Abstract
In 2008, artemether-lumefantrine was introduced in Guinea-Bissau, West Africa, but quinine has also been commonly prescribed for the treatment of uncomplicated Plasmodium falciparum malaria. An efficacious high-dose chloroquine treatment regimen was used previously. Temporal and seasonal changes of genetic polymorphisms associated with altered drug susceptibility to chloroquine, lumefantrine, and quinine have been described. P. falciparum chloroquine resistance transporter (pfcrt) K76T, pfmdr1 gene copy numbers, pfmdr1 polymorphisms N86Y and Y184F, and pfmdr1 sequences 1034 to 1246 were determined using PCR-based methods. Blood samples came from virtually all (n = 1,806) children <15 years of age who had uncomplicated P. falciparum monoinfection and presented at a health center in suburban Bissau (from 2003 to 2012). The pfcrt K76T and pfmdr1 N86Y frequencies were stable, and seasonal changes were not seen from 2003 to 2007. Since 2007, the mean annual frequencies increased (P < 0.001) for pfcrt 76T (24% to 57%), pfmdr1 N86 (72% to 83%), and pfcrt 76 + pfmdr1 86 TN (10% to 27%), and pfcrt 76T accumulated during the high transmission season (P = 0.001). The pfmdr1 86 + 184 NF frequency increased from 39% to 66% (from 2003 to 2011; P = 0.004). One sample had two pfmdr1 gene copies. pfcrt 76T was associated with a lower parasite density (P < 0.001). Following the discontinuation of an effective chloroquine regimen, probably highly artemether-lumefantrine-susceptible P. falciparum (with pfcrt 76T) accumulated, possibly due to suboptimal use of quinine and despite a fitness cost linked to pfcrt 76T. (The studies reported here were registered at ClinicalTrials.gov under registration no. NCT00137514 [PSB-2001-chl-amo], NCT00137566 [PSB-2004-paracetamol], NCT00426439 [PSB-2006-coartem], NCT01157689 [AL-eff 2010], and NCT01704508 [Eurartesim 2012].)
INTRODUCTION
Plasmodium falciparum malaria is a major cause of morbidity and mortality in sub-Saharan Africa (1). Due to the development and spread of P. falciparum resistance to commonly available monotherapies, the World Health Organization recommends the use of artemisinin-based combination therapy (ACT) for the treatment of uncomplicated malaria (2). Therefore, Guinea-Bissau, in West Africa, replaced an apparently efficacious high-dose chloroquine (CQ) regimen with an equally efficacious artemether-lumefantrine (AL) regimen in 2008 (3). Quinine was and is recommended for the treatment of severe malaria in Guinea-Bissau. However, in clinical practice, it is also commonly used for nonsevere malaria (4). The P. falciparum incidence and prevalence decreased to all-time lows between 2003 and 2007, prior to the change in treatment policy, and then resurged in an epidemic fashion, particularly in older children between 2008 and 2011, following the introduction of AL (5).
CQ resistance and reduced tolerance to lumefantrine have been linked to single nucleotide polymorphisms (SNPs) in the P. falciparum CQ resistance transporter gene (pfcrt) and multidrug resistance gene 1 (pfmdr1) (6–10). pfcrt 76T is essential for resistance to the standard dose of CQ, and additional SNPs in pfcrt and pfmdr1 86Y appear to compensate for fitness loss and to modulate CQ susceptibility (11). In Guinea-Bissau, CQ resistance was linked to the pfcrt haplotype 72–76 CVIET + 271E + 326S, whereas the haplotype 72–76 CVMNK + Q271 + N326 was linked to CQ sensitivity (12, 13). pfcrt K76 and pfmdr1 N86 are selected for after treatment with AL and are linked to reduced lumefantrine susceptibility (7–9, 14). The pfmdr1 86 + 184 + 1246 NFD haplotype has been selected for by AL (7, 15, 16). Furthermore, an increased pfmdr1 copy number has been linked to reduced susceptibility to artemisinin derivatives, lumefantrine, piperaquine, and mefloquine and to artesunate plus mefloquine treatment failure (17–20). In addition to these well-characterized resistance-associated SNPs, pfmdr1 polymorphisms N86Y, S1034C, N1042D, and D1246Y have been suggested to modulate levels of CQ and quinine resistance (21, 22).
P. falciparum in Guinea-Bissau recently had to adapt to changing drug pressure and changing epidemiology (5). These factors are likely to affect the prevalence of P. falciparum through genetic polymorphisms associated with reduced drug susceptibility, especially as CQ resistance, at least, has been associated with a cost to parasite fitness (23). As part of the clinical trials conducted within the Bandim Health and Demographic Surveillance Site (HDSS), blood has been collected for genotyping. The primary aim of this study was to determine the temporal and seasonal frequencies of genotypes associated with reduced susceptibilities to CQ, AL, and quinine between 2003 and 2012.
MATERIALS AND METHODS
Study area and population.
The studies were conducted at the Bandim HDSS in suburban Bissau, the capital of Guinea-Bissau. The Bandim Health Centre is located centrally within the HDSS, and nurses see pediatric patients in the mornings, afternoons, and weekends. There is a laboratory manned by trained technicians.
Data collection.
This report is based on five clinical trials. Three trials conducted between 2001 and 2008 have been published (3, 12, 24, 25). One trial conducted between 2010 and 2012 has been completed, and analyses are ongoing. Another ongoing trial started in November 2012. Thus, part of the data collected between 2003 and 2008 has been published; however, the data form a necessary background for this paper with a different aim and are therefore included (3, 12, 24–27). No clinical trial was conducted in 2009, so there are no data from that year. The inclusion criteria used in all the studies were an age of <15 years, residence within the HDSS, fever or history of fever, monoinfection with P. falciparum, a parasite density of ≥800/μl, and no signs or symptoms of severe malaria.
Malaria diagnoses.
Thick and thin Giemsa-stained smears were made from blood obtained by finger-pricks to identify species and quantify asexual parasite density (per 200 white blood cells) using ×1,000 magnification and a sunlit microscope. A slide was considered negative after examination of 100 high-power fields. Rapid diagnostic tests have been available since 2010, but all stains from the children with possible malaria were also screened using microscopy.
