Abstract
The emergence and transmission of extensively drug-resistant tuberculosis (XDR-TB) pose an increasing threat to global TB control. This study aimed to identify the patterns of evolution and transmission dynamics of XDR-TB in populations in a region of China where TB is highly endemic. We analyzed a total of 95 XDR-TB isolates collected from 2003 to 2009 in Chongqing, China. Eight drug resistance genes covering 7 drugs that define XDR-TB were amplified by PCR followed by DNA sequencing. Variable-number tandem repeat 16-locus (VNTR-16) genotyping and genotypic drug resistance profiles were used to determine the evolution or transmission patterns of XDR-TB strains. Our results indicated that the Beijing genotype was predominant (85/95 [89.5%]) in XDR-TB strains, and as many as 40.0% (38/95) of the isolates were distributed into 6 clusters based on VNTR-16 genotyping and drug resistance mutation profiles. All isolates of each cluster harbored as many as six identical resistance mutations in the drug resistance genes rpoB, katG, inhA promoter, embB, rpsL, and gidB. Among the nine cases with continuous isolates from multidrug-resistant (MDR) to XDR-TB, 4 cases represented acquired drug resistance, 4 cases were caused by transmission, and 1 case was due to exogenous superinfection. The XDR-TB epidemic in China is mainly caused by a high degree of clonal transmission, but evolution from MDR to XDR and even superinfection with a new XDR strain can also occur.
INTRODUCTION
The emergence of multidrug-resistant tuberculosis (MDR-TB), which is resistant to at least isoniazid (INH) and rifampin (RIF), and more recently, of extensively drug-resistant tuberculosis (XDR-TB), defined as MDR-TB with additional resistance to fluoroquinolones and at least one of the second-line injectable drugs, including amikacin (AMK), kanamycin (KAN), and capreomycin (CAP), poses a significant threat to TB control worldwide (1). XDR-TB was first reported in South Africa in 2006 (2). According to the World Health Organization (WHO), XDR-TB was reported in 92 countries by 2012 and is a major concern for global health (3, 4). The mortality rates of XDR-TB patients are generally high but depend on the study population and their HIV status and vary in different countries (5–7).
The emergence of XDR-TB may be due to acquired drug resistance by evolution or primary drug resistance by active transmission. A recent report in the Western Cape of South Africa showed that although MDR-TB was spreading by transmission, XDR-TB appeared to be acquired but not clonally derived (8). The diversity of the resistance mutations in Beijing strains has also been observed in Russia (9) and Japan (10), indicating independent acquisition. This is in contrast to the highly publicized outbreak of XDR-TB in HIV-positive individuals due to transmission in Tugela Ferry, South Africa. Similar to the outbreaks of MDR-TB in the 1990s (11–13), XDR-TB cases among HIV-infected patients often occurred as a result of transmission, because 55% of the cases had no history of TB treatment (14). In China, a recent national survey in 2007 showed that 5.7% of new cases and 25.6% of previously treated cases had MDR-TB, and 8% of MDR-TB cases had developed XDR-TB. Inadequate treatment in both the public health system and the hospital system, especially TB hospitals, has made drug-resistant TB a serious epidemic, as primary transmission accounted for most cases (15). An analysis of the different causes for the emergence of XDR-TB will impact clinical practice, public health, and health system interventions and has important implications for TB infection control.
To better understand the molecular epidemiology, evolution, and transmission patterns of XDR-TB in the regions where TB is highly endemic in China, we analyzed the genetic lineages of 95 XDR-TB isolates collected from 2003 to 2009 in Chongqing, one of the areas with highest tuberculosis disease burden, by variable-number tandem repeat (VNTR) genotyping and drug resistance mutation profiling. This is the first such report of high transmission of XDR-TB in China. The scale and severity of the XDR-TB problem may have long been underestimated, and measures to prevent and contain the primary transmission of XDR-TB are urgently needed.
MATERIALS AND METHODS
Study population and Mycobacterium tuberculosis strains.
We performed a study in patients who received a diagnosis of XDR-TB in Chongqing Pulmonary Hospital, China, from 2003 through 2009. XDR-TB was diagnosed in 90 patients, of which 33 (37%) were female and 57 (63%) were male, and the median age was 45 years (range, 15 to 82 years). A total of 95 XDR-TB strains were isolated from 90 patients, consisting of 85 isolates from 85 patients and 10 isolates from 5 patients at different time points (see Table S1 in the supplemental material). In addition, 9 MDR-TB strains were isolated before they subsequently developed into XDR-TB strains from 9 of the 90 XDR-TB patients. The identification of M. tuberculosis isolates was carried out using the MTB/NTM ACE detection kit (Seegene, Inc., Seoul, South Korea), according to the manufacturer's instructions. The study protocol was approved by the ethics committee of Huashan Hospital, Fudan University.
Drug susceptibility testing.
