Abstract
Tedizolid, a novel oxazolidinone with activity against a wide range of Gram-positive pathogens, was evaluated in two noninferiority phase 3 acute bacterial skin and skin structure infection trials. The data from individual trials showed its noninferior efficacy compared to that of linezolid and a favorable tolerability profile. To evaluate potential differences, the pooled data were analyzed. The patients received 200 mg of tedizolid once daily for 6 days or 600 mg of linezolid twice daily for 10 days. Efficacy was evaluated at 48 to 72 h (primary endpoint), on days 11 to 13 (end of therapy [EOT]), and 7 to 14 days after the EOT (posttherapy evaluation). Treatment-emergent adverse events and hematologic and clinical laboratory parameters were collected. The baseline characteristics were comparable between the treatment groups: 852/1,333 (64%) patients were from North America, and the majority of infections were caused by Staphylococcus aureus. Tedizolid was noninferior to linezolid (early clinical responses, 81.6% versus 79.4%, respectively). The early responses remained relatively consistent across various host/disease factors and severity measures. Nausea was the most frequently reported adverse event (tedizolid, 8.2%; linezolid, 12.2%; P = 0.02), with onset occurring primarily during the first 6 days. Fewer tedizolid than linezolid patients had platelet counts of <150,000 cells/mm3 at the EOT (tedizolid, 4.9%; linezolid, 10.8%; P = 0.0003) and during the postbaseline period through the last day of active drug visit (tedizolid, 6.4%; linezolid, 12.6%; P = 0.0016). Efficacy was achieved with a 6-day once-daily course of therapy with the option of an intravenous/oral regimen, and fewer low platelet counts and gastrointestinal side effects were reported with tedizolid than with linezolid, all of which aligns well with antimicrobial stewardship principles. (These studies have been registered at ClinicalTrials.gov under registration no. NCT01170221 and NCT01421511.)
INTRODUCTION
Serious bacterial skin infections are a significant problem in inpatient and outpatient settings and constitute a growing health care burden (1, 2). Acute bacterial skin infections are most frequently caused by Gram-positive pathogens (3, 4); methicillin-resistant Staphylococcus aureus (MRSA) is the predominant causative pathogen in many parts of the United States (5–7) and is endemic in Europe and other geographic regions (8–10). Current therapies for MRSA infection are associated with dosing- or administration-related complexities, a lack of oral formulations, safety issues, drug-drug interactions, and resistance (11–14).
Tedizolid is a new addition to the armamentarium against serious skin infections caused by Gram-positive pathogens. Tedizolid is a novel oxazolidinone antibacterial with potent activity (MIC effective on 90% of isolates [MIC90], 0.25 to 0.5 μg/ml) against a wide range of Gram-positive pathogens, including resistant strains, such as MRSA, and against vancomycin-resistant enterococci (VRE) (15). The pharmacokinetic and pharmacodynamic properties of tedizolid, a bacterial protein synthesis inhibitor, allow for once-daily administration, either orally or intravenously (i.v.), at equivalent doses (16–18).
Tedizolid has been evaluated in two randomized controlled noninferiority phase 3 trials (registered at ClinicalTrials.gov under registration no. NCT01170221 and NCT01421511) in patients with acute bacterial skin and skin structure infection (ABSSSI), conducted and analyzed in accordance with 2013 ABSSSI Food and Drug Administration and European Medicines Agency guidance (19–22). Although the positive results from the individual trials are consistent, there were differences (demographic, clinical characteristics, epidemiological, and geographical) between the study populations and in treatment strategy (oral therapy only [19] versus i.v.-to-oral sequential therapy [20]). Because of the similarity in the overall study design, the data from both trials lend well to a pooled analysis. The main value of conducting a pooled analysis lies in its ability to better detect potential safety signals in a larger and more diverse patient population and to further evaluate treatment efficacy.
Therefore, to better evaluate the efficacy and safety of tedizolid and linezolid, we analyzed pooled data from both trials according to a prespecified analysis plan, focusing on clinically important subgroups and on known safety issues with antibacterial agents in general (gastrointestinal [GI] side effects) and oxazolidinones in particular (myelosuppression).
(The data were partially presented at the 53rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC] meeting, 10 to 13 September 2013, Denver, CO, and at IDWeek 2013, 2 to 6 October 2013, San Francisco, CA.)
MATERIALS AND METHODS
Study design and participants.
ESTABLISH-1 and ESTABLISH-2 were randomized double-blind double-dummy multicenter multinational phase 3 noninferiority trials; the methods and results of the individual studies have been previously reported in detail (19, 20). Patients were randomly assigned 1:1 to receive either tedizolid or linezolid, and randomization was stratified by clinical syndrome (cellulitis/erysipelas, wound infection, or major cutaneous abscess [<30% of enrollees]) and geographic region. In ESTABLISH-1, randomization was also stratified by the presence or absence of fever at baseline (19). Both trials enrolled patients who were ≥12 years of age (ESTABLISH-1 enrolled patients ≥18 years [19]) who had ABSSSI (cellulitis/erysipelas, major cutaneous abscess, or wound infection) with a minimum lesion area of 75 cm2 that was suspected or documented to be associated with a Gram-positive pathogen. Patients who received systemic antibiotics with Gram-positive coccal activity within the preceding 96 h or for whom antibiotic therapy was ineffective for the primary ABSSSI site were ineligible. Patients with any of the following were also excluded: uncomplicated skin/skin structure infection, infection associated with prosthetic devices or vascular catheter sites, thrombophlebitis, diabetic foot infection, infected burn, chronic skin ulcer, nonclean surgery, known bacteremia at screening, septic shock/severe sepsis, history of opportunistic infection with the underlying cause still active, long-term systemic immunosuppressive therapy or antipyretic medication (other than ≤200 mg/day of aspirin), severe renal disease, or severe hepatic disease.
