Abstract
MK7655 is a newly developed beta-lactamase inhibitor of class A and class C carbapenemases. Pharmacokinetics (PK) of imipenem-cilastatin (IMP/C) and MK7655 were determined for intraperitoneal doses of 4 mg/kg to 128 mg/kg of body weight. MIC and pharmacodynamics (PD) studies of MK7655 were performed against several beta-lactamase producing Pseudomonas aeruginosa and Klebsiella pneumoniae strains to determine its effect in vitro and in vivo. Neutropenic mice were infected in each thigh 2 h before treatment with an inoculum of approximately 5 × 106 CFU. They were treated with IMP/C alone (every 2 hours [q2h], various doses) or in combination with MK7655 in either a dose fractionation study or q2h for 24 h and sacrificed for CFU determinations. IMP/MK7655 decreased MICs regarding IMP MIC. The PK profiles of IMP/C and MK7655 were linear over the dosing range studied and comparable with volumes of distribution (V) of 0.434 and 0.544 liter/kg and half-lives (t1/2) of 0.24 and 0.25 h, respectively. Protein binding of MK7655 was 20%. A sigmoidal maximum effect (Emax) model was fit to the PK/PD index responses. The effect of the inhibitor was not related to the maximum concentration of drug in serum (Cmax)/MIC, and model fits for T>MIC and area under the concentration-time curve (AUC)/MIC were comparable (R2 of 0.7 and 0.75), but there appeared to be no significant relationship of effect with dose frequency. Escalating doses of MK7655 and IMP/C showed that the AUC of MK7655 required for a static effect was dependent on the dose of IMP/C and the MIC of the strain, with a mean area under the concentration-time curve for the free, unbound fraction of the drug (fAUC) of 26.0 mg · h/liter. MK7655 shows significant activity in vivo and results in efficacy of IMP/C in otherwise resistant strains. The exposure-response relationships found can serve as a basis for establishing dosing regimens in humans.
INTRODUCTION
Antimicrobial resistance is an emerging problem worldwide (1), and until this decade, the development of new classes of antibiotics had been an important weapon for combating this. Carbapenems are one of these classes, represented by drugs such as imipenem and meropenem. As broad-spectrum antibiotics, these drugs have been the mainstay of treatment against multiply resistant Gram-negative bacilli and infections caused by them. These infections are difficult to treat and include those due to Pseudomonas aeruginosa and multidrug-resistant Klebsiella spp. (2). However, strains have recently emerged that carry carbapenemases (enzymes that destroy carbapenems), rendering them resistant to all carbapenems (3). One approach to overcoming this mode of resistance is by inhibition of these enzymes. This approach has successfully been applied in the past for other beta-lactamases, for instance clavulanic acid (4, 5) and tazobactam (6, 7). The new compound MK7655 is a beta-lactamase inhibitor (BLI) that has been shown to inhibit several types of beta-lactamases, including carbapenemases (8). In vitro studies have shown that the MICs for resistant strains were drastically reduced in the presence of MK7655 and thereby became susceptible to imipenem (8, 9). The next step in determining the feasibility of MK7655 is to determine its effect in vivo and the doses and concentrations that are required to overcome resistance. Pharmacokinetics (PK) and pharmacodynamics (PD) studies to determine exposure-effect relationships of antimicrobials have become a mainstay in optimizing dosing regimens and determining breakpoints (10–12). PK/PD relationships of combinations pose a specific challenge. In this study, we determined the pharmacodynamics of MK7655 during imipenem-cilastatin (IMP/C) treatment of experimental infections with IMP/C-resistant bacteria. Our strategy was to first determine the PK/PD relationship of IMP/C. This allowed choosing doses of IMP/C to result in a moderate increase in the d CFU of two selected P. aeruginosa and Klebsiella pneumoniae strains (i.e., a change of 1- to 2-log10 growth). Doses of MK7655 were then varied in a dose fractionation study to determine the pharmacodynamic index (PI) of the inhibitor. In subsequent experiments, the initial observations were further validated by documenting the efficacy of MK7655 against a number of other IMP/C-resistant strains.