Treatment.
Although the official treatment dose was 25 mg/kg of body weight of CQ over 3 days, an efficacious high-dose CQ treatment regimen was used until June 2008, when AL was introduced as part of national policy for the treatment of uncomplicated malaria (3, 13, 28). The origin of the high-dose treatment regimen is not certain but was probably a misinterpretation of the 25-mg/kg dose over 3 days as 25 mg/kg per day for 3 days. Quinine remained the drug of choice for severe malaria throughout this time. CQ was also commonly used for the home treatment of a fever presumed to be caused by malaria, at least until the introduction of AL. The introduction of AL was widely announced by the media, and people were told to stop using CQ.
The drugs used as part of the studies were CQ or amodiaquine (2001 to 2004), CQ (2004 to 2006), CQ or AL (2006 to 2008), and dihydroartemisinin-piperaquine or AL (November 2012 to present). As part of an effectiveness study (2010 to 2012), antimalarial drug use was monitored in 799 children with uncomplicated malaria; AL was prescribed to 47% of them, quinine to 43%, quinine-AL to 9%, and CQ to 1%. Children with recurrent parasitemia during the study follow-up period were re-treated with sulfadoxine-pyrimethamine, CQ (50 mg/kg), or AL, depending on the study.
Sample collection, storage, and DNA extraction.
After inclusion on day 0, blood was put onto filter papers (Whatman 3MM), dried, and stored in individual plastic bags until DNA extraction was performed. Approximately 25 μl of blood was cut from the filter papers and extracted on an Applied Biosystems Prism 6100 Nucleic Acid PrepStation for studies conducted between 2003 and 2011. The extraction was performed according to the manufacturer's protocol for the isolation of DNA from whole blood, with minor modifications. Since mid-2011, DNA was extracted using a Chelex-based method. The extracted DNA was stored at −20°C until use.
PCR analyses.
Previously described multiplex restriction fragment length polymorphism PCR methods were used to detect pfcrt K76T and pfmdr1 N86Y and Y184F SNPs (6, 29, 30).
Pfmdr1 codons 1034 to 1246 were ascertained by PCR amplification, followed by sequencing, using previously described PCR protocols (7). The Sequencher software version 4.6 (Gene Codes Corporation, Ann Arbor, MI) was used for sequencing analysis. The sequences of the P. falciparum 3D7 clone obtained from the NCBI database were used as references for pfmdr1 (accession no. XM_001351751.1). The PCR and restriction products were resolved on 2% agarose gels (Amresco, Solon, OH). All the gels were stained with a nucleic acid gel stain (ethidium bromide or GelRed; Biotium, Inc., Hayward, CA, USA) and visualized under UV transillumination (Gel Doc; Bio-Rad, Hercules, CA, USA). The PCR products were purified and sequenced commercially (Macrogen, Inc., Seoul, South Korea).
Statistics.
SNP frequency was calculated as the number of samples with a certain allele. Thus, mixed infections were counted twice. Haplotype frequency was calculated after excluding mixed infections. The yearly and seasonal changes of allele and haplotype frequencies were estimated using logistic regression with bootstrapping (100 repeats), with year or month as the continuous covariate and the earliest time point as the baseline. For the calculation of seasonal changes, February was the earliest time point, as the high-transmission season ends in January. Parasite densities and ages were estimated and compared using quantile regression with bootstrapping (100 repeats). The linkage disequilibrium was calculated as a correlation coefficient (r) between pairs of loci after excluding mixed infections. A correlation coefficient of 1 indicated absolute correlation, 0 indicated no correlation, and −1 indicated total negative correlation.
Ethics statement.
Each patient was included in the respective clinical studies after informed consent was received from his or her caretaker. Ethical approval was granted by the ethical review board in Bissau, Guinea-Bissau (Parecet NCP/N19/2006, 019/DHE/2004, and 064/DGSP/2006), the regional ethics committee in Stockholm, Sweden (2005/111-31/1, 2006/1151-31/1, and 2011/832-32/2), and the central scientific ethics committee in Denmark (624-01-0042). All these studies were also registered at ClinicalTrials.gov under registration no. NCT00137514 (PSB-2001-chl-amo), NCT00137566 (PSB-2004-paracetamol), NCT00426439 (PSB-2006-coartem), NCT01157689 (AL-eff 2010), and NCT01704508 (Eurartesim 2012).
RESULTS
A total of 1,806 children aged less than 15 years with uncomplicated P. falciparum monoinfection were included from 2003 to 2012. The basic demographic characteristics are shown in Table 1. There were 921 male and 832 female children. Sex was not recorded at the end of 2008, which accounts for the discrepancy in the total number of children. Prior to 2008, malaria cases were detected throughout the year, with peaks at the beginning (June) and end (October to November) of the rainy season. Between 2008 and 2012, malaria became epidemic, with a single high peak in November. Between 2003 and 2012, the annual median age increased from 5 years 8 months to 10 years (P < 0.001).
TABLE 1.
Baseline characteristics of children who had P. falciparum monoinfection and attended the Bandim Health Centre between 2003 and 2012
| Yr | No. of children | Sex ratio (M:F)a | Age (95% CI)b |
|---|---|---|---|
| 2003 | 342 | 160:182 | 5.7 (5.1–6.2) |
| 2004 | 179 | 96:83 | 5 (3.8–6.2) |
| 2005 | 173 | 85:88 | 5.4 (4.9–5.9) |
| 2006 | 89 | 47:42 | 5.0 (4–5.9) |
| 2007 | 40 | 22:18 | 5.1 (3.9–6.2) |
| 2008 | 141 | 52:37 | 6.5 (5.7–7.4) |
| 2010 | 345 | 180:165 | 8.8 (8–9.5) |
| 2011 | 360 | 205:154 | 9.0 (8.3–9.7) |
| 2012 | 137 | 74:63 | 10.0 (9.3–10.7) |
Sex was not recorded at the end of 2008, and data were missing for one patient in 2011.
CI, confidence interval.
pfcrt K76T allele frequencies over time.
