Abstract
To evaluate the in vitro effects of the combination of ceftazidime and avibactam on the MICs of both compounds, checkerboard assays were performed for 81 clinical strains, including 55 Enterobacteriaceae strains (32 Klebsiella pneumoniae, 19 Escherichia coli, 1 Citrobacter freundii, and 3 Enterobacter cloacae) and 26 strains of Pseudomonas aeruginosa, all with known resistance mechanisms such as extended-spectrum β-lactamases (ESBLs) and carbapenemases, phenotypically or molecularly determined. Phenotypically ceftazidime-resistant strains (n = 69) were analyzed in more detail. For the Enterobacteriaceae strains, a concentration-dependent effect of avibactam was found for most strains with a maximum effect of avibactam at a concentration of 4 mg/liter, which decreased all ceftazidime MICs to ≤4 mg/liter. Avibactam alone also showed antibacterial activity (the MIC50 and MIC90 being 8 and 16 mg/liter, respectively). For the ceftazidime-resistant P. aeruginosa strains, considerable inhibition of β-lactamases by avibactam was acquired at a concentration of 4 mg/liter, which decreased all ceftazidime MICs except one to ≤8 mg/liter (the CLSI and EUCAST susceptible breakpoint). Increasing the concentration of avibactam further decreased the MICs, resulting in a maximum effect for most strains at 8 to 16 mg/liter. In summary, for most strains, the tested addition of avibactam of 4 mg/liter restored the antibacterial activity of ceftazidime to a level comparable to that of wild-type strains, indicating full inhibition, and strains became susceptible according to the EUCAST and CLSI criteria. Based on these in vitro data, avibactam is a promising inhibitor of different β-lactamases, including ESBLs and carbapenemases.
INTRODUCTION
Antibiotic resistance is a worldwide problem. Until the past decade, the development of new classes of antibiotics was an important weapon against development of resistance. However, strains that carry extended-spectrum β-lactamases and/or carbapenemases have emerged (1, 2). In some countries, the resistance levels to these drugs are now >50% (3). One approach for overcoming this mechanism of resistance is by inhibition of these enzymes. This approach has been taken in the past against β-lactamase-carrying strains using drug combinations consisting of a β-lactam agent and a β-lactamase inhibitor (4–11), such as piperacillin-tazobactam and amoxicillin-clavulanic acid. These have been and still are among the most successful antimicrobials available.
Among several new β-lactamase inhibitors and combinations with β-lactams that are currently being developed is avibactam (12, 13). This compound is active against Ambler class A extended-spectrum β-lactamases (ESBLs) (e.g., TEM, SHV, and CTX-M types), KPC class A enzymes, class C (AmpC), and some class D enzymes. Studies in vitro (14–24) have shown that the MICs of ceftazidime for many resistant strains were markedly reduced in the presence of avibactam and thereby became susceptible.
An important step in the development of a combination β-lactam–β-lactamase inhibitor is to determine the concentration-effect relationship and the concentration at which maximal in vitro inhibition is achieved. These relationships can subsequently be used to determine the optimal conditions to correlate the in vitro testing with the efficacy of the combination. Therefore, we employed a checkerboard assay (25) to evaluate the in vitro effects of ceftazidime combined with avibactam over a range of concentrations for a selection of clinical Gram-negative isolates, including Enterobacteriaceae and Pseudomonas aeruginosa.
MATERIALS AND METHODS
Antibacterials.
Ceftazidime (lot no. G263848; potency 77.0%) and avibactam (lot no. AFCH005151; potency 91.7%) were provided by AstraZeneca Pharmaceuticals LP (Waltham, MA, USA). The drugs were reconstituted in sterile water to a stock solution of 5,120 mg/liter, and further solutions were prepared in Mueller-Hinton broth (Difco; Brunschwig Chemie, Amsterdam, The Netherlands).
Bacterial strains.
Originally, 81 ceftazidime-resistant strains were obtained, all from clinical samples from a wide variety of infections, one strain per patient. The strains were chosen because they represent different β-lactamase-mediated mechanisms of resistance and thus did not quantitatively represent the presence of such resistance mechanisms in the general population, i.e., unselected clinical isolates. Of the isolates, 69 were retained after susceptibility testing, because they were phenotypically ceftazidime resistant or intermediate according to the MIC and EUCAST interpretive criteria. A list describing the strains is provided in Table 1. Included in this selection were 51 Enterobacteriaceae (18 Escherichia coli, 29 Klebsiella pneumoniae, 3 Enterobacter cloacae, and 1 Citrobacter freundii) and 18 P. aeruginosa isolates.
TABLE 1.