MATERIALS AND METHODS
Setting.
In vivo experiments were carried out in the Central Animal Facility (Centraal Dierenlab) at Radboud University Nijmegen Medical Centre. The animal studies were conducted in accordance with the recommendations of the European Community Directive 86/609/EEC, 24 November 1986. The study was approved by the animal welfare committee of Radboud University (approval no. RU-DEC 2009-060).
Drugs.
For the in vitro susceptibility testing, imipenem (IMP) and MK7655 were provided by Merck. The drugs were reconstituted in phosphate buffer (0.01 mol/liter, pH 7.2) to a stock solution of 5,120 mg/liter. Working solutions were prepared in Mueller-Hinton broth (MH) (Difco, Brunschwig Chemie, Amsterdam, The Netherlands). For the in vivo studies, MK7655 was supplied by Merck. Imipenem-cilastatin was obtained commercially as powder for injection. The drugs were reconstituted in 0.9% NaCl to a final concentration of 14.08 mg/ml, except for the highest concentration of IMP/C (128 mg/ml), where the final concentration was 28.16 mg/ml. Subsequently, solutions were combined and/or diluted with 0.9% NaCl to their final concentrations. The solutions were stored at −80°C until use and were used within 5 days.
Strains.
P. aeruginosa ATCC 27853 and K. pneumoniae ATCC 43816 were used for infections in the PK experiments. For the PK/PD studies, 5 different Pseudomonas and 2 Klebsiella strains with various MICs and beta-lactamase expressions were selected from strains provided by Merck. The characteristics of these strains are shown in Table 1.
TABLE 1.
Characteristics of the strains used
| Species | Strain | Resistance summarya |
|---|---|---|
| P. aeruginosa | ||
| ATCC 27853 | ||
| 24354 | OprD−, AmpCcon, class A−, class B− | |
| 24227 | OprD−, AmpCind, class A−, class B− | |
| 24226 | OprD−, AmpCcon, class A−, class B− | |
| 24228 | OprD−, AmpCind, class A−, class B− | |
| K. pneumoniae | ATCC 43816 | ATCC (capsular serotype 2) |
| 6755 | KPC-3, OmpK35red, OmpK36red | |
| 6339 | KPC-2, OmpK35−, OmpK35red |
ind, inducible; con, constitutive; red, reduced expression; AmpC and class C, cloxacillin susceptible; class A, clavulanic acid susceptible; class B, EDTA susceptible; KPC-2*, KPC type inferred from ribotyping identity to NYC epidemic clone.
In vitro susceptibility testing.
Susceptibility testing for imipenem was performed by a broth microdilution assay following the ISO 20776-1:2006 international standard protocol against rapidly growing aerobic bacteria (13). MICs for each agent in combination were determined by checkerboard titrations in MH broth. Doubling dilutions of imipenem and MK7655 were freshly prepared in MH broth to give a concentration 4-fold greater than the desired concentration. We dispensed 25 μl of imipenem (range, 512 to 0.25 mg/liter) in the columns of a microdilution tray. We also dispensed 25 μl MK7655 (range, 64 to 0.5 mg/liter) in the rows of the same tray. The trays were stored at −80°C until use. We added 50 μl bacterial suspension (1 × 106 CFU/ml) to the thawed trays and incubated them overnight. Each tray contained a growth control and a negative control. The recorded MIC was the lowest concentration of imipenem/MK7655 that completely inhibited visible growth. All titrations were done at least in duplicate. MK7655 dilutions ranging from 0.125 mg/liter to 16 mg/liter were tested to determine whether they exhibited any antimicrobial activity.
Animals.
Outbred female CD1 mice (weighing 20 to 22 g at arrival) were used in all the experiments and were obtained from Charles River (SulzFeld, Germany). The mice were rendered neutropenic by intraperitoneal injection of cyclophosphamide at 150 mg/kg of body weight 4 days before the experiment (day −4) and 100 mg/kg at day −1. Animals were housed two per cage and received water and food ad libitum.
Infection.