Drug susceptibility testing (DST) for 7 drugs, including 4 first-line drugs, rifampin (RIF), isoniazid (INH), ethambutol (EMB), and streptomycin (SM), and 3 second-line drugs, ofloxacin (OFX), kanamycin (KAN), and capreomycin (CAP), was performed using the absolute concentration method on Lowenstein-Jensen medium and interpreted according to the standards of the Clinical and Laboratory Standards Institute (16); these results were confirmed with a BacT/Alert 3D microbial detection system (bioMérieux, Durham, NC) at the same absolute level of drug concentration. The absolute concentration method was chosen for its simplicity for inoculum preparation and for reading the results. The inoculum size was standardized by suspending 10−2 mg of M. tuberculosis organisms in 1 ml of sterile distilled water, followed by plating 0.1 ml of organisms onto the Lowenstein-Jensen medium containing or not containing the drugs. Growth was defined as the presence of >20 colonies at the end of 4 to 6 weeks. The critical concentrations (judged for low-level and high-level resistance) were 1 μg/ml and 10 μg/ml INH, 50 μg/ml and 250 μg/ml RIF, 5 μg/ml and 50 μg/ml EMB, 5 μg/ml and 50 μg/ml OFX, 10 μg/ml and 100 μg/ml SM, 10 μg/ml and 100 μg/ml KAN, and 20 μg/ml and 100 μg/ml CAP (17–22). Resistance to the 7 drugs refers to resistance to at least the lower level of drug concentrations tested.
DNA extraction, amplification, and sequence analysis.
Genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA), according to the manufacturer's instructions. PCR amplification and sequencing of the following 9 drug resistance genes (23) were performed as described previously (24): rpoB (RIF), katG and inhA promoter (INH), embB (EMB), rpsL, rrs, and gidB (SM), pncA (pyrazinamide [PZA]), gyrA (fluoroquinolone [FQ]), and rrs (KAN and CAP). Of the 9 genes, rpsL, pncA, and gidB were amplified as complete open reading frames and the drug resistance-determining regions of rpoB (rifampin resistance-determining region [RRDR]), embB (ethambutol resistance-determining region [ERDR]), and gyrA (quinolone resistance-determining region [QRDR]), the promoter of inhA, and regions with established resistance-associated mutations of katG and rrs were selected for PCR amplification. The DNA sequences of all samples were compared using the MegAlign software (DNAStar, Inc., Madison. WI). The mutations detected in the respective genes were compared with the corresponding gene sequences from wild-type M. tuberculosis strain H37Rv.
Genotyping methods.
Deletion-targeted multiplex PCR (DTM-PCR) was used to identify the Beijing genotype strains (25). Different PCR-based genotyping was used to detect the variable-number tandem repeat (VNTR) type in the 95 isolates. Mycobacterial interspersed repetitive (MIRU)-VNTR-16 loci were amplified, including Qub-11a, QUB26, Qub-11b, MIRU26, Mtub21, QUB-4156, Mtub04, Mtub24, MIRU10, MIRU16, MIRU31, MIRU39, ETR-F, QUB-1895, VNTR3820, and Qub-18 (26). The PCR products were analyzed on a 2% agarose gel. The sizes of the amplicons were estimated by a comparison with the 50- and 100-bp DNA ladders (Tiangen, Shanghai, China).
Statistical analysis.
The 16-locus MIRU-VNTR analysis of the M. tuberculosis isolates was performed using the BioNumerics 6.6 software (Applied Maths, Sint-Martens-Latem, Belgium). Dendrograms were constructed according to the unweighted-pair group method using arithmetic averages (UPGMA). If two or more isolates showed identical VNTR profiles, they were considered clustered. The clustering rate was defined as (nc − C)/N, where N is the total number of isolates, C is the number of clusters, and nc is the total number of clustered isolates (27).
RESULTS
Drug resistance patterns of the XDR-TB isolates.
The phenotypic drug resistance profiles of the 95 M. tuberculosis isolates for the first- and second-line drugs are summarized in Table 1. All the isolates were resistant to RIF, INH, OFX, and either KAN or CAP. Of the 95 isolates, 74 (77.9%) were resistant to EMB, 94 (98.9%) were resistant to SM, 73 (76.8%) were resistant to all the four first-line drugs tested, and 55 (57.9%) were resistant to all 3 second-line drugs, OFX, KAN, and CAP. Notably, 44 (46.3%) XDR isolates were resistant to all the first- and second-line drugs tested in this study.
TABLE 1.
Drug resistance patterns of the 95 XDR-TB isolates
| No. (%) of isolates | Drug resistance pattern fora: |
|
|---|---|---|
| 1st-line drugs | 2nd-line drugs | |
| 44 (46.3) | RIF, INH, EMB, SM | OFX, KAN, CAP |
| 15 (15.8) | RIF, INH, EMB, SM | OFX, CAP |
| 14 (14.7) | RIF, INH, EMB, SM | OFX, KAN |
| 10 (10.5) | RIF, INH, SM | OFX, KAN, CAP |
| 6 (6.3) | RIF, INH, SM | OFX, KAN |
| 5 (5.3) | RIF, INH, SM | OFX, CAP |
| 1 (1.1) | RIF, INH, EMB | OFX, KAN, CAP |
RIF, rifampin; INH, isoniazid; EMB, ethambutol; SM, streptomycin; OFX, ofloxacin; KAN, kanamycin; CAP, capreomycin.
Genotypic resistance of the 95 XDR M. tuberculosis isolates.