Resistance specifications of the 69 ceftazidime-resistant clinical isolates used in the checkerboard assay
| No. of strains | Genus and species | Resistance specificationsa |
|---|---|---|
| 18 | Pseudomonas aeruginosa | Ceftazidime resistant, carbapenem resistant, AmpCcon, AmpCind, KPC-2 |
| 29 | Klebsiella pneumoniae | OmpK35, OmpK36, KPC, KPC-2 and -3, CTX-M-15, gr. 9, 39, TEM-1 and -84, SHV-1, -2, -5, -11, -12, and -33, OXA-1, -2, and -48, LEN, GES-1, ESBL, possible carbapenemases, AmpC |
| 18 | Escherichia coli | CTX-M-1, -2, -3, and -15, TEM-1 and -84, SHV-1 and -12, OXA-1, ESBL |
| 3 | Enterobacter cloacae | CTX-M-9, TEM-1, SHV-12, OXA-1 |
| 1 | Citrobacter freundii | KPC-2 |
AmpCcon, AmpC derepressed; AmpCind, AmpC inducible.
Susceptibility testing.
The MICs of ceftazidime and avibactam were determined by microdilution according to the ISO guidelines (26). The drugs were reconstituted in sterile water to a stock solution of 5,120 mg/liter, and further solutions were prepared in Mueller-Hinton broth (Difco; Brunschwig Chemie). Checkerboards were set up with 2-fold dilutions of ceftazidime (0.032 to 256 mg/liter) and avibactam (0.016 to 16 mg/liter). Freshly prepared trays were stored at −80°C until use. Every tray contained a negative control and a growth control. Each set of MIC determinations included three control strains: E. coli ATCC 25922, P. aeruginosa ATCC 27853, and K. pneumoniae ATCC 700603. Three to 5 μl of 1.5 × 107 CFU/ml bacterial suspension was added in each well with an inoculator (INOC 2001; Bitel Mechatronics B.V., INOC B.V., Zevenbergen, The Netherlands). Plates were read after 18 to 20 h of incubation at 35°C in a closed plastic box using a mirrored surface. The MIC was recorded as the lowest concentration of the agent that completely inhibited visible growth. All experiments were performed in duplicate.
Analysis.
The susceptibility of the combination was interpreted following the EUCAST criteria for ceftazidime (27) (Enterobacteriaceae: susceptible, ≤1 mg/liter; resistant, >4 mg/liter; P. aeruginosa: susceptible, ≤8 mg/liter; resistant, >8 mg/liter). Results are expressed as the MIC50 and MIC90 of ceftazidime and the concentrations of avibactam required to reduce the MIC of ceftazidime (Prism, version 6, 2013; GraphPad Software, San Diego, CA).
RESULTS
Avibactam alone did not have a significant inhibitory effect on P. aeruginosa nor on C. freundii isolates, whereas it did have an inhibitory effect on other Enterobacteriaceae strains, with a MIC of 8 mg/liter (the majority of E. coli and K. pneumoniae strains) or 16 mg/liter (1 of the 3 E. cloacae strains) for avibactam in the absence of ceftazidime (data not shown).
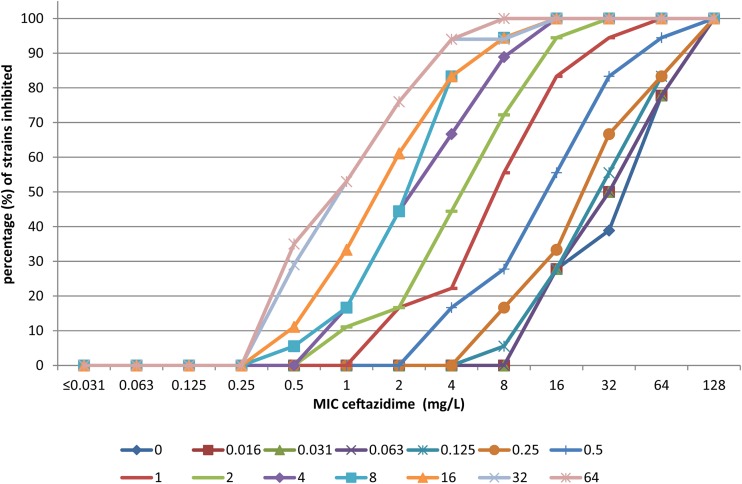
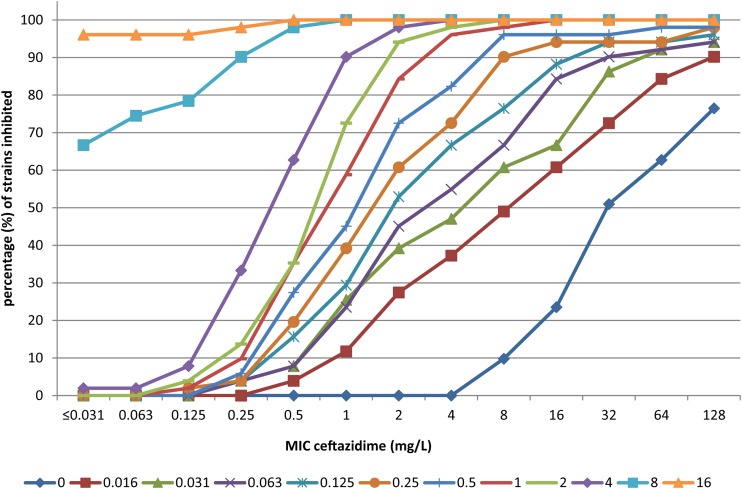
Figures 1 and 2 show the effects of increasing concentrations of avibactam on the MIC of ceftazidime as cumulative inhibition for P. aeruginosa isolates and Enterobacteriaceae strains, respectively. The cumulative inhibition plots are shifted to the left for increasing concentrations of avibactam, indicating a clear effect: the higher the concentration of avibactam present, the lower the resulting MIC for ceftazidime.