On the day of the experiment, animals were infected with an inoculum of 0.1 ml in both thighs, each with a different strain. The inoculum was prepared by standard procedures. In brief, for each strain, a batch of 50 ml was prepared in MH broth (Difco, Brunschwig Chemie, Amsterdam, The Netherlands) by inoculation and growth to a final concentration of 108 to 109 bacteria/ml. Each batch was divided equally into 50 microtubes and stored at 80°C. Growth curves were generated on separate days to determine the reproducibility and colony count of the inoculum after thawing. On the day of infection, a 0.5-ml aliquot was thawed and added to 4.5 ml prewarmed MH broth. After incubation for 1 h, this was diluted to a final inoculum of approximately 5 × 106 bacteria. Antimicrobial treatment (t = 0 h) was started 2 h after infection.
Pharmacokinetics.
Single-dose plasma pharmacokinetic studies were performed for IMP/C and MK7655 in thigh-infected mice. The mice were injected intraperitoneally with 0.1 ml (0.2 ml for the highest dose) of a solution containing IMP/C and MK7655 in combination 2 h after infection. The following doses were applied: 8/4, 16/8, 32/16, 64/32, and 128/64 mg/kg, respectively. Blood samples were taken before injection (t = 0 h) and at 0.167, 0.33, 0.5, 0.75, 1, 1.5, 2, 3, 4, and 6 h after injection. Before collection by retro-orbital bleeding, the mice were anesthetized by using isoflurane. Samples were immediately put on ice and subsequently centrifuged. The supernatant, approximately 0.25 ml, was added to a 0.5-ml morpholineethanesulfonic acid (MES) buffer (pH 6.0). The exact amount of plasma was determined by weighing the samples. Samples were stored at 80°C, and concentrations were determined by high-pressure liquid chromatography (HPLC) at Merck (USA). The analytical methods for determination of MK7655 and IMP/C are based on the extraction of drug from plasma via protein precipitation. MK7655, IMP/C, and internal standards are separated using hydrophilic interaction chromatography (HILIC) and detected via tandem mass spectrometry in the turbo-ion spray (TIS) mode. The lower limit of quantification (LLOQ) for each analyte was 0.25 μg/ml, with a linear calibration range of 0.25 to 100 μg/ml. For each time point, 2 mice were used.
Pharmacokinetic analysis.
Concentrations were plotted against time. One- and two-compartment models with and without an absorption phase were fitted to the data using WinNonlin (version 2.1; Pharsight Corp., St. Louis, MO) and GraphPad Prism version 5.0 (GraphPad, Inc., San Diego, CA). Using the parameter estimates obtained, simulations of concentration-time profiles of IMP/C and MK7655 in mice were carried out using MicLab 2.33 (Medimatics, Maastricht, The Netherlands). From these, the fT>MIC was determined for a range of MICs and dosing regimens of IMP/C and MK7655. For IMP/C and MK7655, a protein binding of 20% was taken (Merck, data on file [14]).
Pharmacodynamic studies.
Animals were treated with IMP/C or sham treated with a control (saline) every 2 h for 24 h with or without MK7655 at various doses and frequencies (see below). At 0 h, 2 mice were humanely sacrificed to determine the number of CFU just before treatment. All the other animals were sacrificed at 24 h unless the welfare of the animals necessitated earlier termination, in accordance with animal welfare regulations. Excised thighs were transferred to a precooled 10-ml plastic tube (Transport Tube, Omnilabo, The Netherlands) containing 2 ml PBS and were homogenized using an Ultra-Turax homogenizer (IKA Labortechnik, Germany). A 10-fold dilution series was prepared, and 3 × 10 μl was plated per dilution. The following day, the CFU were counted. The drug effect was determined as the difference between the 10-log CFU values at 24 h and 0 h (mean value of 2 mice) expressed as d log CFU. For the dose fractionation study of MK7655, IMP/C was given at a fixed dose every 2 h, while MK7655 was then varied in such a way that an optimal variation in exposure would be obtained for the three major PK/PD indices maximum concentration of free, unbound drug in serum (fCmax)/MIC, %fT>MIC, and area under the concentration-time curve for the free, unbound fraction of drug (fAUC)/MIC. All dosing regimens were performed with at least two animals. For the dose fractionation study of MK7655, two dosing regimens of IMP/C were chosen, based on the efficacy of IMP/C in monotherapy experiments, corresponding to doses producing a static effect and a 2-log10 increase in the number of CFU with IMP/C alone. For further quantification of the effects of MK7655 for other strains, a dosing regimen of 8 mg/kg IMP/C every 2 h was used, corresponding to %fT>MIC values of 46.1% at an MIC of 1 mg/liter, 34.2% at 2 mg/liter, and 22.4% at 4 mg/liter.