The genotypic drug resistances to RIF, INH, EMB, SM, OFX, KAN, and CAP in the 95 XDR-TB isolates were analyzed, and the results are shown in Fig. 1. Of the 95 isolates, 48 harbored identical or highly similar resistance mutations and therefore were grouped into 6 clusters, a to f, which contain 15, 13, 6, 7, 4, and 3 isolates, respectively, and the isolates in each cluster shared an identical array of drug resistance mutations for all 4 first-line drugs (RIF, INH, EMB, and SM). The mutation combination profiles in all six genes (rpoB, katG, inhA promoter, embB, rpsL, and gidB) in each cluster were distinguished from each other. The distinct mutation profile for first-line drugs for the most prominent cluster (cluster a) was a combination of rpoB(D516G), katG(S315T), inhA promoter(T8C), rpsL(K43R), and gidB(E92D).
FIG 1.
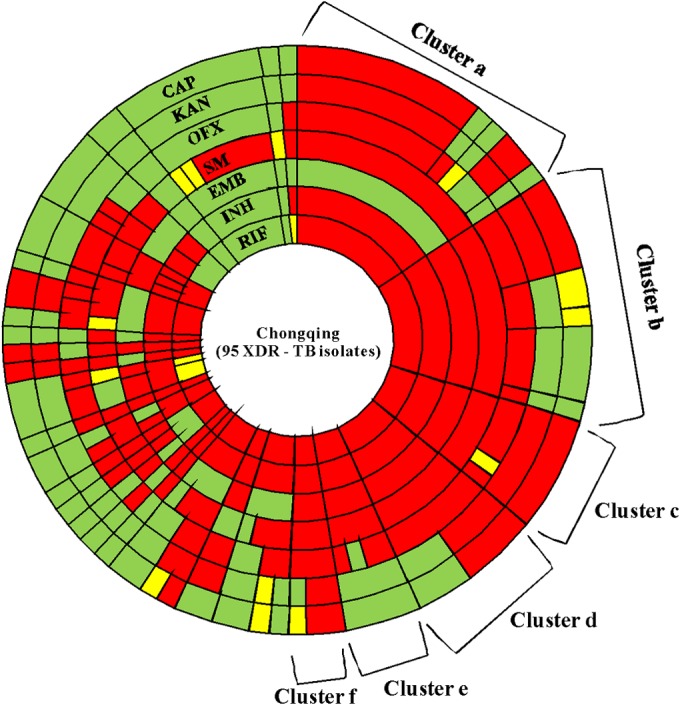
Radial plot of distribution of genotypic drug resistance mutations in 95 XDR-TB isolates from Chongqing. The area of a wedge corresponds to the number of isolates with the given drug resistance mutation signature. There are 6 clusters (clusters a, b, c, d, e, and f) that exhibit significant clustering, sharing exactly the same mutations for resistance to all the 4 first-line drugs. From clusters a to f, the corresponding numbers of each cluster are 15, 13, 6, 7, 4, and 3 isolates, respectively. The presence of mutations conferring resistance to RIF, INH, EMB, SM, OFX, KAN, and CAP is indicated by segments in various shades of yellow to red. The red segments correspond to the more common mutations [e.g., katG(S315T) for INH and rpoB(S531L) for RIF], while yellow tones indicate rarer mutations. The green segments indicate wild type and no mutation.
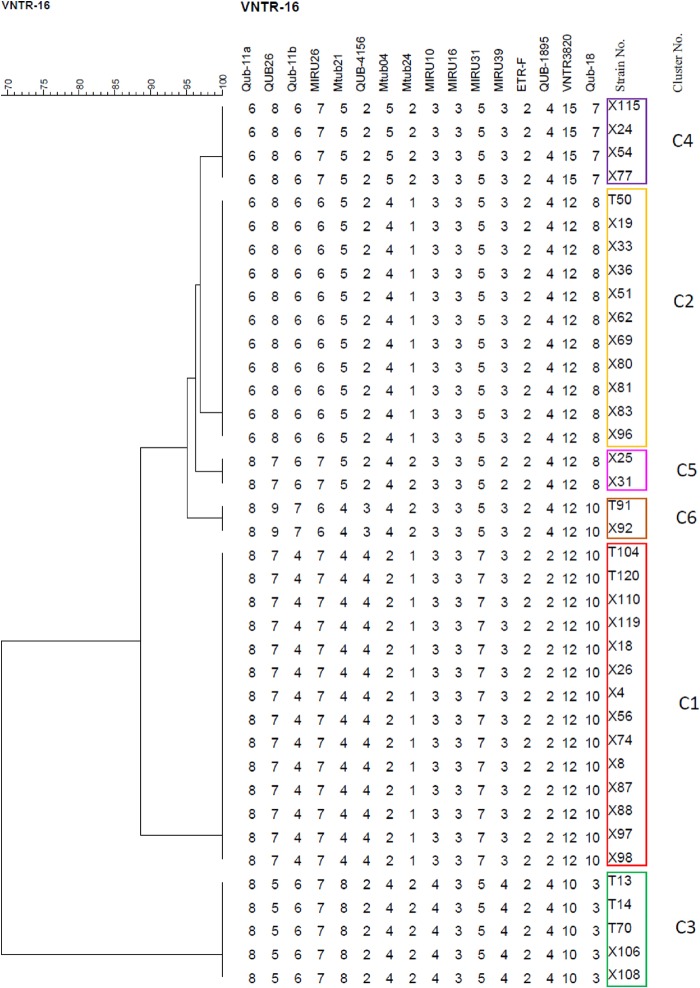
MIRU-VNTR-16 genotyping of the 95 XDR-TB strains.