Figure 1, top, and Table 2 show the pfcrt K76T frequencies over time, the number of children with malaria each year, and the recommended 1st-line antimalarial drugs used. The pfcrt K76 and 76T frequencies were stable at ∼80% and ∼24%, respectively, between 2003 and 2007. After 2007, the pfcrt K76 frequency decreased (P < 0.001), and the mean frequency between 2008 and 2012 was 67% (598/896). The pfcrt 76T frequency increased significantly (P < 0.001) between 2007 and 2012. More specifically, the pfcrt 76T frequency gradually increased to 57% between 2007 and 2011 and then appeared to decrease to 34% in 2012.
FIG 1.
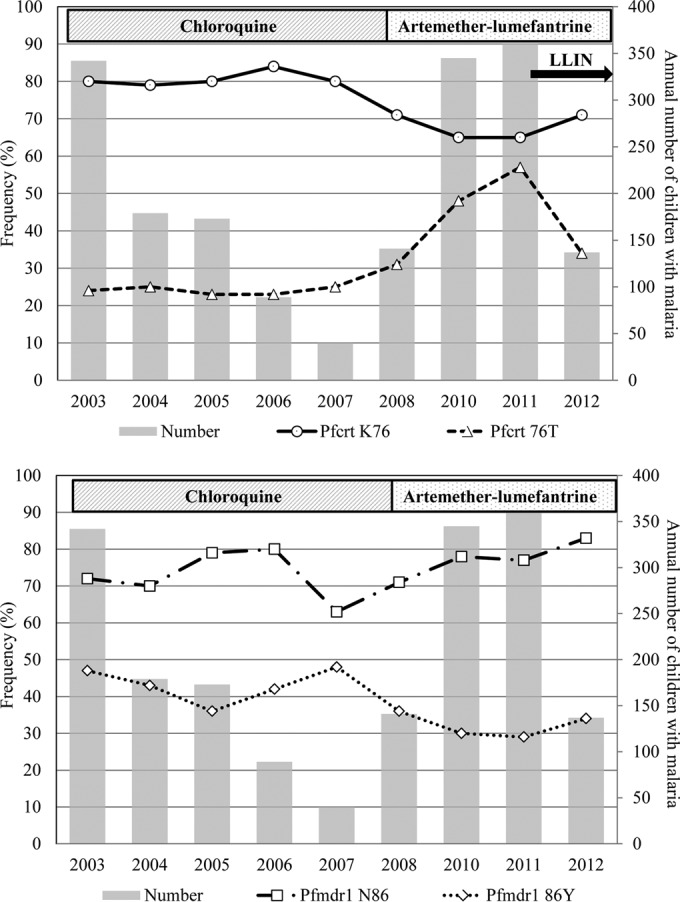
pfcrt K76T and pfmdr1 N86Y allele frequencies in children aged less than 15 years who attended the Bandim Health Centre between 2003 and 2012. LLIN, mass distribution of long-lasting insecticide-treated bed nets in November 2011.
TABLE 2.
pfcrt K76T frequencies, pfmdr1 N86Y frequencies, and pfmdr1 gene copy numbers between 2003 and 2012 among children diagnosed with P. falciparum malaria at the Bandim Health Centre
| Yr | Frequency (% [no. with a specific allele/total no. of successfully amplified samples]) for: |
No. of samples with pfmdr1 copy no. of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
pfcrt |
pfmdr1 |
||||||||
| K76 | 76T | N86 | 86Y | F184 | 184Y | 1 | 1.5 | 2 | |
| 2003 | 79 (253/318) | 24 (75/318) | 72 (227/317) | 47 (149/317) | 79 (251/317) | 37 (118/317) | 311 | ||
| 2004 | 79 (119/151) | 25 (37/151) | 70 (108/155) | 43 (66/155) | 152 | ||||
| 2005 | 80 (136/169) | 23 (39/169) | 79 (134/170) | 36 (61/170) | 173 | ||||
| 2006 | 84 (74/88) | 23 (20/88) | 80 (70/88) | 42 (37/88) | 88 | ||||
| 2007a | 80 (32/40) | 25 (10/40) | 63 (25/40) | 48 (19/40) | 39 | ||||
| 2008 | 71 (95/134) | 31 (42/134) | 71 (96/135) | 36 (49/135) | 138 | 1 | |||
| 2010 | 65 (193/296) | 48 (141/296) | 78 (232/297) | 30 (89/297) | 76 (218/288) | 42 (121/288) | 271 | 1 | 1 |
| 2011 | 65 (224/345) | 57 (195/345) | 77 (249/322) | 29 (93/322) | 74 (245/332) | 41 (136/332) | 337 | ||
| 2012 | 71 (86/121) | 34 (41/121) | 83 (105/126) | 34 (43/126) | 79 (73/92) | 46 (42/92) | 126 | ||
pfcrt K76T and pfmdr1 N86Y frequencies did not change significantly before 2007. pfcrt K76 decreased (P < 0.001) and pfcrt 76T increased (P < 0.001) after 2007. pfmdr1 N86 increased and pfmdr1 86Y decreased significantly (both P < 0.001) between 2007 and 2012. pfmdr1 Y184F did not change significantly.
pfmdr1 N86Y allele frequencies over time.
The pfmdr1 N86Y allele frequencies did not change significantly before 2007. The pfmdr1 N86 frequency increased significantly (63% to 83%, P < 0.001) and the pfmdr1 86Y frequency decreased significantly (48% to 34%, P < 0.001) between 2007 and 2012, as shown in Fig. 1, bottom, and Table 2.
Other pfmdr1 SNPs over time.
The pfmdr1 Y184F allele frequencies did not change significantly over time, as shown in Table 2. The SND haplotype was found in pfmdr1 1034, 1042, and 1246 in 137 of 138 samples between 2003 and 2004 and in 315 of 316 in 2011. During the same years, the SNY haplotype was found in 1 of 138 and 1 of 316 samples, respectively.
Sequencing of all samples collected in 2011 identified the nonsynonymous SNPs 1082tcc→gcc (2/316), 1199aat→aag (5/316), and 1241gat→aat (2/316) and the synonymous SNPs 1069act→acg (40/317), 1158aca→acg (1/317), and 1243aac→aat (1/316). The pfcrt and pfmdr1 haplotypes for the novel SNPs listed above are shown in Table S1 in the supplemental material. The pfmdr1 SNP 1069acg was found together with pfcrt K76 in 27/146 (18%) samples and with pfcrt 76T in 8/104 (8%) samples (P = 0.015) after the exclusion of mixed infections. No other significant associations were found.
pfmdr1 amplifications.