FIG 1.
Cumulative % inhibition of 18 P. aeruginosa isolates by ceftazidime for increasing concentrations (symbols below the figure, mg/liter) of avibactam. The % inhibition of growth of P. aeruginosa is presented on the y axis with the MIC for ceftazidime on the x axis as related to the added concentrations of avibactam in differently colored lines.
FIG 2.
Cumulative % inhibition of 51 Enterobacteriaceae isolates by ceftazidime for increasing concentrations (symbols below the figure, mg/liter) avibactam. The % inhibition of growth of Enterobacteriaceae is presented on the y axis with the MIC for ceftazidime on the x axis as related to the added concentration of avibactam in differently colored lines.
Table 2 shows the MIC50 and MIC90 values of ceftazidime at various concentrations of avibactam and provides an indication of the concentration of avibactam required to reduce the MICs for ceftazidime for Enterobacteriaceae and P. aeruginosa strains. A reasonable shift of MICs was already attained with the addition of only 0.016 mg/liter avibactam. The impact of avibactam on the Enterobacteriaceae strains was more pronounced than for the P. aeruginosa isolates. A maximum concentration of avibactam of 4 mg/liter resulted in susceptibility for Enterobacteriaceae, whereas for P. aeruginosa 8 to 16 mg/liter avibactam was required to reach susceptible levels for all strains.
TABLE 2.
MIC50 and MIC90 values of ceftazidime at increasing concentrations of avibactam
| Avibactam concn (mg/liter) |
P. aeruginosa (n = 18) |
All Enterobacteriaceae (n = 51) |
E. coli (n = 18) |
K. pneumoniae (n = 29) |
||||
|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/liter) | MIC90 (mg/liter) | MIC50 (mg/liter) | MIC90 (mg/liter) | MIC50 (mg/liter) | MIC90 (mg/liter) | MIC50 (mg/liter) | MIC90 (mg/liter) | |
| 0 | 32 | 128 | 32 | ≥512 | 32 | 64 | 128 | ≥512 |
| 0.016 | 32 | 128 | 8 | 128 | 2 | 16 | 32 | 128 |
| 0.031 | 32 | 128 | 4 | 64 | 1 | 8 | 16 | 128 |
| 0.063 | 32 | 128 | 2 | 16 | 1 | 2 | 8 | 128 |
| 0.125 | 32 | 128 | 2 | 16 | 1 | 2 | 8 | 64 |
| 0.25 | 32 | 128 | 2 | 8 | 1 | 2 | 4 | 64 |
| 0.5 | 16 | 64 | 2 | 8 | 0.5 | 2 | 2 | 8 |
| 1 | 8 | 32 | 1 | 4 | 0.5 | 1 | 1 | 4 |
| 2 | 4 | 16 | 1 | 2 | 0.5 | 1 | 1 | 2 |
| 4 | 4 | 16 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 2 |
| 8 | 2 | 8 | ≤0.031 | 0.25 | ≤0.031 | 0.063 | ≤0.031 | 0.25 |
| 16 | 2 | 8 | ≤0.031 | ≤0.031 | ≤0.031 | ≤0.031 | ≤0.031 | ≤0.031 |
| 32 | 1 | 4 | ||||||
| 64 | 1 | 4 | ||||||
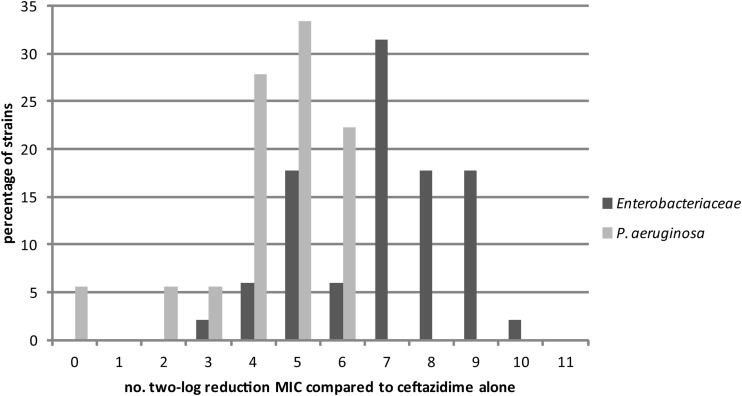