Pharmacodynamic analysis.
d log CFU was plotted against total daily doses, area under the concentration-time curve (AUC), fT>C, and Cmax of MK7655 and the fT>MIC of IMP/C alone. The Hill model with variable slope was fitted to the data using GraphPad Prism 5.0 (San Diego, CA). The static PIs were determined from the model fit.
RESULTS
In vitro studies.
Table 2 provides the results of the checkerboard experiments. At low concentrations, there was no effect of the inhibitor, as indicated by a 0-fold difference in MIC compared to the control. Increasing the concentration of the inhibitor resulted in an increasing effect until a certain plateau was reached. The MK7655 concentration at which the plateau was reached and the plateau itself varied for each strain. MK7655 alone showed no antibacterial activity.
TABLE 2.
MICs of strains used for imipenem at different concentrations of MK7655a
| Strain | MIC of IMPb (mg/liter) combined with MK7655c concn (mg/liter) of: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | |
| P. aeruginosa | |||||||||
| ATCC 27853 | 1 | 1/2 | 1 | 0.5 | 0.25/0.5 | 0.25 | 0.25 | 0.25 | 0.25 |
| 24354 | 32 | 32 | 32 | 16/32 | 8/32 | 8/16 | 2/4 | 2 | 1 |
| 24227 | 4 | 4 | 2/4 | 2 | 1 | 0.5 | 0.25/0.5 | 0.25 | 0.25 |
| 24226 | 8 | 8 | 4/8 | 2/4 | 0.5/2 | 0.5/1 | 0.5 | 0.5 | 0.25/0.5 |
| 24228 | 8 | 4/8 | 4 | 4 | 2 | 1 | 0.5 | 0.5 | 0.5 |
| K. pneumoniae | |||||||||
| ATCC 43816 | 0.25 | 0.125 | 0.125/0.25 | 0.125 | 0.125 | 0.125/0.25 | 0.125 | 0.125 | 0.125 |
| 6755 | 4 | 0.5 | 0.25 | 0.25 | 0.125 | 0.125 | 0.125 | 0.06/0.125 | 0.0625 |
| 6339 | 16 | 4 | 4 | 2 | 0.5/1 | 0.25 | 0.125/0.25 | 0.125 | 0.0625 |
Current EUCAST breakpoints: susceptible, ≤2 mg/liter (K. pneumoniae) and 4 mg/liter (P. aeruginosa); resistant, >8 mg/liter. Current CLSI breakpoints: susceptible, ≤1 mg/liter (K. pneumoniae) or ≤2 (P. aeruginosa); intermediate, 2 mg/liter (K. pneumoniae) or 4 mg/liter (P. aeruginosa); resistant, ≥4 mg/liter (K. pneumoniae) or ≥8 mg/liter (P. aeruginosa).
IMP, imipenem.
MICs were obtained in duplicate; two values indicate incongruent results.
Pharmacokinetics of imipenem and MK7655.
A one-compartment model best fit the data. IMP/C and MK7655 showed no dose-dependent pharmacokinetics. The PK profiles of IMP/C and MK7655 were linear over the dosing range studied. The IMP/C and MK7655 V values were 0.434 and 0.544 liter/kg, and the elimination half-lives were 0.24 and 0.25 h, respectively.
Pharmacodynamics studies.