The studies were conducted in accordance with the 2008 Declaration of Helsinki and all relevant international, European Union, national, and local rules and legislation. Institutional review board or ethics committee approval was obtained at each participating center. All participants provided written informed consent.
Patients received 200 mg of tedizolid once daily for 6 days or 600 mg of linezolid twice daily for 10 days. A double-dummy design with placebo unique to each active treatment arm was used to maintain the blind for the full 10 days of study drug treatment. The patients in ESTABLISH-1 received oral study drug, whereas the patients in ESTABLISH-2 received two or more i.v. doses, after which they could be switched to oral drug when prespecified criteria were met. The patients were evaluated for efficacy endpoints at 48 to 72 h (primary endpoint evaluation; early clinical response), days 7 to 9, days 11 to 13 (end of therapy [EOT] visit), and 7 to 14 days after EOT (posttherapy evaluation [PTE], days 18 to 25). The patients were evaluated for safety through the long-term follow-up visit (18 to 25 days after EOT).
Endpoints.
The primary efficacy outcome for this pooled analysis was early clinical response at 48 to 72 h after the initiation of therapy in the intent-to-treat (ITT) population. Patients were classified as responders if they exhibited a ≥20% reduction in area (length × width of erythema, edema, or induration [ESTABLISH-1 erythema only was used to determine the lesion size]) of the primary ABSSSI lesion compared with that at baseline, did not receive systemic concomitant antibiotics with Gram-positive activity, and did not die as a result of any cause within 72 h of the first dose. The definition of this endpoint differs slightly between the two studies based on the differences between the U.S. Food and Drug Administration draft 2010 guidance and the final 2013 ABSSSI guidance. This pooled analysis reflects the final guidance for the primary endpoint (21). The key secondary endpoints included the (i) response at day 7 (investigator assessed), (ii) EOT (programmatically defined as no fever or fever unrelated to primary skin infection, decrease from baseline in size of the primary lesion, no worse than mild tenderness or absence of it, absence of or reduced purulent drainage from wound infection, not having received concomitant antibiotics, lack of unplanned surgical intervention, and lack of osteomyelitis), and (iii) PTE (7 to 14 days after EOT, investigator assessed; this is the primary evaluation per European Medicines Agency guidance [22]). Exploratory analyses for early clinical response (48 to 72 h) were conducted in a variety of subgroups defined by clinically relevant host and disease factors and baseline severity measures. The clinically relevant host and disease factor subgroups included clinical syndrome (cellulitis/erysipelas, wound infection, or major cutaneous abscess), body mass index (BMI), i.v. drug use, age, presence of diabetes mellitus, liver disease, and renal impairment. The baseline severity factor subgroups included divisions by baseline lesion area size, systemic signs of infection (presence of fever, increased white blood cell [WBC] count, and band neutrophils), and the presence of systemic inflammatory response syndrome (SIRS) or lymphadenopathy.
The clinical response by key causative pathogen was evaluated at early (48 to 72 h) and late (PTE) visits for S. aureus (including MRSA, MRSA USA300 strain, methicillin-susceptible S. aureus [MSSA], and Panton-Valentine leukocidin [PVL]-positive S. aureus), Streptococcus pyogenes, and Streptococcus anginosus group pathogens.
The collection of adverse events (AEs), physical examination, evaluation of hematologic and chemistry laboratory parameters, and electrocardiography were conducted in the safety population. The effects on hematologic parameters were evaluated using prespecified count-level analyses for 3 key hematologic cell lines, platelets, neutrophils, and hemoglobin, at patient visits on days 7 to 9 and days 11 to 13 (EOT), as well as for the period after baseline through the visit associated with the last day of active therapy (days 7 to 9 visit for tedizolid; days 11 to 13 visit for linezolid). The times to onset of GI AEs were collected and grouped in three periods for evaluation: 0 to 6 days, 7 to 10 days, and >10 days.
Statistical analysis.
The ITT population, all of which consisted of randomized patients, was the primary population for the efficacy analyses. The safety population, all of which consisted of randomized patients who received any amount of study drug, was the primary analysis population for the safety analyses. The microbiologic ITT population, all of which consisted of randomized patients with a baseline Gram-positive pathogen known to cause ABSSSI, was the population used for evaluating the clinical response by pathogen.