One gene copy was found in 1,635 samples, and 1.5 copies were found in two samples. One sample had two copies, as shown in Table 2.
Haplotype frequencies.
The pfcrt K76T and pfmdr1 N86Y haplotype frequencies are shown in Table 3 and Fig. 2. The numbers are lower than the individual SNP frequencies, as mixed infections were excluded. pfcrt 76 + pfmdr1 86 KN (P = 0.002) and KY (P < 0.001) decreased significantly, whereas TY remained stable at ∼10% between 2003 and 2012. The TN frequency was ∼10% between 2003 and 2007 but then increased to 27% by 2012 (P = 0.001).
TABLE 3.
Pfcrt K76T and pfmdr1 N86Y haplotype frequencies between 2003 and 2012 among children diagnosed with P. falciparum malaria at the Bandim Health Centre
| Yr |
pfcrt K76T + pfmdr1 N86Y haplotype |
pfmdr1 N86Y + pfmdr1 F184Y haplotype |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. of samples | Frequency (% [no. of samples with haplotype]) for: |
Total no. of samples | Frequency (% [no. of samples with haplotype]) for: |
|||||||
| KNa | KYa | TNb | TY | NFc | NY | YFd | YY | |||
| 2003 | 254 | 56 (142) | 23 (59) | 9 (24) | 11 (29) | 223 | 39 (87) | 22 (49) | 37 (82) | 2 (5) |
| 2004 | 129 | 56 (72) | 21 (27) | 9 (11) | 15 (19) | |||||
| 2005 | 139 | 65 (91) | 14 (20) | 10 (14) | 10 (14) | |||||
| 2006 | 65 | 63 (41) | 15 (10) | 11 (7) | 11 (7) | |||||
| 2007 | 36 | 47 (17) | 31 (11) | 11 (4) | 11 (4) | |||||
| 2008 | 121 | 46 (56) | 23 (28) | 22 (27) | 8 (10) | |||||
| 2010 | 263 | 50 (132) | 10 (26) | 26 (68) | 14 (37) | 221 | 52 (116) | 25 (55) | 19 (41) | 4 (9) |
| 2011 | 263 | 46 (122) | 12 (31) | 29 (75) | 13 (35) | 246 | 46 (114) | 27 (66) | 22 (54) | 5 (12) |
| 2012 | 89 | 54 (48) | 10 (9) | 27 (24) | 9 (8) | 50 | 66 (33) | 24 (12) | 10 (5) | 0 (0) |
KN (P = 0.002) and KY (P < 0.001) frequencies decreased over time.
TN frequency increased between 2007 and 2012 (P = 0.001).
NF frequency increased (P = 0.004).
YF frequency decreased over time (P < 0.001).
FIG 2.
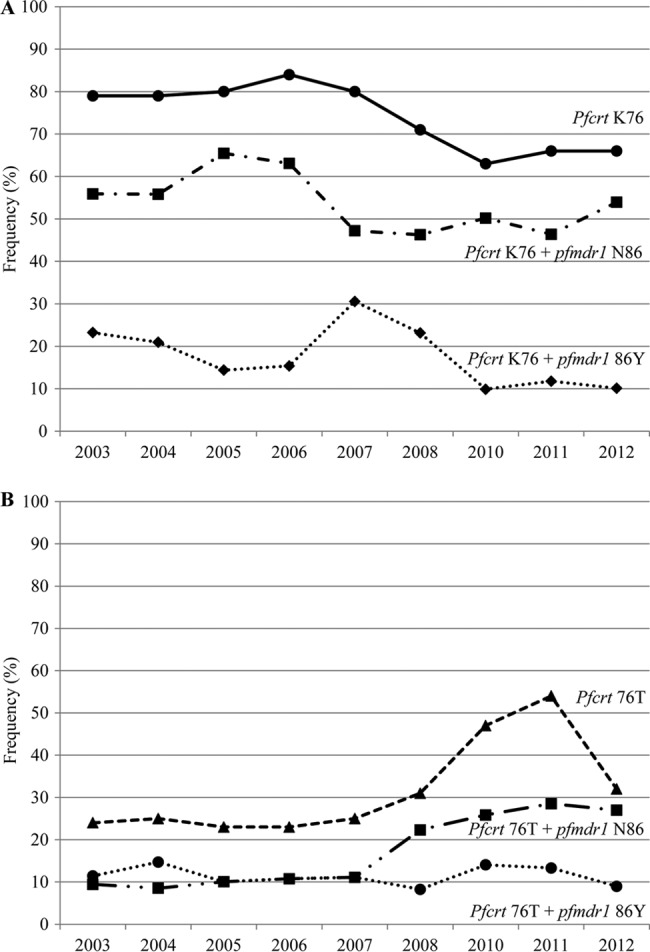
Frequencies of pfcrt K76 and pfcrt 76 + pfmdr1 86 KN or KY haplotypes (A) and pfcrt 76T and pfcrt 76 + pfmdr1 86 TN or TY haplotypes (B) between 2003 and 2012.
The pfmdr1 86 + 184 NF frequency increased significantly over time (P = 0.004), while that of YF decreased significantly (P < 0.001). NY and YY frequencies did not change significantly.
The triple haplotypes pfcrt 76 + pfmdr1 86 + pfmdr1 184 were determined in 219, 200, 191, and 42 samples in 2003, 2010, 2011, and 2012, respectively. During these years, the KNF frequencies were 32%, 37%, 25%, and 45%, respectively, and did not change significantly (P = 0.8). KYF decreased from 24% to 7%, 11%, and 7% from 2003 to 2010, 2011, and 2012, respectively (P < 0.001). From 2003 to 2010, 2011, and 2012, TNF increased from 7% to 17%, 17%, and 21%, respectively (P = 0.002), and TNY increased from 3% to 10%, 13%, and 7%, respectively (P = 0.001).
Linkage disequilibrium.