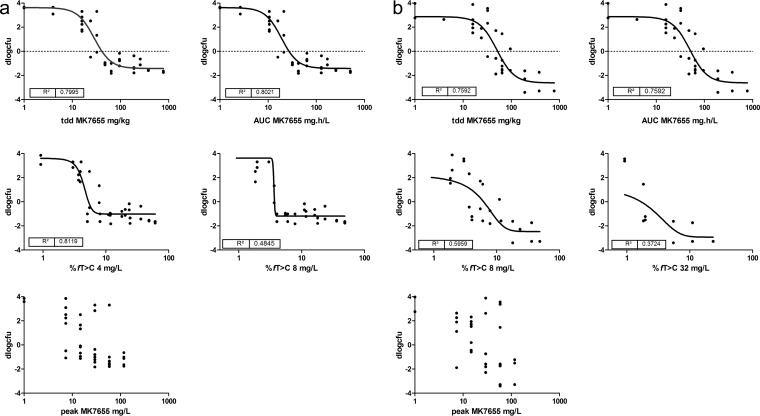
The PD of IMP/C alone was determined for each of the seven strains studied. Figure 1 shows examples of the dose-response relationship of IMP/C given every 2 h (q2h) for two strains, whereas Table 3 provides the pharmacodynamic characteristics of IMP/C for each strain. Subsequently, a dose fractionation study was performed for MK7655. Strains P. aeruginosa 24228 and K. pneumoniae 6755 were chosen to evaluate exposure-response relationships. Doses of MK7655 (from 4 to 64 mg/kg) and the frequencies of administration (1 to 12 times daily) were varied. The IMP/C dosing regimens used were determined from the exposure-response relationship of IMP/C alone (see above) and set at the two doses that would result in an approximately 2-log10 increase in CFU and a static effect, respectively. The efficacy of MK7655 would then be indicated as a decrease in CFU relative to the controls and would be relatively sensitive to changes in concentration of the inhibitor. Figure 2a and b show the relationship between exposure and effect for various PIs for strains K. pneumoniae 6755 and P. aeruginosa 24228. The effect was clearly dependent on the presence of MK7655 in a dose-dependent manner. There did not appear to be any relationship between MK7655 peak concentration (Cmax) and efficacy. There appears to be a stronger relationship between AUC and dose compared to that between T>MIC and dose, indicating that the total daily dose and AUC are the primary drivers of outcome. In addition, a separate analysis of the dose fractionation study fitting the maximum effect (Emax) model for each dose frequency showed no significant relationship between 50% effective concentration (EC50) and dose frequency, indicating that the effect was primarily dependent on total daily dose and therefore AUC. The effect of MK7655 was studied further in five other strains, primarily to further validate and quantify the relationship between MK7655 concentration and effect. Because the dose fractionation study had indicated that the total daily dose (or AUC) was the PK/PD index that best correlated with outcome, MK7655 doses were administered at the same time as those of IMP/C. Figure 3 shows the exposure-response relationships for 5 strains treated with 8 mg/kg IMP/C every 2 h (22.4%fT>C at 4 mg/liter; 0%fT>C at 16 mg/liter). This IMP/C dose was chosen because it resulted in %fT>MIC values relevant for clinically achievable concentrations. For each strain, a clear exposure effect of MK7655 was observed.
FIG 1.
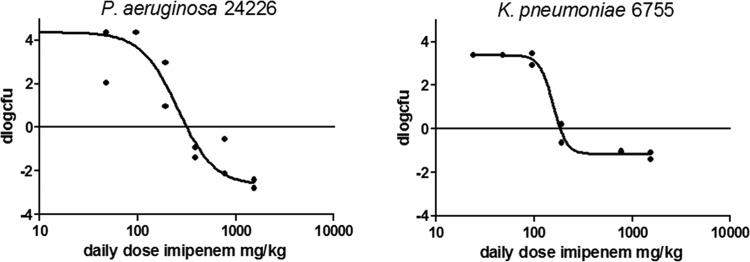
Dose-response relationship for IMP/C given q2h in thigh-infected mice. (Left) P. aeruginosa 24226; (Right) K. pneumoniae 6755.
TABLE 3.