DTM-PCR genotyping indicated that 89.5% (85/95) of the isolates belonged to Beijing genotype strains, while 10.5% (10/95) of the isolates were non-Beijing genotype strains. We chose the MIRU-VNTR 16-locus (MIRU-VNTR-16) genotyping to efficiently differentiate Beijing genotype strains, as its discriminatory power has been shown in a previous study (26) to be comparable to that of IS6110 random fragment length polymorphism (RFLP) for differentiating Beijing genotype strains. The cluster analysis grouped 38 (40.0%) isolates in 6 clusters, resulting in a clustering rate of 33.7% [calculated as (38 − 6)/95], while the remaining 57 (60.0%) strains had unique VNTR patterns (Fig. 2). All the clustered strains belonged to the Beijing genotype. From clusters 1 to 6, the corresponding numbers of each cluster were 14, 11, 5, 4, 2, and 2, respectively (Fig. 2). Figure 3 shows the phylogenetic relationships with representative branch lengths, which clearly demonstrates the clustering of 6 clustered XDR-TB isolates based on the VNTR-16 pattern.
FIG 2.
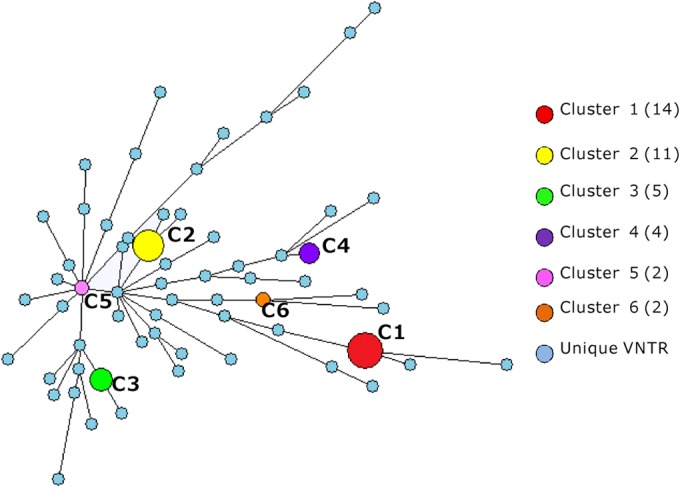
Minimum spanning tree analysis of the 95 XDR-TB isolates based on VNTR-16 genotyping. The 95 XDR-TB isolates are distributed into 6 clusters and 57 unique VNTR types. Each vertex (circle) corresponds to a certain VNTR genotype. The circle size corresponds to the number of isolates with a particular genotype. The circle color represents a different VNTR type. The number in parentheses next to the cluster code corresponds to the number of isolates in a cluster.
FIG 3.
Phylogenetic tree based on VNTR-16 pattern of 6 clustered XDR-TB isolates. The boxes with different colors indicate the 6 clusters (numbered and color-coded as in Fig. 2) of XDR-TB. In turn, the VNTR-16 loci are Qub-11a, QUB26, Qub-11b, MIRU26, Mtub21, QUB-4156, Mtub04, Mtub24, MIRU10, MIRU16, MIRU31, MIRU39, ETR-F, QUB-1895, VNTR3820, and Qub-18.
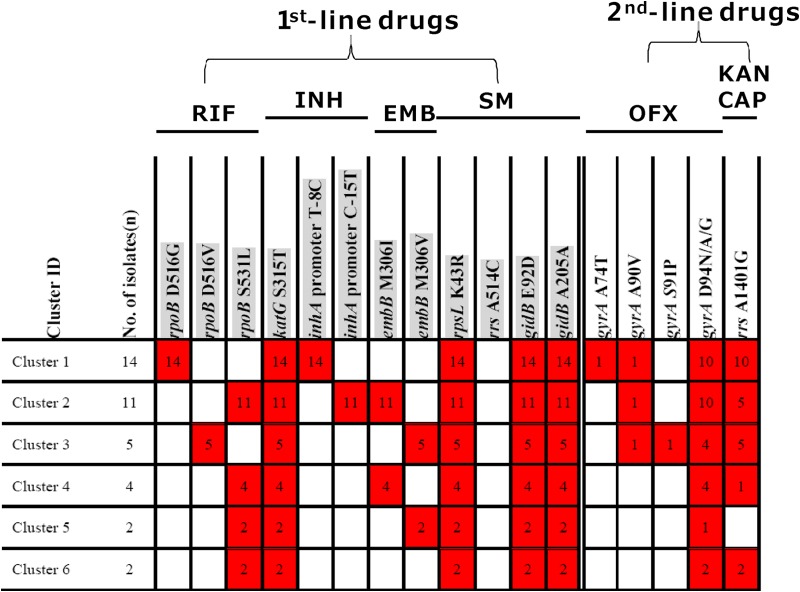
Relationship between drug resistance mutation profile and VNTR-16 genotyping in 6 clustered XDR-TB strains.