Correlation coefficients for pfcrt 76 and pfmdr1 86 decreased for KN and TY and increased from negative values (indicating negative correlation) for KY and TN over time. When the data were pooled, the correlation coefficients were 0.26 (2003 to 2007) and 0.12 (2008 to 2012) for KN and TY and −0.26 and −0.12 for KY and TN. No changes were seen with pfcrt K76T and pfmdr1 Y184F. The pfmdr1 86 + pfmdr1 184 NY and YF correlation coefficients decreased from 0.34 in 2003 to 0.15 in 2010 to 2012, while NF and YY increased from −0.34 to −0.15 over the same time period.
Seasonal trends.
There were no seasonal variations between 2003 and 2007. Between 2008 and 2012, pfcrt 76T increased from an average of 37% (50/136) during the low-transmission period (February to July) to 55% (69/126) at the end of the high-transmission period (December; P = 0.03). In parallel, pfcrt 76K possibly decreased from an average of 74% (100/136) during the low-transmission period (February to July) to 59% (74/126) in December (P = 0.05), as shown in Fig. 3, top. The pfcrt 76 + pfmdr1 86 TN frequency increased significantly from 18% (17/97, February to July) to 31% in December (P = 0.045) (Fig. 3, bottom). The pfmdr1 N86Y, pfcrt K76T + pfmdr1 F184Y, and pfmdr1 N86Y + pfmdr1 F184Y frequencies showed no consistent seasonal variations.
FIG 3.
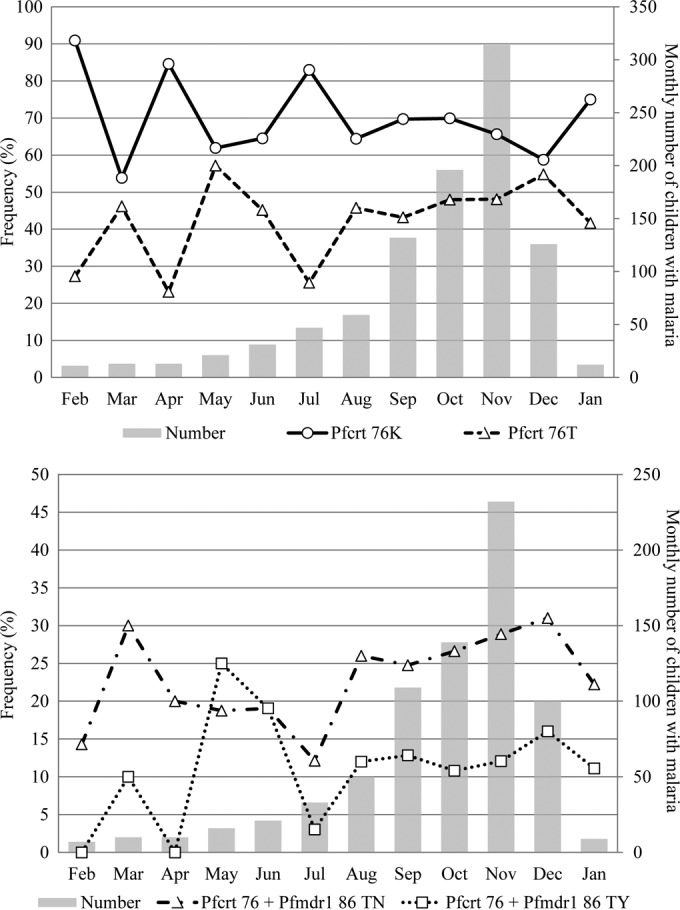
Monthly pfcrt K76T allele, pfcrt 76T + pfmdr1 N86 haplotype, and pfcrt 76T + pfmdr1 86Y haplotype frequencies between 2008 and 2012.
Parasite densities.
The median parasite densities (P. falciparum parasites/μl) with pfcrt K76T and pfmdr1 N86Y alleles and haplotypes are shown in Table 4. pfcrt K76 was associated with a significantly higher day 0 parasite density than pfcrt 76T (P = 0.005). The parasite density was nonsignificantly higher with pfmdr1 N86 than with pfmdr1 Y86 (P = 0.16). There were no differences between the pfmdr1 Y184F alleles. The median parasite densities were significantly higher with pfcrt 76 + pfmdr1 86 KN (P = 0.008) and KY (P = 0.004) than with TY, but there were no significant differences between KN or KY and TN.
TABLE 4.
P. falciparum parasite densities correlated to pfcrt K76T and pfmdr1 N86Y alleles and haplotypes
| SNPa or pfcrt 76 + pfmdr1 86 haplotype | No. | Median density (P. falciparum parasites/μl) | 95% confidence interval | P value |
|---|---|---|---|---|
| pfcrt K76 | 1,062 | 18,000 | 16,773–19,227 | 0.005b |
| pfcrt 76T | 450 | 15,120 | 13,805–16,434 | |
| pfmdr1 N86 | 1,041 | 17,600 | 15,778–19,421 | 0.16c |
| pfmdr1 86Y | 205 | 15,960 | 14,325–17,594 | |
| KN | 689 | 18,000 | 16,432–19,568 | |
| KY | 217 | 18,400 | 16,484–20,316 | |
| TN | 240 | 15,840 | 12,917–18,762 | |
| TY | 158 | 14,640 | 13,093–16,187 | <0.01d |
SNP, single nucleotide polymorphism.
pfcrt K76 was associated with a higher parasite density than pfcrt 76T.
Parasite density did not differ significantly between pfmdr1 N86Y alleles.
The pfcrt 76T + pfmdr1 86Y haplotype was associated with significantly lower parasite densities than the pfcrt K76 + pfmdr1 N86 (P < 0.008) or pfcrt K76 + pfmdr1 86Y (P < 0.004) haplotypes.
DISCUSSION
We present here unique data on resistance-associated genetic polymorphisms collected from virtually all children aged <15 years with uncomplicated malaria and who attended a Guinea-Bissau health center each year in a 9-year period. During this period, P. falciparum epidemiology changed dramatically, the treatment policy changed from an efficacious high-dose CQ regimen to ACT in 2008, and long-lasting insecticide-treated bed nets were mass distributed in 2011 (5).