For the primary efficacy outcome of early clinical response, the noninferiority of tedizolid to linezolid was concluded if the lower limit of the 95% confidence interval (CI), calculated using the Miettinen and Nurminen method with stratification (by study) for the weighted difference in early clinical response, was greater than −10%. For the secondary outcomes, two-sided 95% CIs were constructed for the weighted differences in the response rates, with stratification by study. Exploratory subgroup efficacy analyses of responders at 48 to 72 h were performed; the treatment differences were weighted by study, and adjusted (for study) 95% CIs were calculated using the method of Miettinen and Nurminen. Analyses of treatment differences in treatment-emergent AEs (TEAEs), hematologic parameters, and time to onset of GI TEAEs were conducted using the Cochran-Mantel-Haenszel test, stratified by study, with two-sided P values reported as descriptive statistics. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Patients.
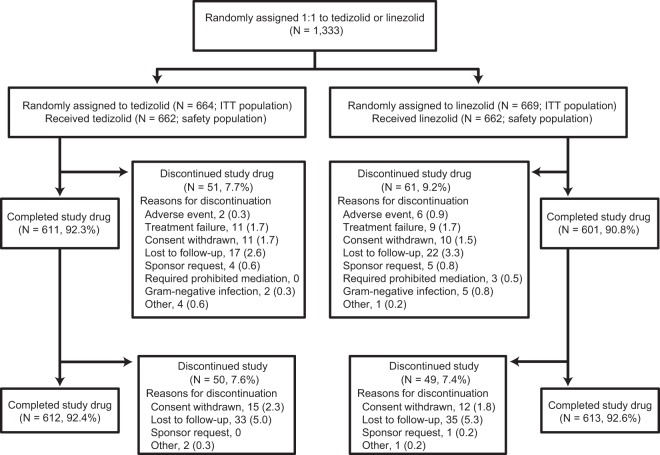
In the two randomized double-blind double-dummy multicenter phase 3 studies, 1,333 patients were randomly assigned to receive treatment with either 200 mg of tedizolid once daily or 600 mg of linezolid twice daily (Fig. 1). The majority of patients (n = 1,225 [91.9%]) completed the study. The baseline characteristics were comparable between the treatment arms, with 64% of the patients enrolled in North America, and with a median patient age of 44 years (Table 1). The most common ABSSSI type was cellulitis/erysipelas, and 75% of all ABSSSI lesions occurred on the extremities. Of those patients with a pathogen isolated, ABSSSI was most frequently caused by S. aureus (82%); the overall rate of MRSA was 35%, and the majority (279/287 [97%]) of the patients were from North America. The overall population enrolled in these trials included a substantial proportion of difficult-to-treat patients, such as obese patients (32%), i.v. drug users (29%), patients with hepatitis C (27%), and patients with concomitant secondary skin infections (14%). Disease severity was also generally substantive, as evidenced by ≥20% of patients who had large lesions (>300 cm3), fever, SIRS, lymphadenopathy, or increased WBC count.
FIG 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram showing patient disposition for the pooled analysis of ESTABLISH-1 and ESTABLISH-2 phase 3 trials.
TABLE 1.
Baseline demographics, clinical characteristics, and microbiology in the ITT populationa
| Characteristicb | 200 mg tedizolid once daily | 600 mg linezolid twice daily |
|---|---|---|
| n | 664 | 669 |
| Age | 44.5 (17–86) | 44.0 (15–100) |
| Median (range) (yr) | ||
| ≥65 | 72 (10.8) | 59 (8.8) |
| Male sex | 429 (64.6) | 412 (61.6) |
| Region of enrollment | ||
| USA/Canada | 426 (64.2) | 426 (63.7) |
| Europe | 165 (24.8) | 166 (24.8) |
| Other region | 73 (11.0) | 77 (11.5) |
| Temperature ≥ 38°C (fever) | 155 (23.3) | 157 (23.5) |
| WBC count ≥ 10,000 cells/mm3 or <4,000 cells/mm3 | 140 (21.1) | 133 (19.9) |
| SIRS | 163 (24.5) | 128 (19.1) |
| Lymphadenopathy | 524 (78.9) | 524 (78.3) |
| >10% immature neutrophils | 76 (11.4) | 56 (8.4) |
| ≥1 Gram-positive pathogen from baseline primary infection site or blood culturec | ||
| Staphylococcus aureusd | 329 (81.0) | 342 (83.0) |
| MRSA | 141 (34.7) | 146 (35.4) |
| USA300 strain | 127 (90.1) | 110 (75.3) |
| MSSA | 188 (46.3) | 198 (48.1) |
| PVL-positive S. aureus | 190 (46.8) | 181 (43.9) |
| Streptococcus pyogenes | 33 (8.1) | 20 (4.9) |
| Streptococcus anginosus group | 30 (7.4) | 28 (6.8) |
| Bacteremia | 11 (1.7) | 16 (2.4) |
| Comorbidities | ||
| Obesity | 200 (30.1) | 232 (34.7) |
| History of diabetes mellitus | 58 (8.7) | 67 (10.0) |
| Hepatic impairment/disease | 14 (2.1) | 12 (1.8) |
| Renal impairment (moderate/severe) | 20 (3.0) | 29 (4.3) |
| HIV positive | 13 (2.0) | 8 (1.2) |
| Hepatitis C | 165 (24.8) | 194 (29.0) |
| Concurrent secondary ABSSSI lesion | 96 (14.5) | 90 (13.5) |
| Risk factors | ||
| Current/recent i.v. drug use | 183 (27.6) | 206 (30.8) |
| Poor living conditions | 40 (6.0) | 34 (5.1) |
| Prior ABSSSI lesion | 146 (22.0) | 144 (21.5) |
| Type of ABSSSI | ||
| Cellulitis/erysipelas | 301 (45.3) | 307 (45.9) |
| Major cutaneous abscess | 168 (25.3) | 166 (24.8) |
| Wound infection | 195 (29.4) | 196 (29.3) |
| Anatomical location of ABSSSI | ||
| Lower extremity | 270 (40.7) | 282 (42.2) |
| Upper extremity | 226 (34.0) | 226 (33.8) |
| Groin/buttocks/back | 93 (14.0) | 93 (13.9) |
| Chest/abdomen | 39 (5.9) | 33 (4.9) |
| Head/neck | 36 (5.4) | 35 (5.2) |
| Lesion surface area (cm3) | ||
| Mean (range) | 347.8 (22.5–5,572.8) | 347.8 (27.0–5,220.0) |
| >300 | 228 (34.4) | 220 (32.9) |
Data are presented as no. (%) unless otherwise stated. ITT, intent-to-treat.