Figure 3 shows the maximum decrease in ceftazidime MICs in number of 2-fold dilutions. Addition of avibactam to ceftazidime reduced the MIC of ceftazidime up to 7 to 9 2-fold dilutions for the majority of the Enterobacteriaceae strains, and the maximum effect was reached at an avibactam concentration of 4 mg/liter. For the P. aeruginosa strains, the maximum effect was less pronounced: 5 to 6 doubling dilutions and higher concentrations of avibactam, up to 8 to 16 mg/liter, were required. One P. aeruginosa isolate did not show any change in the MIC of ceftazidime in combination with the highest concentration of avibactam added.
FIG 3.
Distribution of the maximum effect of ceftazidime-avibactam combinations over the tested concentration range expressed as a 2-fold dilution of the MIC decrease of ceftazidime. The maximum MIC-lowering effect of the addition of avibactam to ceftazidime (compared to the MIC of ceftazidime alone) for % strains of both Enterobacteriaceae and P. aeruginosa is presented on a 2-log transformed x axis.
Table 3 displays target MICs of ceftazidime for Enterobacteriaceae and P. aeruginosa isolates and the required concentrations of avibactam expressed as the 50th and 90th percentiles. The median and 90th percentile concentrations of avibactam required to reduce the MIC of ceftazidime to 4 mg/liter were 4 and >16 mg/liter for P. aeruginosa strains, 0.016 and 0.063 mg/liter for E. coli strains, and 0.250 and 1 mg/liter for K. pneumoniae strains respectively. The median and 90th percentile concentrations of avibactam required to reduce the MIC of ceftazidime to 1 mg/liter were 32 and ≥64 mg/liter (i.e., the measurable MIC90 was not attained) for P. aeruginosa strains, 0.031 and 2 mg/liter for E. coli strains, and 2 and 8 mg/liter for K. pneumoniae strains, respectively.
TABLE 3.
Concentration of avibactam required to reach a given MIC of ceftazidime for the median and 90th percentile of strains
| Ceftazidime target MIC (mg/liter) | MICs of avibactam (50th/90th percentiles [mg/liter]) for: |
|||
|---|---|---|---|---|
| P. aeruginosa (n = 18) | Enterobacteriaceae all (n = 51) | E. coli (n = 18) | K. pneumoniae (n = 29) | |
| 1 | 32/≥64 | 1/4 | 0.031/2 | 2/8 |
| 4 | 4/16 | 0.063/1 | 0.016/0.063 | 0.250/1 |
| 8 | 1/8 | 0.031/0.25 | 0.016/0.031 | 0.125/0.5 |
At an avibactam concentration of 1 mg/liter, the MIC50 of ceftazidime against the isolates of P. aeruginosa was at or below the ceftazidime resistance breakpoint of 8 mg/liter (as shown in Table 2). The MIC90, however, was much higher (32 mg/liter). At a concentration of avibactam of 4 mg/liter (as currently used for in vitro broth dilution susceptibility testing) (28), the MIC90 for the ceftazidime-avibactam combination was 16 mg/liter, one dilution higher than both the EUCAST and CLSI breakpoints of ceftazidime for P. aeruginosa. The median and 90th percentile concentrations of avibactam for P. aeruginosa isolates for three different MICs of the ceftazidime-avibactam combination indicate that avibactam is particularly effective with P. aeruginosa at concentrations of 8 to 16 mg/liter (Table 3).