Perhaps the most striking finding was the decreasing pfcrt K76 frequency and increasing pfcrt 76T frequency following the introduction of AL in 2008. pfcrt K76 has been associated with reduced susceptibility to lumefantrine and has been selected for after treatment with AL (3, 8, 14). Furthermore, pfcrt 76T has been associated with a fitness cost, and it rapidly disappeared in other settings when CQ use was discontinued (23, 31, 32). Therefore, the increased pfcrt 76T and decreased pfcrt K76 frequencies contradicted expectations.
Although AL was the recommended drug, quinine was prescribed to 43% of the children diagnosed with P. falciparum malaria between 2010 and 2012 (our unpublished data). Furthermore, the preliminary data indicate that treatment with quinine selected for pfcrt 76T among recrudescing P. falciparum. Other studies have indicated that pfcrt is involved in the transport of quinine and that SNPs in pfcrt, including 76T, decrease P. falciparum susceptibility to quinine (22, 33–35). Thus, it seems highly probable that the increased pfcrt 76T frequency was due to the increased use of quinine. The accumulation of pfcrt 76T during the high-transmission season (Fig. 3), indicating that pfcrt 76T conferred a selective advantage as drug pressure increased, further supports this. Interestingly, this was not seen when high-dose CQ was used, indicating that 76T provided a greater selective advantage when quinine was used than when high-dose CQ was used.
An alternative explanation for the accumulation of pfcrt 76T is selective pressure by AL. However, a study completed in 2008 indicated that AL selected for pfcrt K76 in Bissau as it does elsewhere, suggesting that selective pressure is unlikely (3, 8). Continued CQ use might also select for pfcrt 76T. However, based on prescription data collected from 2010 to 2012 and informal questioning, CQ is hardly used anymore. Furthermore, if CQ was the driving force, a novel lower and less efficacious CQ dose than that routinely used in the past would have had to have been used. The increased pfcrt 76T frequency might also be the result of selection after the 2007 bottleneck event (when malaria drastically decreased) due to a selective advantage in older children or better inherent fitness. For example, older children may be more likely to self-medicate with CQ at home prior to seeking care at the Bandim Health Centre, or pfcrt 76T may be accompanied by other fitness-restoring genetic changes not examined in this study (36). However, SNP frequencies did not vary with age, and proxies for fitness, such as a lower parasite density and lower pfcrt 76T frequency during the low-transmission period, suggest that pfcrt 76T was associated with a fitness cost, not a benefit.
Further analysis revealed that pfcrt 76 + pfmdr1 86 TN increased between 2007 and 2012. The increased TN haplotype might be due to direct selection by quinine. However, decreased quinine susceptibility has more often been linked with the TY haplotype (22, 35). The discrepancy may be due to the probable multigenic basis of quinine resistance (22, 37). Alternatively, the TN haplotype is inherently fitter than TY, at least in Guinea-Bissau. The significantly lower parasite density found with TY (but not with TN) compared to that with KN or KY might support this, assuming that the day 0 parasite density is a proxy for inherent parasite fitness.
Reduced susceptibility to lumefantrine and recurring parasites after treatment with AL have been linked to pfmdr1 N86, the pfmdr1 86 + 184 + 1246 NFD haplotype, and pfmdr1 amplifications in addition to pfcrt K76 (3, 7–9, 14–16, 38). Increased pfmdr1 N86 and pfmdr1 86 + 184 NF haplotype frequencies following the introduction of AL are in line with these previous observations. As pfmdr1 D1246 was fixed at ∼100% frequency in our study area, the selection of pfmdr1 86 + 184 NF actually represented a selection of the pfmdr1 NFD haplotype that has been linked to a 15-fold higher in vivo AL tolerance (7). As quinine probably selected the pfcrt 76 + pfmdr1 TN haplotype and both the pfcrt 76 + pfmdr1 86 + 184 TNF and TNY haplotypes increased, it seems likely that neither pfmdr1 Y184F allele conferred a selective advantage against quinine. The increased NF frequency was therefore more likely driven by AL than by quinine.
Despite the detection of the first sample with two pfmdr1 gene copies in 2008, the frequency did not subsequently increase, suggesting that the increased copy number did not provide P. falciparum with a great selective advantage in Guinea-Bissau. Alternatively, finding so few samples with increased copy numbers might well be within the margin of error when doing PCRs, suggesting that no samples exist in Bissau. Either way, P. falciparum with multiple pfmdr1 copies has not become widespread in Bissau, contrary to the situation in Ghana, where relatively high frequencies were recently reported (38).
A concern when using AL as a first-line antimalarial is that reduced susceptibilities to artemether and lumefantrine have been linked to some of the same genetic polymorphisms (17–19, 21). The use of multiple first-line therapies that select for opposing genetic polymorphisms might therefore be of value. Furthermore, modeling has suggested that the use of multiple first-line therapies may prolong the total lifetime of the first-line drugs in use over that achieved with sequential use (39). Although by no means solid evidence, our findings of an increased pfcrt 76T frequency that probably reflects the increased prevalence of highly AL-susceptible P. falciparum (compared to that of 76K) when AL and quinine are used concurrently support that hypothesis. Beyond the use of multiple first-line therapies, this balancing of opposing forces is of relevance for the design of antimalarial therapies that aim to decrease the risk of drug resistance. However, it is important to note that our results were probably a consequence of the poor effectiveness of quinine as used in Guinea-Bissau.
The low and stable frequencies of pfcrt 76T when CQ was used (2003 to 2007) were probably due to the routine use of approximately three times the standard dose of CQ that cured the majority of children infected with pfcrt 76T-carrying P. falciparum (3, 12, 13, 28). Thus, pfcrt 76T provided little advantage over pfcrt K76 and did not accumulate, as discussed in detail in a previous paper (13). Our present results showing that the pfcrt 76T frequency increased when the use of high-dose CQ was stopped support the previous data and are of particular interest whenever the reintroduction of CQ is considered (32). If standard-dose CQ is reintroduced, it is likely that the pfcrt 76T frequency will rapidly increase, whereas the introduction of a higher efficacious dose may avoid this.
