by microplate assay. In addition, the light and confocal laser scanning microscopic (CLSM) analyses confirmed the reduction in the biofilm architecture of Proteus biofilms treated with 2 μM AiiA AuNPs. This result is consistent with the findings of Choi et al. (42), who reported that interactions of nanosilver with biofilm-forming cells resulted in significant inhibition.
Inhibition of Proteus biofilm formation is essential to overcome urinary tract infection, as the exopolysaccharide formed reduces the susceptibility of the organism to an administered drug. Uropathogens have been shown to produce 80 types of exopolysaccharide capsule as a means of avoiding the immune response and thereby contributing to serum resistance (43). EPS production assay results showed that AiiA AuNPs inhibited exopolysaccharide production by Proteus and also disturbed the architecture of biofilms. We propose that the synergistic interactions between AiiA protein and the gold nanoparticles may inhibit the quorum-sensing molecules, which further leads to the inhibition of biofilm formation. Yan et al. (44) reported that strong antibacterial activity against MDR bacteria was observed for gold NPs due to their multiple targets and inherent elemental properties.
EPS and cell surface hydrophobicity play an important role in bacterium-host cell interactions and biofilm architecture in microbes (45, 46). Generally, targeting the hydrophobicity index is a novel way of inhibiting biofilm formation. Furthermore, in our study we did not see any significant changes in macrophage cell morphology when treated with AiiA AuNPs at concentrations of 2 to 8 μM. The cells were adherent to the surface, which further indicates that AiiA AuNPs have no cytotoxic effect on mouse macrophages. These results are consistent with previous investigations performed with HeLa cells (47, 48). The cell viability test showed no cytotoxic effects of AiiA AuNPs on macrophages at concentrations up to 6 μM AuNPs. A reduced cytotoxic effect may be due to the surface coating of AiiA protein in AuNPs.
Conclusion.
In the present study, gold nanoparticles coated with functional AHL lactonase protein from Bacillus licheniformis were prepared and characterized by UV-visible spectra, FTIR, TEM, and XRD. AiiA AuNPs inhibited the in vitro biofilm formation as well as the virulence factor (exopolysaccharide) production and metabolic activity of Proteus. The results of cytotoxicity studies showed no changes in macrophage cell morphology when treated with AiiA AuNPs at 2 to 8 μM. The outcome of these results may be development of potential biomaterials against urinary tract infection by MDR and biofilm-forming Proteus strains.
ACKNOWLEDGMENTS
This work was supported by the University Grants Commission (UGC), New Delhi, India, under Project Grants code F.No 36-5/2008(sr) and by grant SR/NM/NS-1085/2011 from Department of Science and Technology, Government of India, to A.S. R.P. is grateful to the Department of Science and Technology, Government of India, for a DST-INSPIRE fellowship.
REFERENCES
- 1.Mobley HL, Jones BD, Penner JL. 1987. Urease activity of Proteus penneri. J Clin Microbiol 25:2302–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stickler DJ. 2008. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol 5:598–608. doi: 10.1038/ncpuro1231. [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 4.Vlastarakos PV, Nikolopoulos TP, Maragoudakis P, Tzagaroulakis A, Ferekidis E. 2007. Biofilms in ear, nose, and throat infections: how important are they? Laryngoscope 117:4668–4673. doi: 10.1097/MLG.0b013e318030e422. [DOI] [PubMed] [Google Scholar]
- 5.Fey PD, Olson ME. 2010. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol 5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison C, Lai HC, Gygi D, Hughes C. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemo attractant for swarming cells. Mol Microbiol 8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 7.Schneider R, Lockatell CV, Johnson D, Belas R. 2002. Detection and mutation of a luxS-encoded autoinducer in Proteus mirabilis. Microbiology 148:773–782. [DOI] [PubMed] [Google Scholar]
- 8.Sturgill G, Rather PN. 2004. Evidence that putrescine acts as an extracellular signal required for swarming in Proteus mirabilis. Mol Microbiol 51:437–446. doi: 10.1046/j.1365-2958.2003.03835.x. [DOI] [PubMed] [Google Scholar]
- 9.Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care associated infections and deaths in U.S. hospitals. Public Health Rep 122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentzer M, Givskov M. 2003. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112:1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid G, Habash M. 2001. Oral fluoroquinolone therapy results in drug adsorption on ureteral stents and prevention of biofilm formation. Int J Antimicrob Agents 17:317–320. doi: 10.1016/S0924-8579(00)00353-8. [DOI] [PubMed] [Google Scholar]
- 12.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen TB, Givskov M. 2006. Quorum sensing inhibitors: a bargin of effects. Microbiology 152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 14.Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. 2008. Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J 2:19–26. doi: 10.1038/ismej.2007.92. [DOI] [PubMed] [Google Scholar]
- 15.Czajkowski R, Jafra S. 2009. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol 56:1–16. [PubMed] [Google Scholar]
- 16.Dobretsov S, Teplitski M, Paul V. 2009. Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 25:413–427. doi: 10.1080/08927010902853516. [DOI] [PubMed] [Google Scholar]