According to the VNTR-based genotyping, 38 out of 95 (40.0%) XDR-TB isolates formed 6 clusters. All members of each cluster had the same mutation profile in its drug resistance genes (rpoB + katG + inhA promoter + embB + rpsL + gidB) against first-line drugs and a slightly different mutation profile in genes (gyrA and rrs) for resistance to second-line drugs (Fig. 4). In contrast, the remaining 57 XDR-TB isolates showed unique MIRU-VNTR patterns that were distinct from those of the others. To further define if the 6 clusters of the XDR-TB strains with identical drug resistance mutations for rpoB + katG + inhA promoter + embB + rpsL + gidB are due to the same or different strains, we analyzed mutations in the pncA gene for pyrazinamide resistance in the 6 clustered XDR-TB isolates, as pncA mutations are known to have high diversity in PZA-resistant strains (23), which can be used as a further genotyping tool. Interestingly, the majority of the pncA mutations were clustered in the 6 clustered isolates, with some different mutations being identified in clusters 1, 3, and 6, suggesting that the mutations are perhaps due to microevolution in response to PZA pressure in such patients (see Table 2). The majority of the pncA mutations were clustered in the region of nucleotide (nt) 64, between nt 407 and 408, and at nt 146, nt 185, nt 226, and nt 559 in 42.9%, 100.0%, 40.0%, 100.0%, 100.0%, and 50.0% of the 6 clustered isolates, respectively.
FIG 4.
Drug resistance mutation profile of MIRU typing-clustered strains. The numbers in the red boxes correspond to the number of strains harboring the mutation of a designated drug resistance gene, while the white boxes indicate no mutation in the designated gene. The mutations involved in resistance to 1st-line drugs are shaded gray.
TABLE 2.
Mutations in the pncA gene for pyrazinamide resistance in the 6 clustered XDR-TB isolates
| Cluster | No. of isolates | Locus (nt) | Nucleotide changea | No. of isolates | Mutation rate (%) |
|---|---|---|---|---|---|
| 1 | 14 | 64 | Del A | 6 | 42.9 |
| 40 | T→C | 2 | 14.3 | ||
| 137 | C→A | 2 | 14.3 | ||
| WT | 2 | 14.3 | |||
| 211 | C→A | 1 | 7.1 | ||
| 329 | A→G | 1 | 7.1 | ||
| 2 | 11 | Between 407 and 408 | Ins A | 11 | 100.0 |
| 3 | 5 | WT | 3 | 60.0 | |
| 146 | A→G | 2 | 40.0 | ||
| 4 | 4 | 185 | C→T | 4 | 100.0 |
| 5 | 2 | 226 | A→C | 2 | 100.0 |
| 6 | 2 | 559 | T→C | 1 | 50.0 |
| WT | 1 | 50.0 |
Del, deletion; Ins, insertion; WT, wild type.
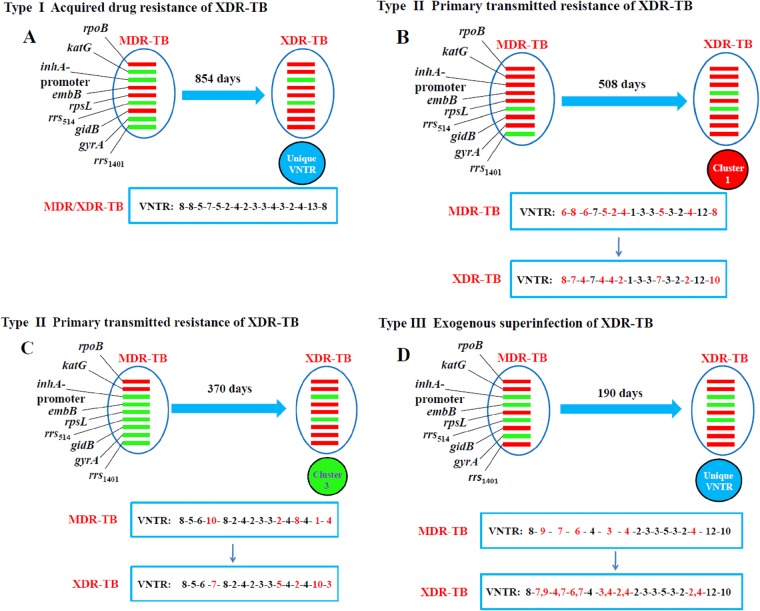
Evolution versus transmission of XDR strains in patients.
To provide insight into how XDR-TB is derived from MDR-TB, we examined the VNTR-16 genotypes and drug resistance mutation profiles of multiple isolates that evolved from MDR to XDR collected from the same patient at different times. Nine patients were included in the analysis, and it was found that they could be divided into type I acquired drug resistance through the evolution of increasing drug resistance mutations (4 cases), type II primary transmitted resistance (4 cases), and type III exogenous superinfection with a different XDR-TB strain (1 case). Figure 5 illustrates the three different scenarios. Case A belonged to type I. In case A, the MDR isolate evolved into an XDR isolate over 854 days, and the two had the same VNTR-16 genotype (Fig. 5A). Cases B and C belonged to type II. Case B was a patient with MDR-TB that progressed to XDR-TB 508 days later. In this case, the fingerprint of the XDR isolate with a MIRU type belonging to cluster 1 was different from the fingerprint of the previous MDR isolate (Fig. 5B). Similarly, in case C, the patient had two distinct VNTR-16 genotypes for the MDR and XDR isolates, and the XDR isolate had a MIRU type belonging to cluster 3 (Fig. 5C). Case D belonged to type III (Fig. 5D). In this case, the patient had two distinct VNTR-16 genotypes for the MDR and XDR isolates. Notably, the fingerprint of this XDR isolate had two alleles in six VNTR loci, which indicates mixed infections, i.e., exogenous superinfection (28).