WBC, white blood cell; SIRS, systemic inflammatory response syndrome; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; PVL, Panton-Valentine leukocidin; HIV, human immunodeficiency virus; ABSSSI, acute bacterial skin and skin structure infection.
The number of patients fitting this criterion were 406 in the tedizolid group and 412 in the linezolid group.
MRSA and MSSA totals may not add up to 100% because of a lack of susceptibility data for all isolates; one patient in ESTABLISH-1 may have had both MRSA and MSSA.
All baseline isolates were susceptible to tedizolid (MIC range, 0.06 to 0.5 μg/ml) and linezolid. Against the MRSA isolates, the MIC50 of tedizolid was 0.25 μg/ml, and the MIC90 was 0.5 μg/ml (range, 0.12 to 0.5 μg/ml); the MIC50 of linezolid was 2 μg/ml, and the MIC90 was 2 μg/ml (range, 1 to 2 μg/ml). In addition, all isolates were vancomycin susceptible; no testing for heterogeneous vancomycin-intermediate S. aureus (hVISA) was performed.
Efficacy.
In the pooled ITT population, tedizolid was noninferior to linezolid for the primary endpoint: 81.6% of the tedizolid and 79.4% of the linezolid patients exhibited early clinical response (48 to 72 h), with a difference of 2.2% (95% CI, −2.0 to 6.5). In addition, the treatment outcomes were similar for the key secondary endpoints of programmatic clinical response at EOT (tedizolid, 87.0%; linezolid, 87.9%; difference, −0.8%; 95% CI, −4.4 to 2.7) and investigator-assessed clinical response at PTE (tedizolid, 86.7%; linezolid, 86.8%; difference, −0.1%; 95% CI, −3.8 to 3.6). The clinical response at the 48- to 72-h visit was highly predictive of later success at the PTE visit (i.e., 93% of patients with an early clinical response also had clinical cure at PTE) (23).
The early clinical and late clinical responses at PTE were similar between the treatment groups and supportive of the primary efficacy analysis across all subgroups prespecified for analysis: age, sex, race, BMI, geographic region, clinical syndrome, history of i.v. drug use, receipt of nonsteroidal anti-inflammatory drugs (NSAIDs)/oral steroid use, baseline lesion size, and anatomic location of infection. Furthermore, the treatment differences for early clinical response remained relatively consistent across a variety of clinically relevant host and disease factors (Fig. 2A) and baseline severity measures (Fig. 2B). Numeric differences were observed, sometimes in favor of tedizolid (e.g., wound infection) and sometimes in favor of linezolid (e.g., diabetes and renal impairment; Fig. 2A). Although there are no commonly accepted standard definitions of disease severity in bacterial skin infections, the impact of a number of potential severity measures on clinical outcomes was evaluated, including lesion size, systemic signs of infection (presence of fever, increased WBC counts, and bands), presence of SIRS, and lymphadenopathy. Among these baseline disease severity strata, there were no substantive variances in the treatment differences for early response between the treatment groups (Fig. 2B). Early clinical response and clinical response at PTE were also similar between the treatment arms, regardless of the causative pathogen (Table 2). Finally, among the patients with bacteremia at baseline, unknown at the time of enrollment in the study (known bacteremia at screening was an exclusion criterion), early clinical response (in the ITT population) was achieved in 11 (100%) of 11 tedizolid-treated and 11 (69%) of 16 linezolid-treated patients.
FIG 2.
Differences in early clinical response between tedizolid and linezolid according to clinically relevant host and disease factors (A) and baseline severity measures (B) in the ITT population. The results are the weighted (by study) difference and adjusted confidence interval. [n/n], number of subjects in the subgroup in the tedizolid arm/number of subjects in the subgroup in the linezolid arm. BMI, body mass index; ITT, intent-to-treat; IV, intravenous; LZD, linezolid; SIRS, systemic inflammatory response syndrome; TZD, tedizolid; WBC, white blood cell.