Likewise the MIC50 and MIC90 of ceftazidime-avibactam for Enterobacteriaceae strains decreased with increasing concentrations of avibactam. Of note, avibactam showed some moderate antibacterial activity without ceftazidime (range, 8 to >16 mg/liter; MIC50 and MIC90 of avibactam, respectively, 8 and 16 mg/liter). This resulted in a very low MIC50 and MIC90 of the ceftazidime-avibactam combination for Enterobacteriaceae strains. For all the Enterobacteriaceae strains tested, at a concentration of 1 mg/liter of avibactam, the MIC90 of the combination was at or below the ceftazidime-susceptible breakpoint of 4 mg/liter. The effect of avibactam was species dependent, the MICs of the combination against E. coli isolates being reduced to a MIC90 of 2 mg/liter when the concentration of avibactam was 0.063 mg/liter, whereas 1 mg/liter avibactam was required to bring the MIC90 of the combination against K. pneumoniae isolates to 4 mg/liter.
DISCUSSION
In this study, it was shown that the addition of avibactam renders ESBL-producing strains susceptible to ceftazidime, but that the concentration of avibactam required is species and strain dependent. Although the efficacy of the combination of avibactam and ceftazidime in vitro was described in earlier studies for some concentrations of avibactam (14, 16, 18, 21, 23), we here present the results of complete checkerboard experiments.
It should be noted that the isolates of Enterobacteriaceae and P. aeruginosa studied in the present work were selected for further investigation due to ceftazidime resistance, i.e., the ceftazidime or ceftazidime-avibactam MIC distributions of the isolates studied are not representative of the normal MIC distribution observed in a routine clinical laboratory. The median and 90th percentile tables and the cumulative frequency plots are ways of displaying the collected properties of the sample of isolates chosen. They are not in any way estimates of the MIC distribution statistics of any current population of clinical isolates. The value of this collection is as a challenge set of bacteria that can be used to study the concentration-effect profile of avibactam when combined with ceftazidime against β-lactamase-producing isolates. These baseline results were important for ensuing pharmacokinetic/pharmacodynamic (PK/PD) studies (e.g., reference 29).
The results of the checkerboard experiments were analyzed in different ways. First, the maximum effective concentration of avibactam was determined for the different microorganisms and species. From these data, it can be concluded that the activity of avibactam in combination with ceftazidime is more pronounced for Enterobacteriaceae than for P. aeruginosa strains. The possible explanations include the existence of more and different resistance mechanisms present in Pseudomonas species compared to the Enterobacteriaceae, such as resistance due to changes in porins or efflux pumps (30, 31).
Except for one strain of P. aeruginosa, avibactam restored the antibacterial activity of ceftazidime to a level comparable to that of wild-type strains (even though a concentration of 32 mg/liter avibactam was required to reach this for strain 11), which renders them susceptible according to both the CLSI and the EUCAST criteria. This is consistent with what was observed for a collection of unselected clinical isolates of P. aeruginosa where the addition of avibactam at 4 mg/liter restored the antibacterial activity of ceftazidime (32) and reverted the frequency distribution of the MICs of ceftazidime to one resembling the EUCAST “wild-type” distribution (33).
Second, the optimum inhibitory combinations of ceftazidime and avibactam in vitro were derived from the data. Ideally, the concentration of avibactam to use is such that the majority of strains are inhibited by ceftazidime-avibactam and, on the other hand, that distinguishes between strains that harbor enzymes that are susceptible or resistant to avibactam inhibition at the putative breakpoint level of ceftazidime-avibactam. This is not necessarily the same as the breakpoint for ceftazidime owing to considerations of dosing, human population pharmacokinetics, PK/PD indices and magnitudes, MIC frequency distributions in surveillance studies, activity against key multidrug-resistant strains, and clinical data.
At an avibactam concentration of 1 mg/liter, the MIC50 of ceftazidime against the isolates of P. aeruginosa was at or below the ceftazidime resistance breakpoint of 8 mg/liter (note that this breakpoint is associated with the 2-g, every 8 h [q8h] dose of ceftazidime, whereas the ceftazidime breakpoint for Enterobacteriaceae of 4 mg/liter was set based on the 1-g, q8h dose [EUCAST ceftazidime breakpoint rationale document (34)]). The MIC90 of ceftazidime at the avibactam concentration of 1 mg/liter was much higher (32 mg/liter).
In the presence of a fixed amount of 4 mg/liter avibactam, 92% of all of the bacterial strains tested displayed a MIC to the combination of ≤4 mg/liter. However, a number of strains of P. aeruginosa required significantly higher concentrations of avibactam in the range of 8 to 32 mg/liter (in vitro) to achieve the ceftazidime-susceptible breakpoint. The current dosing (2,000 mg ceftazidime and 500 mg avibactam q8h, 2-h infusion per dose [35]) will most likely not result in concentrations high enough or long enough to inhibit these specific strains in vivo.