FIG 5.
Three different patterns of XDR-TB development from MDR-TB. Each pattern represents a typical type of XDR-TB development, belonging to type I (acquired drug resistance) (A), II (primary transmitted resistance) (B and C), and III (exogenous superinfection) (D). All four cases had one MDR isolate and one XDR isolate at different times of treatment. Their drug resistance mutation profiles and VNTR-16 types were compared between the two different isolates. Each red or green bar represents drug resistance genes with or without mutations. The numbers in the rectangles represent the VNTR-16 genotyping results. In turn, the VNTR-16 loci are Qub-11a, QUB26, Qub-11b, MIRU26, Mtub21, QUB-4156, Mtub04, Mtub24, MIRU10, MIRU16, MIRU31, MIRU39, ETR-F, QUB-1895, VNTR3820, and Qub-18.
DISCUSSION
This is a longitudinal study of XDR-TB based on molecular genotypic and drug resistance mutation analyses in China. The present study suggests that there is high transmission of XDR-TB in the area with a high burden of tuberculosis disease in China, as shown by 40.0% (38/95) of the XDR-TB isolates being highly clustered based on their drug resistance mutation patterns and VNTR genotypes. The remaining 57 XDR-TB isolates harbored unique MIRU-VNTR patterns, which suggests the independent development of XDR-TB in different strains. These results have important implications for tuberculosis infection control, especially in settings with prevalent drug-resistant tuberculosis.
We found the Beijing family genotype was predominant and represented 89.5% (85/95) of the XDR-TB strains, which is consistent with the data from Shanghai, where >80% of the tuberculosis patients were infected with the Beijing genotype strains (29). Therefore, we selected VNTR-16 typing to more efficiently differentiate the Beijing genotype strains (26).
Our previous comparative analysis showed that M. tuberculosis isolates circulating in regions of China with high and low endemicity possessed different mutation profiles (30). For example, no multiresistance profile was observed in more than two occurrences in the Shanghai isolates, whereas the MDR-TB isolates from Chongqing exhibited significant clustering (30). The present study also showed the clustering of XDR-TB isolates from Chongqing. The result suggested that the isolates of 6 clusters may be caused by active transmission of the XDR-TB strains, i.e., due to primary transmitted resistance. Consistent with this, all isolates of each cluster harbored identical resistance mutations in the genes rpoB, katG, inhA promoter, embB, rpsL, and gidB (Fig. 4), suggesting that these 6 clusters represent a transmitted pre-XDR strain resistant to RIF, INH, EMB, SM, OFX, or one of the injectable second-line drugs (KAN or CAP). The slightly different mutation profiles in the genes (gyrA and rrs) for resistance to second-line drugs suggested that the mutations are perhaps due to microevolution caused by inappropriate treatment in the patients with primary pre-XDR-TB. In addition, Fig. 5B and C also display the active transmission of XDR-TB strains that occurred. Our study suggests that active transmission of XDR-TB strains is a significant problem in regions of high endemicity for TB and contributes to the high rate of XDR-TB.
Our genetic data provide support for a recent report from the Chinese CDC that the majority of cases of MDR-TB in China are due to active transmission (15). Another study from Shanghai indicated that 84% of retreated TB patients with drug-resistant disease had primary drug resistance, not acquired drug resistance (31). Most of the MDR/XDR cases in South Africa were also due to primary drug resistance (32). In several central Asian and eastern European countries, >30% of the newly diagnosed patients had MDR-TB, suggesting active transmission of primary drug-resistant strains (33). In several other countries, the primary transmission of MDR-/XDR-TB has been well documented (5, 34–37). These reports showed that primary transmission played a major role in the epidemic of drug-resistant tuberculosis. Our results suggested that multifocal transmission existed, rather than there being a single point-source outbreak from one patient. The transmission of these clustered XDR strains may indicate that M. tuberculosis strains with such mutation profiles can multiply and transmit with no or a low fitness cost and thus become dominant strains over time. The association of genotypic characteristics of transmissible XDR-TB strains with their fitness cost and virulence needs to be further elucidated in future studies.
Besides active transmission, the evolution and stepwise acquisition of resistance is also an important cause of the emergence of XDR-TB. The stepwise acquisition of XDR-TB has been reported in South Africa (8), South Korea (38), Portugal (39), and other countries. Recently, the molecular mechanisms underlying the evolution and transmission of MDR-/XDR-TB in a population were investigated through whole-genome sequencing of 1,000 prospectively obtained patient isolates from Russia (40). In our study, we found the acquisition of resistance leading to XDR-TB in 4 patients (Fig. 5A). Figure 5A depicts the ability of some strains to evolve continuously in individuals and transmit readily in a population. In addition, Fig. 5D indicates that exogenous superinfection drives the emergence of XDR-TB caused by active transmission or evolution of MDR-/XDR-TB. This dual ability of M. tuberculosis would obviously pose a challenge to our current strategy for controlling MDR-/XDR-TB. The XDR-TB epidemic in China is mainly caused by a high degree of clonal transmission, but evolution from MDR to XDR, and even superinfection with a new XDR strain, can also occur.