TABLE 2.
Clinical response by key causative pathogen in microbiologic ITT populationa
| Pathogen by response timeb | 200 mg tedizolid once daily (n = 406) |
600 mg linezolid twice daily (n = 412) |
||
|---|---|---|---|---|
| No./total no.c | % | No./total no.c | % | |
| Early clinical response | ||||
| Staphylococcus aureus | 280/329 | 85.1 | 276/342 | 80.7 |
| MRSA | 114/141 | 80.9 | 111/146 | 76.0 |
| USA300 strain | 100/127 | 78.7 | 83/110 | 75.5 |
| MSSA | 166/188 | 88.3 | 167/198 | 84.3 |
| PVL-positive S. aureus | 157/190 | 82.6 | 139/181 | 76.8 |
| Streptococcus pyogenes | 25/33 | 75.8 | 16/20 | 80.0 |
| Streptococcus anginosus group | 22/30 | 73.3 | 25/28 | 89.3 |
| Clinical response at PTE | ||||
| S. aureus | 331/374 | 88.5 | 303/342 | 88.6 |
| MRSA | 151/178 | 84.8 | 119/146 | 81.5 |
| USA300 strain | 105/127 | 82.7 | 92/110 | 83.6 |
| MSSA | 180/196 | 91.8 | 186/198 | 93.9 |
| PVL-positive S. aureus | 164/190 | 86.3 | 153/181 | 84.5 |
| S. pyogenes | 30/33 | 90.9 | 19/20 | 95.0 |
| S. anginosus group | 21/30 | 70.0 | 25/28 | 89.3 |
ITT, intent-to-treat.
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; PVL, Panton-Valentine leukocidin; PTE, posttherapy evaluation.
No. of patients with clinical response/total no. of patients with the particular causative pathogen.
Safety and tolerability.
Forty-three percent of patients reported at least one TEAE, the majority of which were mild (2% severe), with few leading to discontinuation in either arm (tedizolid, 0.5%; linezolid, 0.9%). The most commonly reported TEAEs were nausea, headache, and abscess, of which nausea was reported less frequently in the tedizolid group than in the linezolid group (Table 3). Three patients died, two in the tedizolid treatment group (one with Gram-negative pneumonia with septic shock and one with myocardial infarction) and one in the linezolid treatment group (tubercular meningitis); none of the deaths was considered to be treatment related. Although approximately one-third of patients had hepatic impairment or disease (tedizolid, 189/662 [28.5%]; linezolid, 221/662 [33.4%]), no patients with hepatic impairment/disease withdrew from the study because of a TEAE. In addition, similar to what was observed in the overall population, the mean values of the chemistry parameters evaluated were stable over the course of the studies, and increases of two toxicity grades or more (defined according to the Division of Microbiology and Infectious Diseases adult toxicity table [24]) from baseline to the worst postbaseline result were infrequent in both the tedizolid (alanine aminotransferase, 2.2%; alkaline phosphatase, 0.5%; aspartate aminotransferase, 1.7%; glucose, 4.3%) and linezolid (alanine aminotransferase, 2.0%; alkaline phosphatase, 0%; aspartate aminotransferase, 4.5%; glucose, 6.7%) treatment groups in patients with hepatic impairment/disease.
TABLE 3.
Most commonly reported treatment-emergent adverse events (≥1% in either treatment group) in the safety population
| TEAEa | No. (%) of patients with the indicated TEAE |
|
|---|---|---|
| 200 mg tedizolid once daily | 600 mg linezolid twice daily | |
| Total no. of patients | 662 | 662 |
| Gastrointestinal disorders | 106 (16.0)b | 152 (23.0) |
| Nausea | 54 (8.2)b | 81 (12.2) |
| Diarrhea | 26 (3.9) | 35 (5.3) |
| Vomiting | 19 (2.9)b | 37 (5.6) |
| Constipation | 9 (1.4) | 6 (0.9) |
| Dyspepsia | 4 (0.6) | 8 (1.2) |
| Infections and infestations | 91 (13.7) | 78 (11.8) |
| Abscess | 35 (5.3) | 26 (3.9) |
| Cellulitis | 17 (2.6) | 14 (2.1) |
| Vulvovaginal mycotic infection | 2 (0.3) | 9 (1.4) |
| Nervous system disorders | 65 (9.8) | 67 (10.1) |
| Headache | 41 (6.2) | 39 (5.9) |
| Dizziness | 12 (1.8) | 14 (2.1) |
| Skin and subcutaneous tissue disorders | 47 (7.1) | 40 (6.0) |
| Pruritus generalized | 11 (1.7) | 7 (1.1) |
| Pruritus | 3 (0.5) | 9 (1.4) |
| General disorders and administration site conditions | 36 (5.4) | 39 (5.9) |
| Fatigue | 9 (1.4) | 12 (1.8) |
| Psychiatric disorders | 17 (2.6) | 8 (1.2) |
| Insomnia | 10 (1.5) | 5 (0.8) |
TEAE, treatment-emergent adverse event.
P < 0.05.