It should be noted that the P. aeruginosa isolates selected for this study represented isolates from the high end of the MIC distribution of current clinical isolates (14, 32) in order to identify strains suitable for PK/PD analyses in murine infection studies (e.g., see reference 29). The concentration of avibactam used in susceptibility testing cannot be related directly to the fluctuating avibactam concentrations in vivo. Rather, the in vitro test is designed so that a constant concentration of avibactam inhibits β-lactamases as much as is practicable in order to reveal the intrinsic susceptibility to ceftazidime which is diluted in 2-fold increments, read as the “MIC.” In vivo, the question becomes what PK time course of concentrations of avibactam will inhibit β-lactamases and support the underlying intrinsic PK/PD of avibactam? (29, 36, 37). That is outside the scope of the present work, but suffice it to say that it need not be related exactly to the 4 mg/liter avibactam adopted as the fixed susceptibility test concentration (28).
The resulting data provide a basis for determining the optimal avibactam concentration in routine susceptibility testing in microdilution assays. The data indicate that a concentration of avibactam of 4 mg/liter will give the best result while ensuring that the concentration remains below the point at which avibactam begins to show some antibacterial activity alone in the case of Enterobacteriaceae strains (Table 1), which might potentially yield in vitro test results of false susceptibility (i.e., very major errors). This is consistent with the currently adopted standard method set by the CLSI (28, 38).
The MIC-lowering effect was far more pronounced for the Enterobacteriaceae strains. For most strains, 1 mg/liter of avibactam was enough to reach the maximum β-lactamase inhibition, and the β-lactamases of virtually all strains were inhibited at 4 mg/liter. The β-lactamases expressed in E. coli isolates in particular were very susceptible to avibactam with concentrations as low as 0.063 mg/liter reinstating susceptibility to ceftazidime, whereas for the K. pneumoniae and other Enterobacteriaceae isolates, 1 mg/liter was necessary. The stepwise increase in ceftazidime susceptibility with increasing avibactam concentration leads to the hypothesis that multiple steps interact in the restoration of ceftazidime activity by avibactam and that each step contributes to a different extent in different strains. For K. pneumoniae, avibactam at 4 mg/liter brought MIC values of ceftazidime against all of the challenge isolates in this selected sample to ≤4 mg/liter (Table 1) and of 90% to ≤2 mg/liter (Table 2). In the attempt to relate this observation to the clinical standards of the MIC interpretive criteria, it is relevant to note that the PK/PD target attainment based cutoff MIC for the 1-g, q8h dose of ceftazidime is 4 mg/liter (28, 34, 38). The current EUCAST ceftazidime-susceptible breakpoint of ≤1 mg/liter was lowered from the PK/PD cutoff of 4 mg/liter in order to avoid categorization of ESBL producers inhibited by 2 or 4 mg/liter as susceptible (34). However, the combination of ceftazidime with avibactam is designed to overcome the hydrolysis by ESBLs, and, thus, the PK/PD target attainment based on the 1-g, q8h ceftazidime dose might support a susceptible breakpoint of ≤4 mg/liter. Indeed, this is currently the CLSI MIC-based interpretive criterion of ceftazidime susceptible (28). In the case of ceftazidime-avibactam, the dose is 2 g ceftazidime (plus 0.5 g avibactam), q8h, and the infusion time is 120 min rather than the standard 30 min used for ceftazidime, so the PK/PD cutoff is proportionately higher (35, 39). It should be noted that the PK/PD cutoff for the 2-g, 8-h dose of ceftazidime supports the clinical susceptible interpretive criterion of ≤8 mg/liter applied to P. aeruginosa by both the CLSI (28, 38) and the EUCAST (34). In summary, when one is attempting to relate the ceftazidime-avibactam MIC values against the challenge isolates reported here to any putative susceptibility interpretive criterion, the appropriate value to use ultimately will be the PK/PD cutoff of the q8h 120-min infusion dose of 2 g ceftazidime combined with 0.5 g avibactam, the definitive analysis of which has yet to be performed, because phase 3 clinical trials are in progress (but see references 35 and 39 for pre-phase 3 models and analyses).
The MICs for ceftazidime were also lowered for phenotypically ceftazidime-susceptible strains (data not shown). This can possibly be explained by a baseline level of β-lactamase production that elevates the baseline MIC of ceftazidime, in which case avibactam potentially removes that baseline activity. Hitherto, the mechanism of synergy between avibactam and ceftazidime had not been investigated.
Since it might be possible that there was a specific trend in the results, depending on the selected microorganisms or on the resistance mechanism these microorganisms possessed, we specifically looked at whether this was the case. No clear differences related to the type of ESBL present or to the presence of one or more ESBLs were visible, although there are biochemical kinetic differences in the inhibition of different β-lactamases in vitro (12). Another factor that might affect the ability of avibactam to lower the MICs of ceftazidime is that the number of copies per cell may differ, requiring more or less avibactam.