This study has some potential limitations. The study analyzed strains in only one region of China where TB is endemic. In addition, the number of strains analyzed is relatively small, even though this is probably the largest number of XDR-TB isolates from China analyzed so far. The six clusters of patients with identical drug resistance mutations and genotype patterns were identified from 38 XDR-TB patients, but epidemiological links between them were difficult to establish. This study was limited to an evaluation of the hospital setting only, and transmission that may have occurred in households or communities was not determined.
In summary, this work showed 40.0% of XDR-TB isolates being highly clustered from one of the areas with high tuberculosis incidence in China, suggesting that primary transmitted resistance is one of the critical factors driving the XDR-TB epidemic. While XDR-TB was spreading by transmission in this region, additional drug resistance mutations were independently acquired. The evolution from MDR to XDR and high transmission patterns with a new XDR strain can occur in China. In the context of XDR-TB prevention, rapid molecular methods to detect drug resistance should be highly recommended for populations that are in areas where XDR-TB is highly endemic and that lack access to DST. Control strategies must include not only efforts to improve cure rates for susceptible and MDR-TB cases but also infection control programs that can prevent the transmission of drug-resistant M. tuberculosis strains. Sufficient funding, adequate human resource development, staff training, and patient education may all contribute to the management of XDR-TB. Collaborative efforts among the government, CDC, physicians, and patients are the only way to control the serious problem of XDR-TB in regions in China that are highly endemic for XDR-TB.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients, nurses, lab staff and physicians at Chongqing Pulmonary Hospital for participating in this study.
This work was supported by the Key Technologies Research and Development Program for Infectious Diseases of China (grants 2011ZX09102-008, 2013ZX10003001-002, and 2013ZX10003008-003), the Foundation from the Science and Technology Commission of Shanghai (grant 12ZR1441500), the Specialized Project on Scientific Research within the health care industry of China (grant 201302010), and the National Natural Science Foundation of China (grant 81373064).
We report no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03504-14.
REFERENCES
- 1.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep 55:301–305. [PubMed] [Google Scholar]
- 3.Raviglione MC, Smith IM. 2007. XDR tuberculosis—implications for global public health. N Engl J Med 356:656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 4.WHO. 2012. Global tuberculosis control report 2012. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf. [Google Scholar]
- 5.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2007. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis 45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Upshur R, Padayatchi N. 2007. XDR-TB in South Africa: no time for denial or complacency. PLoS Med 4:e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioerger TR, Feng Y, Chen X, Dobos KM, Victor TC, Streicher EM, Warren RM, Gey van Pittius NC, Van Helden PD, Sacchettini JC. 2010. The non-clonality of drug resistance in Beijing-genotype isolates of Mycobacterium tuberculosis from the Western Cape of South Africa. BMC Genomics 11:670. doi: 10.1186/1471-2164-11-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toungoussova OS, Caugant DA, Sandven P, Mariandyshev AO, Bjune G. 2004. Impact of drug resistance on fitness of Mycobacterium tuberculosis strains of the W-Beijing genotype. FEMS Immunol Med Microbiol 42:281–290. doi: 10.1016/j.femsim.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto T, Yoshida S, Suzuki K, Wada T. 2008. Population structure analysis of the Mycobacterium tuberculosis Beijing family indicates an association between certain sublineages and multidrug resistance. Antimicrob Agents Chemother 52:3805–3809. doi: 10.1128/AAC.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. 1991. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons—Florida and New York, 1988–1991. MMWR Morb Mortal Wkly Rep 40:585–591. [PubMed] [Google Scholar]
- 12.Edlin BR, Tokars JI, Grieco MH, Crawford JT, Williams J, Sordillo EM, Ong KR, Kilburn JO, Dooley SW, Castro KG, Jarvis WR, Holmberg SD. 1992. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med 326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 13.Rullán JV, Herrera D, Cano R, Moreno V, Godoy P, Peiró EF, Castell J, Ibañez C, Ortega A, Agudo LS, Pozo F. 1996. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis in Spain. Emerg Infect Dis 2:125–129. doi: 10.3201/eid0202.960208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi NR, Weissman D, Moodley P, Ramathal M, Elson I, Kreiswirth BN, Mathema B, Shashkina E, Rothenberg R, Moll AP, Friedland G, Sturm AW, Shah NS. 2013. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis 207:9–17. doi: 10.1093/infdis/jis631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X, Pang Y, Song Y, Zhao B, Zhang H, He G, Guo J, Wang Y. 2012. National survey of drug-resistant tuberculosis in China. N Engl J Med 366:2161–2170. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. 2003. Susceptibility testing of mycobacteria, Nocardiae, and other aerobic actinomycetes; approved standard. NCCLS document M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed] [Google Scholar]