The effects on hematologic parameters were evaluated for three key hematologic cell lines: platelets, neutrophils, and hemoglobin. The most notable differences between the treatment groups were observed for platelets (Fig. 3), with 3.7% of the tedizolid patients and 5.6% of the patients (P = 0.0585) with platelet counts below the lower limit of normal (LLN) (150,000 cells/mm3) at the days 7 to 9 visit, and 4.9% versus 10.8% for tedizolid versus linezolid, respectively (P = 0.0003), at the EOT (days 11 to 13) visit. In the period after baseline through the visit for the last dose of active drug for each group, 6.4% of the tedizolid patients and 12.6% of the linezolid patients (P = 0.0016) had platelet counts below the LLN. The proportions of patients with absolute neutrophil counts below the LLN were 1.7% and 2.8% for the patients in the tedizolid and linezolid groups, respectively, on days 7 to 9, and 1.9% and 3.3%, respectively, at EOT (days 11 to 13). The proportions of patients with hemoglobin levels below the LLN were 29.4% and 33.3% for the patients in the tedizolid and linezolid groups, respectively, on days 7 to 9, and 28.9% and 31.1%, respectively, at EOT (days 11 to 13). Similar trends for all cell lines were noted when patients with abnormal or missing values at baseline were excluded from the analysis. In both treatment groups, very few patients experienced toxicity grade shifts (defined according to the Division of Microbiology and Infectious Diseases adult toxicity table [24]) of two or more for all three hematologic cell lines through the EOT visit (tedizolid: platelets, 1.4%; neutrophils, 0.5%; hemoglobin, 1.0%; linezolid: platelets, 0.7%; neutrophils, 1.3%; hemoglobin, 0.7%). Moreover, no bleeding-related AEs were reported in either treatment arm.
FIG 3.

Patients with platelet counts below the lower limit of normal (LLN) (<150,000 cells/mm3) over time. *, P < 0.05. EOT, end-of-therapy.
The most frequently reported class of AEs in these phase 3 trials was GI AEs, which were reported in 16% of the tedizolid patients and 23% of the linezolid patients (P = 0.0015). Nausea, vomiting, and diarrhea occurred primarily during the first 6 days, when both groups were receiving the active drug; a significantly lower proportion of patients in the tedizolid group reported any one of those, or nausea or vomiting individually, than did the patients in the linezolid group (Fig. 4). The severity of nausea and vomiting was generally mild and was similar between the treatment groups, with 1% to 3% experiencing moderate events and <0.5% experiencing severe events. The severity of diarrhea was generally mild, with only 1% of the patients in each treatment arm experiencing moderate diarrhea and none with severe diarrhea.
FIG 4.
Time to onset of gastrointestinal treatment-emergent adverse events. *, P < 0.05.
DISCUSSION
Once-daily 200-mg tedizolid for 6 days showed noninferior efficacy to twice-daily 600-mg linezolid for 10 days in the treatment of ABSSSI, based on the primary endpoint of early clinical response at 48 to 72 h (19, 20). The clinical success rates were also similar between the treatment arms at all subsequent time points. Early clinical response was highly predictive of later success, highlighting that this early endpoint is a reliable indicator of overall cure. Furthermore, the results from these pooled analyses suggest a relatively consistent efficacy response, regardless of causative pathogen, disease severity, and underlying host factors.
A substantial proportion of patients considered difficult to treat (e.g., obese) or with conditions suggesting severe disease (e.g., large lesion size, adjacent lymphadenopathy, SIRS, or systemic signs of infection) were enrolled in these trials. The numeric differences for subgroup analyses were not unexpected and sometimes favored tedizolid and sometimes favored linezolid, which reflects the inherent difficulty in interpreting subgroup analyses (i.e., the balance of prognostic factors between the treatment groups may not be maintained because not all subgroups are randomization stratification factors, are not adjusted for multiple comparisons, and have insufficient statistical power). Therefore, these subgroup analyses can be viewed as hypothesis generating and should be interpreted cautiously. In previously presented separate population pharmacokinetic and exposure analyses, no baseline covariate (i.e., age, body weight, BMI, sex, race, renal function, liver function, or preexisting diabetes) had an impact on tedizolid exposure levels in a way that was expected to affect either efficacy or safety (S. Flanagan, S. L. Minassian, D. Morris, R. Ponnuraj, T. C. Marbury, H. W. Alcorn, E. Fang, and P. Prokocimer, unpublished data; S. Flanagan, J. Passarell, Q. Lu, J. Fiedler-Kelly, E. Ludwig, and P. Prokocimer, unpublished data).