A further noteworthy observation is that the pfcrt K76T alleles did not vary over the seasons prior to 2008. This was probably due to the relatively low frequency of pfcrt 76T, the >95% efficacy of high-dose CQ, and the lack of a selective advantage of 76T when CQ that hindered its accumulation during the peak malaria transmission season was used. The seasonality seen since 2008 is probably largely an effect of the poor effectiveness of quinine in routine practice. In Bissau, quinine is typically given for 3 days (4), and this was a common dosage from 2010 to 2012 (our unpublished data). In a previous efficacy study in Bissau, 33% of children had recurrent parasitemia by day 28 when treated with 15 mg/kg of quinine twice daily for 3 days (4). It is thus probable that treatment failure following the use of quinine resulted in the accumulation of pfcrt 76T during the high-transmission season.
The decreased pfcrt 76T frequencies between 2011 and 2012 occurred concurrently with a halving of the P. falciparum incidence that probably represented a major selective event due to mass distribution of long-lasting insecticide-treated bed nets at the end of 2011. In addition, an efficacy study started in November 2012 possibly reduced quinine use. The decreased pfcrt 76T frequencies might thus reflect decreased quinine use partly due to a new study starting but also because of fewer occurrences of malaria. As pfcrt 76T accumulated during the transmission season, the smaller epidemic seen in 2012 probably resulted in less 76T accumulation and therefore a lower frequency compared to those in the larger epidemics in 2010 and 2011.
We also found a number of novel synonymous and nonsynonymous SNPs in pfmdr1. This is in line with previous studies indicating that pfmdr1 can be quite variable (40, 41). None of the SNPs found were common, suggesting that the SNPs did not provide a major selective benefit, at least not yet. The synonymous pfmdr1 1069acg SNP was described previously for a single sample from Madagascar (41) and for 10% of samples collected in Liberia in 1978 (our unpublished data). The more common occurrence of pfmdr1 1069acg with pfcrt 76K than with 76T in this study may suggest greater genetic variability in clones with pfcrt 76K but should be interpreted with considerable caution.
To conclude, we report here the frequencies of major genetic polymorphisms linked to reduced AL and CQ susceptibilities over a 9-year period in Guinea-Bissau. Despite being linked to a probable fitness cost, the pfcrt 76T frequencies increased when AL officially replaced CQ as the first-line therapy. This was probably largely driven by quinine that was used as commonly as AL was. Most probably, highly artemether-lumefantrine-susceptible P. falciparum therefore accumulated, possibly supporting the use of multiple first-line therapies. The pfmdr1 86 + 184 NF and probably D1246 haplotypes also increased, possibly driven by AL. Prior to the changed treatment policy, the pfcrt 76T frequency was stable at ∼24% when an efficacious high dose of CQ was used; this finding will have implications whenever the reintroduction of CQ is considered.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the children and families who participated in the clinical trials and the staff involved in conducting the studies at the Bandim Health Project and Bandim Health Centre.
This work was supported by a reward from The Anthony and Ann Dunne Foundation for World Health. J.U. has a postdoctoral position funded by Stockholms läns landsting. The sponsors of the study had no role in study design, data collection, analyses, interpretation or writing of the report.
J.U., P.-E.K., A.R., and L.R. contributed to the design, coordination, enrollment, and follow-up of patients included in the clinical studies from which the data were taken. I.T.J. and J.U. did the molecular work and data analyses. I.T.J., P.-E.K., and J.U. wrote the first draft. All the authors contributed to the planning of the study and interpretation of the data, and they all read and approved the final version of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
We report no conflicting interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03554-14.
REFERENCES
- 1.World Health Organization. 2012. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Ursing J, Kofoed PE, Rodrigues A, Blessborn D, Thoft-Nielsen R, Bjorkman A, Rombo L. 2011. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: a randomized trial. J Infect Dis 203:109–116. doi: 10.1093/infdis/jiq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kofoed PE, Co F, Johansson P, Dias F, Cabral C, Hedegaard K, Aaby P, Rombo L. 2002. Treatment of uncomplicated malaria in children in Guinea-Bissau with chloroquine, quinine, and sulfadoxine-pyrimethamine. Trans R Soc Trop Med Hyg 96:304–309. doi: 10.1016/S0035-9203(02)90107-0. [DOI] [PubMed] [Google Scholar]
- 5.Ursing J, Rombo L, Rodrigues A, Aaby P, Kofoed PE. 2014. Malaria transmission in Bissau, Guinea-Bissau, between 1995 and 2012: malaria resurgence did not negatively affect mortality. PLoS One 9:e101167. doi: 10.1371/journal.pone.0101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg M, Ferreira PE, Tarning J, Ursing J, Ngasala B, Bjorkman A, Martensson A, Gil JP. 2013. Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. J Infect Dis 207:842–847. doi: 10.1093/infdis/jis747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisowath C, Petersen I, Veiga MI, Martensson A, Premji Z, Bjorkman A, Fidock DA, Gil JP. 2009. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis 199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sisowath C, Stromberg J, Martensson A, Msellem M, Obondo C, Bjorkman A, Gil JP. 2005. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J Infect Dis 191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 10.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 6:861–871. doi: 10.1016/S1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summers RL, Dave A, Dolstra TJ, Bellanca S, Marchetti RV, Nash MN, Richards SN, Goh V, Schenk RL, Stein WD, Kirk K, Sanchez CP, Lanzer M, Martin RE. 2014. Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasite's chloroquine resistance transporter. Proc Natl Acad Sci U S A 111:E1759–E1767. doi: 10.1073/pnas.1322965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. 2007. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am J Trop Med Hyg 76:844–848. [PubMed] [Google Scholar]