Similar to earlier observations, we did not find any intrinsic antipseudomonal activity of avibactam alone (MICs of ≥16 or 64 mg/liter) (18, 24), and the primary use of avibactam is the inhibition of β-lactamases, including multidrug-resistant (MDR) strains. Ceftazidime activity was restored from 40% to 96% in 25 MDR P. aeruginosa isolates (18), where MDR was defined as resistance to at least one antimicrobial agent from three or more different classes. The molecular resistance mechanisms were not mentioned in this study. Similarly, Levasseur et al. (14) showed an increase in the ceftazidime susceptibility from 65% to 94% with the addition of 4 mg/liter avibactam in 126 consecutive clinical P. aeruginosa isolates. The activity against AmpC-mediated resistance was shown by Mushtaq and colleagues when they demonstrated that avibactam reversed the AmpC-mediated ceftazidime resistance in P. aeruginosa isolates, by reducing the MICs for fully derepressed mutants and isolates to ≤8 mg/liter (21). The 6 Pseudomonas isolates carrying AmpC in the present study also turned susceptible with addition of avibactam.
For Enterobacteriaceae, avibactam has shown significant activity against class A, C, and some D β-lactamases but not to metallo-β-lactamases (24). These include KPC-producing strains (23), strains producing the OXA-48 enzyme (16, 20, 40, 41), and AmpC β-lactamases (19). In the present study, we likewise found activity against each of these resistance mechanisms.
The studies discussed above and our own study indicate that avibactam is a promising inhibitor of different β-lactamases, including ESBLs and KPC-type carbapenemases, and might be an alternative treatment option for infections caused by ESBL-harboring strains. The checkerboard approach that we took indicates that there is a concentration-dependent effect of avibactam. Whereas the 4 mg/liter used in susceptibility testing brought the ceftazidime MICs against the Enterobacteriaceae strains to ≤4 mg/liter (the CLSI breakpoint and the EUCAST PK/PD cutoff for the 1-g, q8h dose) and against the majority of the P. aeruginosa isolates in the present study to ≤8 mg/liter (the CLSI and EUCAST breakpoints), the concentration dependence also raises the hypothesis that higher exposures of avibactam, if proven safe, might ultimately lead to more isolates being susceptible in vivo, particularly among multidrug-resistant P. aeruginosa strains.
ACKNOWLEDGMENTS
This study was supported by an unrestricted grant from AstraZeneca and Forest Laboratories Inc. (now a subsidiary of Actavis PLC).
Wright W. Nichols is an employee of AstraZeneca, and Johan W. Mouton has been a consultant and/or received research funding from Angelini, AstraZeneca, Basilea, Jansen-Cilag, Merck & Co., Cubist, Pfizer, Polyphor, and Roche. Johanna Berkhout, Maria J. Melchers, and Anita C. van Mil declare no conflicts of interest.
REFERENCES
- 1.Cantón R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. 2012. Annual epidemiological report 2012. Reporting on 2010 surveillance data and 2011 epidemic intelligence data. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 4.Bebrone C, Lassaux P, Vercheval L, Sohier JS, Jehaes A, Sauvage E, Galleni M. 2010. Current challenges in antimicrobial chemotherapy: focus on β-lactamase inhibition. Drugs 70:651–679. doi: 10.2165/11318430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Reading C, Cole M. 1977. Clavulanic acid: a beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother 11:852–857. doi: 10.1128/AAC.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neu HC, Fu KP. 1978. Clavulanic acid, a novel inhibitor of beta-lactamases. Antimicrob Agents Chemother 14:650–655. doi: 10.1128/AAC.14.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durkin JP, Viswanatha T. 1978. Clavulanic acid inhibition of beta-lactamase I from Bacillus cereus 569/H. J Antibiot (Tokyo) 31:1162–1169. doi: 10.7164/antibiotics.31.1162. [DOI] [PubMed] [Google Scholar]
- 8.Marunaka T, Maniwa M, Matsushima E, Minami Y. 1988. High-performance liquid chromatographic determination of a new beta-lactamase inhibitor and its metabolite in combination therapy with piperacillin in biological materials. J Chromatogr 431:87–101. doi: 10.1016/S0378-4347(00)83072-8. [DOI] [PubMed] [Google Scholar]
- 9.Moosdeen F, Williams JD, Yamabe S. 1988. Antibacterial characteristics of YTR 830, a sulfone beta-lactamase inhibitor, compared with those of clavulanic acid and sulbactam. Antimicrob Agents Chemother 32:925–927. doi: 10.1128/AAC.32.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum PC, Jacobs MR, Spangler SK, Yamabe S. 1986. Comparative activity of beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with beta-lactams against beta-lactamase-producing anaerobes. Antimicrob Agents Chemother 30:789–791. doi: 10.1128/AAC.30.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutmann L, Kitzis MD, Yamabe S, Acar JF. 1986. Comparative evaluation of a new beta-lactamase inhibitor, YTR 830, combined with different beta-lactam antibiotics against bacteria harboring known beta-lactamases. Antimicrob Agents Chemother 29:955–957. doi: 10.1128/AAC.29.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stachyra T, Pechereau MC, Bruneau JM, Claudon M, Frere JM, Miossec C, Coleman K, Black MT. 2010. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother 54:5132–5138. doi: 10.1128/AAC.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levasseur P, Girard AM, Claudon M, Goossens H, Black MT, Coleman K, Miossec C. 2012. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 56:1606–1608. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curcio D. 2011. Activity of a novel combination against multidrug-resistant nonfermenters: ceftazidime plus NXL104. Expert Rev Anti Infect Ther 9:173–176. doi: 10.1586/eri.10.173. [DOI] [PubMed] [Google Scholar]