Both drugs appeared to be well tolerated, with low platelet counts reported less frequently with tedizolid than with linezolid at the EOT visit. This finding was unexpected because the two drugs were administered for a short period (<14 days). Although some of these low counts might reflect artifacts in the measurement of the cell lines (e.g., blood sampling and shipping or measurement techniques), the prevalences of these artifacts would be expected to be similar between the two groups. Hence, the consistent observation of a difference in the number of patients with low platelet counts might be seen as an early signal of a difference between the two study drugs. These results are consistent with the findings of a previous phase 1 study that showed no meaningful effect on any cell line after 21 days of tedizolid dosing in healthy volunteers at the 200-mg once-daily dose (25). However, the clinical relevance of this finding with regard to a potential longer duration of dosing in patients is unknown. Myelosuppression, a known risk of linezolid use that increases after 2 weeks of drug administration, is associated with oxazolidinone-induced impairment of mitochondrial protein synthesis (26–28). A potential explanation for the platelet differences between tedizolid and linezolid seen here is that oxazolidinone-associated mitochondrial toxicity may be related to the time that the free-drug plasma concentrations exceed the 50% inhibitory concentration (IC50) for mitochondrial protein synthesis during the dosing interval (29, 30). Linezolid free-plasma concentrations are above the mitochondrial protein synthesis IC50 during the entire dosing interval, whereas tedizolid free-plasma concentrations dip below its IC50 for a portion of the dosing interval, providing a biological plausibility for the differences in mitochondrial toxicity-related events between the two drugs (28, 31). Nevertheless, further study is warranted in patients receiving longer courses of tedizolid in order to confirm these findings.
Finally, there were differences in the incidences of GI AEs between tedizolid and linezolid. The observed difference in GI events does not seem to reflect the shorter treatment duration with tedizolid because most events occurred early in treatment, that is, within the first 6 days of therapy when both groups were receiving the active drug. In the absence of comparative studies exploring the potential biological mechanisms behind these differences, we can merely speculate why this was the case. The impact of tedizolid on normal intestinal flora was previously shown to be limited and reversible, with no signs of microbiological substitution after 7 days of i.v. or oral administration in healthy subjects (32), while another study showed linezolid to result in marked alterations in gut flora composition (33). In addition, the lower dosing frequency of tedizolid (once daily versus twice daily with linezolid) may also somewhat reduce the risk of developing GI AEs overall.
Future studies to evaluate the efficacy and safety of tedizolid in other infection types, including those that might necessitate longer treatment duration, should be performed.
In conclusion, once-daily 200-mg tedizolid for 6 days showed noninferior efficacy compared with twice-daily 600-mg linezolid for 10 days and was generally well tolerated, with a lower incidence of platelet counts below the LLN and fewer GI side effects in the patients treated with tedizolid. The drug profile of tedizolid is squarely in line with the principles of antimicrobial stewardship (34). Namely, its once-daily antibiotic administration, availability of an oral administration option, achievement of efficacy with short-course treatment, and favorable tolerability profile align with the goals of stewardship programs. As such, tedizolid might become a useful option for the treatment of ABSSSI in the hospital and outpatient settings.
ACKNOWLEDGMENTS
A. F. Shorr has been a consultant for Cubist Pharmaceuticals. T. P. Lodise has been a consultant and grant recipient for Cubist Pharmaceuticals. G. R. Corey was a principal investigator in the tedizolid phase 3 ABSSSI program and has been a consultant for Cubist Pharmaceuticals. C. De Anda, E. Fang, and P. Prokocimer are employees of Cubist Pharmaceuticals. A. F. Das is a consultant for Cubist Pharmaceuticals.
This work was supported by Cubist Pharmaceuticals, the sponsor of the studies. Editorial support was provided by ApotheCom ScopeMedical and was funded by Cubist Pharmaceuticals.
REFERENCES
- 1.Taira BR, Singer AJ, Thode HC Jr, Lee CC. 2009. National epidemiology of cutaneous abscesses: 1996 to 2005. Am J Emerg Med 27:289–292. doi: 10.1016/j.ajem.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 3.Moran GJ, Abrahamian FM, Lovecchio F, Talan DA. 2013. Acute bacterial skin infections: developments since the 2005 Infectious Diseases Society of America (IDSA) guidelines. J Emerg Med 44:e397–e412. doi: 10.1016/j.jemermed.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 5.Tracy LA, Furuno JP, Harris AD, Singer M, Langenberg P, Roghmann MC. 2011. Staphylococcus aureus infections in US veterans, Maryland, USA, 1999–2008. Emerg Infect Dis 17:441–448. doi: 10.3201/eid1703.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ, EMERGEncy ID Net Study Group. 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 7.Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. 2013. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PLoS One 8:e52722. doi: 10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. 2013. Surveillance Report: antimicrobial resistance surveillance in Europe. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2012. European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2012.pdf. [Google Scholar]