Pharmacodynamic properties of 5 P. aeruginosa and 2 K. pneumoniae strains exposed to IMP/C alone
| Strain | Static TDDa (mg/kg) | MIC (mg/liter) | Static TDD/MIC | Static fT>MIC (%) |
|---|---|---|---|---|
| 24226 | 426.6 | 8 | 53.32 | 36.40 |
| 24227 | 25.1 | 4 | 6.28 | 0.00 |
| 24228 | 208.9 | 8 | 26.12 | 23.60 |
| 24354 | 549.5 | 32 | 17.17 | 15.60 |
| 24349 | 45.7 | 16 | 2.86 | 0.00 |
| 6755 | 182.0 | 4 | 45.49 | 33.00 |
| 6339 | 1479.1 | 16 | 92.44 | 45.20 |
TDD, total daily dose.
FIG 2.
Dose-response relationship of MK7655 observed in dose fractionation studies with IMP/C administered q2h at fixed doses. (a) K. pneumoniae 6755; (b) P. aeruginosa 24228. tdd, total daily dose.
FIG 3.
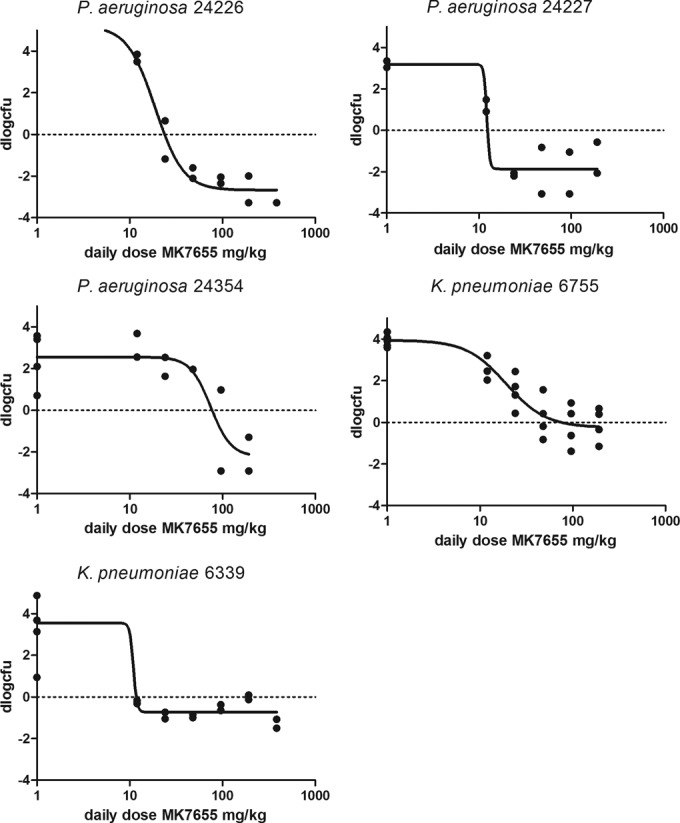
Dose-response relationship of MK7655 in combination with IMP/C 8 mg/kg q2h for 5 strains.
Interaction between imipenem and MK7655.
For two strains, K. pneumoniae 6339 and P. aeruginosa 24226, the effect of MK7655 was studied for four different dosing regimens of IMP/C (1, 2, 4, and 8 mg/kg every 2 h). The main purpose of this experiment was to determine whether the effect of MK7655 was dependent on the IMP/C dose and/or if there was a certain MK7655 AUC threshold for effect. Figure 4 shows the exposure-response relationships for the two strains, indicating that at the lowest dose of IMP/C administered, MK7655 effects were still apparent but marginal.
FIG 4.
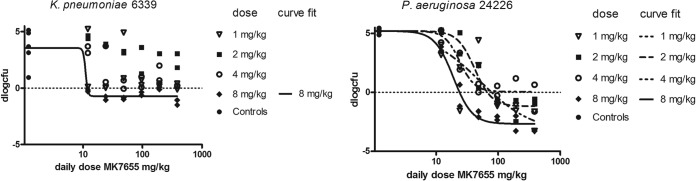
Dose-response relationship of MK7655 in combination with IMP/C q2h at four different dose levels for K. pneumoniae 6339 (left) and P. aeruginosa 24226 (right).
Quantitation of the effect of the inhibitor.