- 16.Aktaş Z, Kayacan C, Oncul O. 2012. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Citron DM, Tyrrell KL, Merriam V, Goldstein EJ. 2011. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother 55:3616–3620. doi: 10.1128/AAC.01682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walkty A, DeCorby M, Lagace-Wiens PR, Karlowsky JA, Hoban DJ, Zhanel GG. 2011. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study). Antimicrob Agents Chemother 55:2992–2994. doi: 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother 55:2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 22.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with beta-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 53:3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnefoy A, Dupuis-Hamelin C, Steier V, Delachaume C, Seys C, Stachyra T, Fairley M, Guitton M, Lampilas M. 2004. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor. J Antimicrob Chemother 54:410–417. doi: 10.1093/jac/dkh358. [DOI] [PubMed] [Google Scholar]
- 25.Pillai SK, Miellering RC Jr, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–440. In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 26.International Organization for Standardization. 2006. Clinical laboratory testing and in vitro diagnostic test systems—susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases ISO 20776-1:2006. International Standards Organization, Geneva, Switzerland. [Google Scholar]
- 27.European Committee on Antimicrobial Susceptibility Testing. 2014. MIC and inhibition zone diameter distributions of microorganisms without and with resistance mechanisms. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing: 24th informational supplement. M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Schuck V, Nichols WW, Mouton JW. 2013. Exposure response relationships of ceftazidime and avibactam in a neutropenic thigh model, abstr A-1023. Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 30.Strateva T, Yordanov D. 2009. Pseudomonas aeruginosa—a phenomenon of bacterial resistance. J Med Microbiol 58:1133–1148. doi: 10.1099/jmm.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 31.Pechère JC, Kohler T. 1999. Patterns and modes of beta-lactam resistance in Pseudomonas aeruginosa. Clin Microbiol Infect 5(Suppl 1):S15–S18. doi: 10.1111/j.1469-0691.1999.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 32.Flamm RK, Farrell DJ, Sader HS, Jones RN. 2014. Ceftazidime/avibactam activity tested against Gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012). J Antimicrob Chemother 69:1589–1598. doi: 10.1093/jac/dku025. [DOI] [PubMed] [Google Scholar]
- 33.Flamm RK, Stone GG, Sader HS, Jones RN, Nichols WW. 2014. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J Chemother 26:333−338. doi: 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 34.European Committee on Antimicrobial Susceptibility Testing. 2010. Ceftazidime: rationale for the EUCAST clinical breakpoints, version 1.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Ceftazidime_Rationale_Document_1.0_2010Nov.pdf.
- 35.Li J, Knebel W, Riggs M, Zhou D, Nichols W, Das S. 2012. Population pharmacokinetic modeling of ceftazidime (CAZ) and avibactam (AVI) in healthy volunteers and patients with complicated intra-abdominal infection (cIAI), abstr A634 Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
- 36.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 58:3366−3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols W, Levasseur P, Li J, Das S. 2012. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, abstr A-1760. [Google Scholar]
- 38.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN, Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute . 2013. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa. I. Cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Zhou D, Nichols W, Das S. 2012. Evaluation of ceftazidime-avibactam (CAZ AVI) dose regimens for phase III study in patients with different renal function, abstr A635 Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
- 40.Mushtaq S, Warner M, Williams G, Critchley I, Livermore DM. 2010. Activity of chequerboard combinations of ceftaroline and NXL104 versus beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 65:1428–1432. doi: 10.1093/jac/dkq161. [DOI] [PubMed] [Google Scholar]
- 41.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]