- 17.Canetti G, Froman S, Grosset J, Hauduroy P, Langerová M, Mahler HT, Meissner G, Mitchison DA, Šula L. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ 29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 18.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ 41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 20.Heifets L. 2000. Conventional methods for antimicrobial susceptibility testing of Mycobacterium tuberculosis, p 133–143. In Bastian I, Portaels F (ed), Multidrug-resistant tuberculosis. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 21.Laszlo A, Rahman M, Espinal M, Raviglione M, WHO/IUATLD Network of Supranational Reference Laboratories. 2002. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994–1998. Int J Tuberc Lung Dis 6:748–756. [PubMed] [Google Scholar]
- 22.Jin J, Shen Y, Fan X, Diao N, Wang F, Wang S, Weng X, Zhang W. 2013. Underestimation of the resistance of Mycobacterium tuberculosis to second-line drugs by the new GenoType MTBDRsl test. J Mol Diagn 15:44–50. doi: 10.1016/j.jmoldx.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Yew WW. 2009. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 13:1320–1330. [PubMed] [Google Scholar]
- 24.Jin J, Zhang Y, Fan X, Diao N, Shao L, Wang F, Hu P, Wang S, Weng X, Zhang W. 2012. Evaluation of the GenoType MTBDRplus assay and identification of a rare mutation for improving MDR-TB detection. Int J Tuberc Lung Dis 16:521–526. doi: 10.5588/ijtld.11.0269. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Tsolaki AG, Shen X, Jiang X, Mei J, Gao Q. 2007. Deletion-targeted multiplex PCR (DTM-PCR) for identification of Beijing/W genotypes of Mycobacterium tuberculosis. Tuberculosis (Edinb) 87:446–449. doi: 10.1016/j.tube.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Chen J, Shen X, Gui X, Mei J, Deriemer K, Gao Q. 2008. Highly polymorphic variable-number tandem repeats loci for differentiating Beijing genotype strains of Mycobacterium tuberculosis in Shanghai, China. FEMS Microbiol Lett 282:22–31. doi: 10.1111/j.1574-6968.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 27.Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, Schecter GF, Daley CL, Schoolnik GK. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med 330:1703–1709. [DOI] [PubMed] [Google Scholar]
- 28.Fang R, Li X, Li J, Wu J, Shen X, Gui X, DeRiemer K, Liu L, Mei J, Gao Q. 2008. Mixed infections of Mycobacterium tuberculosis in tuberculosis patients in Shanghai, China. Tuberculosis (Edinb) 88:469–473. doi: 10.1016/j.tube.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei J, Shen X, Zha J, Sun B, Shen M, Shen GM, Gao Q. 2005. Study on the molecular epidemiology of Mycobacterium tuberculosis in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 26:707e10 (In Chinese.) [PubMed] [Google Scholar]
- 30.Wang F, Massire C, Li H, Cummins LL, Li F, Jin J, Fan X, Wang S, Shao L, Zhang S, Meng S, Wu J, Lu C, Blyn LB, Sampath R, Ecker DJ, Zhang W, Tang YW. 2011. Molecular characterization of drug-resistant Mycobacterium tuberculosis isolates circulating in China by multilocus PCR and electrospray ionization mass spectrometry. J Clin Microbiol 49:2719–2721. doi: 10.1128/JCM.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zhang Y, Shen X, Shen G, Gui X, Sun B, Mei J, Deriemer K, Small PM, Gao Q. 2007. Transmission of drug resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis 195:864–869. doi: 10.1086/511985. [DOI] [PubMed] [Google Scholar]
- 32.Andrews JR, Gandhi NR, Moodley P, Shah NS, Bohlken L, Moll AP, Pillay M, Friedland G, Sturm AW, Tugela Ferry Care and Research Collaboration. 2008. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis 198:1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 33.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, Raviglione M. 2012. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ 90:111–119. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry PM, Gardner TJ, Funk E, Oren E, Field K, Shaw T, Langer AJ. 2012. Multistate outbreak of MDR TB identified by genotype cluster investigation. Emerging Infect Dis 18:11. doi: 10.3201/eid1801.110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen T, Murray M, Abubakar I, Zhang Z, Sloutsky A, Arteaga F, Chalco K, Franke MF, Becerra MC. 2011. Multiple introductions of multidrug-resistant tuberculosis into households, Lima, Peru. Emerging Infect Dis 17:969–975. doi: 10.3201/eid/1706.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavín P, Iglesias MJ, Jiménez MS, Rodríguez-Valín E, Ibarz D, Lezcano MA, Revillo MJ, Martín C, Samper S, Spanish Working Group on MDR-TB. 2012. Long-term molecular surveillance of multidrug-resistant tuberculosis in Spain. Infect Genet Evol 12:701–710. doi: 10.1016/j.meegid.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Kruijshaar ME, Watson JM, Drobniewski F, Anderson C, Brown TJ, Magee JG, Smith EG, Story A, Abubakar I. 2008. Increasing antituberculosis drug resistance in the United Kingdom: analysis of national surveillance data. BMJ 336:1231–1234. doi: 10.1136/bmj.39546.573067.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon CY, Hwang SH, Min JH, Prevots DR, Goldfeder LC, Lee H, Eum SY, Jeon DS, Kang HS, Kim JH, Kim BJ, Kim DY, Holland SM, Park SK, Cho SN, Barry CE III, Via LE. 2008. Extensively drug-resistant tuberculosis in South Korea: risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis 46:42–49. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 39.Perdigão J, Macedo R, Silva C, Machado D, Couto I, Viveiros M, Jordao L, Portugal I. 2013. From multidrug-resistant to extensively drug-resistant tuberculosis in Lisbon, Portugal: the stepwise mode of resistance acquisition. J Antimicrob Chemother 68:27–33. doi: 10.1093/jac/dks371. [DOI] [PubMed] [Google Scholar]
- 40.Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I, Corander J, Bryant J, Parkhill J, Nejentsev S, Horstmann RD, Brown T, Drobniewski F. 2014. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet 46:279–286. doi: 10.1038/ng.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.