- 9.Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, Vega S, Zurita J, Cepparulo M, Castanheira M. 2013. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis 17:672–681. doi: 10.1016/j.bjid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang CI, Song JH. 2013. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother 45:22–31. doi: 10.3947/ic.2013.45.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger A, Oster G, Edelsberg J, Huang X, Weber DJ. 2013. Initial treatment failure in patients with complicated skin and skin structure infections. Surg Infect (Larchmt) 14:304–312. doi: 10.1089/sur.2012.103. [DOI] [PubMed] [Google Scholar]
- 12.Davis SL, Perri MB, Donabedian SM, Manierski C, Singh A, Vager D, Haque NZ, Speirs K, Muder RR, Robinson-Dunn B, Hayden MK, Zervos MJ. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol 45:1705–1711. doi: 10.1128/JCM.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labreche MJ, Lee GC, Attridge RT, Mortensen EM, Koeller J, Du LC, Nyren NR, Treviño LB, Treviño SB, Peña J, Mann MW, Muñoz A, Marcos Y, Rocha G, Koretsky S, Esparza S, Finnie M, Dallas SD, Parchman ML, Frei CR. 2013. Treatment failure and costs in patients with methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med 26:508–517. doi: 10.3122/jabfm.2013.05.120247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tattevin P, Schwartz BS, Graber CJ, Volinski J, Bhukhen A, Bhukhen A, Mai TT, Vo NH, Dang DN, Phan TH, Basuino L, Perdreau-Remington F, Chambers HF, Diep BA. 2012. Concurrent epidemics of skin and soft tissue infection and bloodstream infection due to community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 55:781–788. doi: 10.1093/cid/cis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke JB, Zurenko GE, Shaw KJ, Bartizal K. 2014. Tedizolid for the management of human infections: in vitro characteristics. Clin Infect Dis 58(Suppl 1):S35–S42. doi: 10.1093/cid/cit616. [DOI] [PubMed] [Google Scholar]
- 16.Shaw KJ, Poppe S, Schaadt R, Brown-Driver V, Finn J, Pillar CM, Shinabarger D, Zurenko G. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob Agents Chemother 52:4442–4447. doi: 10.1128/AAC.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke JB, Hilgers M, Shaw KJ. 2009. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob Agents Chemother 53:5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan S, Fang E, Muñoz KA, Minassian SL, Prokocimer PG. 2014. Single- and multiple-dose pharmacokinetics and absolute bioavailability of tedizolid. Pharmacotherapy 34:891–900. doi: 10.1002/phar.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. 2013. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 20.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. 2014. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase III, non-inferiority trial. Lancet Infect Dis 14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. 2013. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071185.pdf. [Google Scholar]
- 22.European Medicines Agency. 2013. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf. [Google Scholar]
- 23.Fang E, De Anda C, Das AF, Prokocimer P. 2014. Concordance between early and late response in ESTABLISH-1 and ESTABLISH-2: pooled results from two tedizolid phase 3 trials in acute bacterial skin and skin structure infection, ePoster eP434 Abstr 24th Annu Meet Eur Congr Clin Microbiol Infect Dis (ECCMID 2014), 10 to 13 May 2014, Barcelona, Spain. [Google Scholar]
- 24.National Institutes of Health (NIH). 2007. Division of Microbiology and Infectious Diseases (DMID) adult toxicity table. National Institute of Allergy and Infectious Disease, National Institutes of Health, Bethesda, MD: http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Documents/dmidadulttox.pdf.. [Google Scholar]
- 25.Prokocimer P, Bien P, Muñoz KA, Aster R. 2008. Hematological effects of TR-701, linezolid and placebo administered for 21 days in healthy subjects, poster F1-2069a Abstr 48th Annu Intersci Conf Antimicrob Agents Chemother Infect Dis Soc Am 46th Annu Meet (ICAAC/IDSA 2008), 25 to 28 October 2008, Washington, DC. [Google Scholar]
- 26.Shaw KJ, Barbachyn MR. 2011. The oxazolidinones: past, present, and future. Ann N Y Acad Sci 1241:48–70. doi: 10.1111/j.1749-6632.2011.06330.x. [DOI] [PubMed] [Google Scholar]
- 27.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC, Vandecasteele S, Boelaert JR. 2006. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 42:1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 28.Pfizer. 2014. Zyvox (linezolid) injection, tablets and oral suspension. Pharmacia & Upjohn Co. Division of Pfizer, Inc., New York, NY: http://labeling.pfizer.com/ShowLabeling.aspx?id=649. [Google Scholar]
- 29.Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM. 2009. Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J Antimicrob Chemother 64:1035–1043. doi: 10.1093/jac/dkp267. [DOI] [PubMed] [Google Scholar]
- 30.Garrabou G, Soriano A, López S, Guallar JP, Giralt M, Villarroya F, Martínez JA, Casademont J, Cardellach F, Mensa J, Miró O. 2007. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob Agents Chemother 51:962–967. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flanagan S, Minassian SL, Muñoz KA, Dreskin H, Fang E, Prokocimer P. Lack of pharmacokinetic drug interaction of tedizolid phosphate with pseudoephedrine in healthy subjects, poster P-921 Abstr 23rd Annu Meet Eur Congr Clin Microbiol Infect Dis (ECCMID 2013), 27 to 30 April 2013, Berlin, Germany. [Google Scholar]
- 32.Tanaka T, Hayashi Y, Okumura K, Yoshikawa K, Kato M, Kanatani KT, Ikushima I. Changes in intestinal flora during 7-day tedizolid phosphate administration in a phase I study in healthy Japanese subjects, poster P1675 Abstr 24th Eur Soc Clin Microbiol Infect Dis (ECCMID 2014), 10 to 13 May 2014, Barcelona, Spain. [Google Scholar]
- 33.Lode H, Von der Höh N, Ziege S, Borner K, Nord CE. 2001. Ecological effects of linezolid versus amoxicillin/clavulanic acid on the normal intestinal microflora. Scand J Infect Dis 33:899–903. doi: 10.1080/00365540110076714. [DOI] [PubMed] [Google Scholar]
- 34.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. 2012. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 33:322–327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]