- 17.Teasdale ME, Liu J, Wallace J, Akhlaghi F, Rowley DC. 2009. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl Environ Microbiol 75:567–557. doi: 10.1128/AEM.00632-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. 2005. Revisiting quorum sensing discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci U S A 102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai F, Han Y, Chen J, Zhang XH. 2008. Disruption of quorum sensing in Vibrio harveyi by the AiiA protein of Bacillus thuringiensis. Aquaculture 274:36–40. doi: 10.1016/j.aquaculture.2007.11.024. [DOI] [Google Scholar]
- 20.Dong YH, Xu JL, Li XZ, Zhang LH. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci U S A 97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong YH, Zhang LH. 2005. Quorum sensing and quorum quenching enzymes. J Microbiol 43:101–109. [PubMed] [Google Scholar]
- 22.Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Applied Environ Microbiol 68:1754–1759. doi: 10.1128/AEM.68.4.1754-1759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Zhou Z, Cao Y, Bai Y, Yao B. 2010. High yield expression of an AHL lactonase from Bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Fact 9:39. doi: 10.1186/1475-2859-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandyopadhyay D, Prashar D, Luk YY. 2011. Anti-fouling chemistry of chiral monolayers enhancing biofilm resistance on racemic surface. Langmuir 27:6124–6131. doi: 10.1021/la200230t. [DOI] [PubMed] [Google Scholar]
- 25.Kishen A, Shi Z, Shrestha A, Neoh KG. 2008. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 34:1515–1520. doi: 10.1016/j.joen.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Ram Kumar Pandian RS, Muniyandi J, Hariharan N, Eom SH. 2009. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloid Surf B 74:3283–3235. [DOI] [PubMed] [Google Scholar]
- 27.Niemeyer CM, Angew. 2001. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Chem Int ed 40:4128–4158. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Devi R, Yadav S, Nehra R, Pundir CS. 2013. An amperometric hypoxanthine biosensor based on Au@FeNPs for determination of hypoxanthine in meat samples. Int J Biol Macromol 62:629–635. doi: 10.1016/j.ijbiomac.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Balazs AC, Emrick T, Russell TP. 2006. Nanoparticle polymer composites: where two small worlds meet. Science 314:1107–1110. doi: 10.1126/science.1130557. [DOI] [PubMed] [Google Scholar]
- 30.Tang EJ, Cheng XL, Ma XL. 2006. Preparation of nano-Zno/PMMA composite particles via grafting of the copolymer on to the surface of zinc oxide nanoparticles. Powder Technol 161:209–214. doi: 10.1016/j.powtec.2005.10.007. [DOI] [Google Scholar]
- 31.Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y. 2006. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev 35:1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 32.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:703–3711. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Pratt LA, Kolter R. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30:3052–3093. [DOI] [PubMed] [Google Scholar]
- 35.Serebryakova EV, Darmov IV, Medvedev NP, Alekseev SM, Rybak SI. 2002. Evaluation of the hydrophobicity of bacterial cells by measuring their adherence to chloroform drops. Mikrobiologiia 71:202–204. [PubMed] [Google Scholar]
- 36.Favre-Bonte S, Kohler T, Delden CV. 2003. Biofilm formation by Pseudomonas aeruginosa role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J Antimicrob Chemother 52:598–604. doi: 10.1093/jac/dkg397. [DOI] [PubMed] [Google Scholar]
- 37.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad P, Mukherjee S, Senapati D, Mandal MI, Khan R, Kumar MS. 2003. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloid Surf B 28:313. doi: 10.1016/S0927-7765(02)00174-1. [DOI] [Google Scholar]
- 39.Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M. 2001. Pepsin-gold colloid conjugates: preparation, characterization and enzymatic activity. Langmuir 17:1674–1679. doi: 10.1021/la001164w. [DOI] [Google Scholar]
- 40.Morohoshi T, Inaba T, Kato N. 2004. Identification of quorum-sensing signal molecules and the LuxRI homologs in fish pathogen Edwardsiella tarda. J Biosci Bioeng 98:274–281. doi: 10.1016/S1389-1723(04)00281-6. [DOI] [PubMed] [Google Scholar]
- 41.Bruhn JB, Dalsgaard I, Nielsen KF, Buchholtz C, Larsen JL, Gram L. 2005. Quorum sensing signal molecules (acylated homoserine lactones) in Gram-negative fish pathogenic bacteria. Dis Aquat Org 65:43–52. doi: 10.3354/dao065043. [DOI] [PubMed] [Google Scholar]
- 42.Choi O, Chang-Ping Yu, Fernandez G, Zhiqiang H. 2010. Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res 44:6095–6103. doi: 10.1016/j.watres.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 43.Johnson JR. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 4:80–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. 2012. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 45.Swiatlo E, Champlin FR, Holman SC, Wilson WW, Watt JM. 2002. Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae. Infect Immun; 70:412–415. doi: 10.1128/IAI.70.1.412-415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flemming HC, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 47.Khan JA, Pillai B, Das TK, Singh Y, Maiti S. 2007. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem 8:1237–1240. doi: 10.1002/cbic.200700165. [DOI] [PubMed] [Google Scholar]
- 48.Hauck TS, Ghazani AA, Chan WC. 2008. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]