- 13.Ursing J, Schmidt BA, Lebbad M, Kofoed PE, Dias F, Gil JP, Rombo L. 2007. Chloroquine resistant P. falciparum prevalence is low and unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infect Genet Evol 7:555–561. doi: 10.1016/j.meegid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, Bull P, Marsh K, Borrmann S, Nzila A. 2009. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob Agents Chemother 53:5069–5073. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen TT, Madsen LB, Hansson HH, Tomas EV, Charlwood D, Bygbjerg IC, Alifrangis M. 2013. Rapid selection of Plasmodium falciparum chloroquine resistance transporter gene and multidrug resistance gene-1 haplotypes associated with past chloroquine and present artemether-lumefantrine use in Inhambane District, southern Mozambique. Am J Trop Med Hyg 88:536–541. doi: 10.4269/ajtmh.12-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malmberg M, Ngasala B, Ferreira PE, Larsson E, Jovel I, Hjalmarsson A, Petzold M, Premji Z, Gil JP, Bjorkman A, Martensson A. 2013. Temporal trends of molecular markers associated with artemether-lumefantrine tolerance/resistance in Bagamoyo district, Tanzania. Malar J 12:103. doi: 10.1186/1475-2875-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui L, Wang Z, Miao J, Miao M, Chandra R, Jiang H, Su XZ. 2012. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol Microbiol 86:111–128. doi: 10.1111/j.1365-2958.2012.08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, Nosten F, Krishna S. 1999. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother 43:2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidhu AB, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis 194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga MI, Ferreira PE, Malmberg M, Jornhagen L, Bjorkman A, Nosten F, Gil JP. 2012. pfmdr1 amplification is related to increased Plasmodium falciparum in vitro sensitivity to the bisquinoline piperaquine. Antimicrob Agents Chemother 56:3615–3619. doi: 10.1128/AAC.06350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 22.Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol Microbiol 49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 23.Ord R, Alexander N, Dunyo S, Hallett R, Jawara M, Targett G, Drakeley CJ, Sutherland CJ. 2007. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine-resistant parasites. J Infect Dis 196:1613–1619. doi: 10.1086/522154. [DOI] [PubMed] [Google Scholar]
- 24.Kofoed PE, Ursing J, Poulsen A, Rodrigues A, Bergquist Y, Aaby P, Rombo L. 2007. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans R Soc Trop Med Hyg 101:231–238. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Kofoed PE, Ursing J, Rodrigues A, Rombo L. 2011. Paracetamol versus placebo in treatment of nonsevere malaria in children in Guinea-Bissau: a randomized controlled trial. Malar J 10:148. doi: 10.1186/1475-2875-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ursing J, Kofoed PE, Rodrigues A, Rombo L. 2009. No seasonal accumulation of resistant P. falciparum when high-dose chloroquine is used. PLoS One 4:e6866. doi: 10.1371/journal.pone.0006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ursing J, Kofoed PE, Rombo L, Gil JP. 2006. No pfmdr1 amplifications in samples from Guinea-Bissau and Liberia collected between 1981 and 2004. J Infect Dis 194:716–718. doi: 10.1086/506456. [DOI] [PubMed] [Google Scholar]
- 28.Ursing J, Kofoed PE, Rodrigues A, Bergqvist Y, Rombo L. 2009. Chloroquine is grossly overdosed and overused but well tolerated in Guinea-Bissau. Antimicrob Agents Chemother 53:180–185. doi: 10.1128/AAC.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veiga MI, Ferreira PE, Bjorkman A, Gil JP. 2006. Multiplex PCR-RFLP methods for pfcrt, pfmdr1 and pfdhfr mutations in Plasmodium falciparum. Mol Cell Probes 20:100–104. doi: 10.1016/j.mcp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Holmgren G, Hamrin J, Svard J, Martensson A, Gil JP, Bjorkman A. 2007. Selection of pfmdr1 mutations after amodiaquine monotherapy and amodiaquine plus artemisinin combination therapy in East Africa. Infect Genet Evol 7:562–569. doi: 10.1016/j.meegid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis 187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 32.Frosch AE, Laufer MK, Mathanga DP, Takala-Harrison S, Skarbinski J, Claassen CW, Dzinjalamala F, Plowe CV. 2014. The return of widespread chloroquine-sensitive Plasmodium falciparum to Malawi. J Infect Dis 210:1110–1114. doi: 10.1093/infdis/jiu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper RA, Lane KD, Deng B, Mu J, Patel JJ, Wellems TE, Su X, Ferdig MT. 2007. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol Microbiol 63:270–282. doi: 10.1111/j.1365-2958.2006.05511.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez CP, Stein WD, Lanzer M. 2008. Dissecting the components of quinine accumulation in Plasmodium falciparum. Mol Microbiol 67:1081–1093. doi: 10.1111/j.1365-2958.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheruiyot J, Ingasia LA, Omondi AA, Juma DW, Opot BH, Ndegwa JM, Mativo J, Cheruiyot AC, Yeda R, Okudo C, Muiruri P, Bidii NS, Chebon LJ, Angienda PO, Eyase FL, Johnson JD, Bulimo WD, Andagalu B, Akala HM, Kamau E. 2014. Polymorphisms in pfmdr1, pfcrt and pfnhe1 genes are associated with reduced in vitro activities of quinine in Plasmodium falciparum isolates from western Kenya. Antimicrob Agents Chemother 58:3737–3743. doi: 10.1128/AAC.02472-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadalla NB, Malmberg M, Adam I, Oguike MC, Beshir K, Elzaki SE, Mukhtar I, Gadalla AA, Warhurst DC, Ngasala B, Martensson A, El-Sayed BB, Gil JP, Sutherland CJ. 24 September 2014. Alternatively spliced transcripts and novel pseudogenes of the Plasmodium falciparum resistance-associated locus pfcrt detected in East African malaria patients. J Antimicrob Chemother doi: 10.1093/jac/dku358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol 52:985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 38.Duah NO, Matrevi SA, de Souza DK, Binnah DD, Tamakloe MM, Opoku VS, Onwona CO, Narh CA, Quashie NB, Abuaku B, Duplessis C, Kronmann KC, Koram KA. 2013. Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar J 12:377. doi: 10.1186/1475-2875-12-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boni MF, Smith DL, Laxminarayan R. 2008. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci U S A 105:14216–14221. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veiga MI, Ferreira PE, Jornhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Bjorkman A, Nosten F, Gil JP. 2011. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One 6:e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andriantsoanirina V, Ratsimbasoa A, Bouchier C, Jahevitra M, Rabearimanana S, Radrianjafy R, Andrianaranjaka V, Randriantsoa T, Rason MA, Tichit M, Rabarijaona LP, Mercereau-Puijalon O, Durand R, Menard D. 2009. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrob Agents Chemother 53:4588–4597. doi 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