Table 4 summarizes the exposures and effects of IMP/C and MK7655 for all the experiments. Since the dose fractionation experiments indicated that fAUC was the parameter best correlated with effect, this index is shown in the table. The static MK7655 fAUC values of the individual experiments ranged from approximately 6 mg · h/liter (P. aeruginosa 24227 and K. pneumoniae 6339) up to 45.2 mg · h/liter (P. aeruginosa 24354); P. aeruginosa 24354 exhibited the highest MIC. The mean fAUC required was 26.0 mg · h/liter. The table also shows the MIC of each strain in the presence of 8 mg/liter MK7655, as well as the %fT>MIC of IMP/C corresponding to the MIC.
TABLE 4.
Doses and fAUC of MK7655 required for a static effect during treatment with IMP/C with various dosing regimens
| Strain | Imipenem |
MK7655 |
|||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | TDDa (mg/kg) | MIC (mg/liter) | fT>MIC (%) | Static TDD (mg/kg) | Static fAUCb (mg · h/liter) | MIC/4c (mg/liter) | |
| 6339 | 1 | 12 | 16 | 0 | 0.25 | ||
| 2 | 24 | 16 | 0 | 0.25 | |||
| 4 | 48 | 16 | 0 | 0.25 | |||
| 8 | 96 | 16 | 0 | 11.8 | 6.3 | 0.25 | |
| 6755 | 8 | 96 | 4 | 22.4 | 75.9 | 40.3 | 0.125 |
| 15.9 | 190.8 | 4 | 34.1 | 39.8 | 21.2 | 0.125 | |
| 24266 | 1 | 12 | 8 | 0 | 0.5 | ||
| 2 | 24 | 8 | 0 | 61.7 | 32.8 | 0.5 | |
| 4 | 48 | 8 | 0 | 79.4 | 42.2 | 0.5 | |
| 8 | 96 | 8 | 10.5 | 23.4 | 12.5 | 0.5 | |
| 24354 | 8 | 96 | 32 | 0 | 85.1 | 45.2 | 2 |
| 24227 | 8 | 96 | 4 | 22.4 | 12 | 6.4 | 0.25 |
| 24228 | 15.9 | 190.8 | 8 | 22.3 | 51.3 | 27.2 | 0.5 |
| Mean | 48.9 | 26.0 | |||||
| SD | 27.1 | 14.4 | |||||
TDD, total daily dose of imipenem.
The static fAUC indicates the exposure of MK7655 to result in a static effect with the imipenem regimes.
MIC/4, imipenem MIC in the presence of 4 mg/liter MK7655.
DISCUSSION
In this study, the effect of MK7655 during treatment with imipenem was determined using doses otherwise too low to demonstrate any efficacy during single-drug therapy. MK7655 in combination with IMP/C showed significant activity in vitro and in vivo, as it resulted in efficacy against imipenem-resistant strains. The PK/PD relationship of MK7655 described indicates that the AUC is the parameter that best correlates with efficacy. In the dose fractionation experiment, the best fit was obtained using the total daily dose or AUC. The results also show that MK7655 efficacy is clearly not related to peak concentration (Cmax). These observations suggest that the timing of MK7655 administration relative to that of imipenem is not critical and supports the potential for coadministration of MK7655 and IMP/C in a single formulation.
The total amount of MK7655 that is needed to render a given strain susceptible to imipenem in vivo is a matter of debate. In the experiments described here, the MK7655 AUC required to result in a static effect of imipenem ranged from 6 to 45 mg · h/liter (mean, 26 mg · h/liter). This value may serve as a basis for determining the doses of MK7655 once the human PK profile of MK7655 has been determined in phase 1 clinical trials (15), taking into consideration the protein binding of MK7655 (20% in mice [14]). While 7 strains have been studied to date and therefore a reasonable characterization of the variation that may be present between strains has been quantified, the effect of MK7655 is not only strain dependent but also dependent on the imipenem dosing regimen. In fact, the doses of imipenem used in this study were relatively low, and the results for MK7655 are therefore conservative. Theoretically, if all enzyme (beta-lactamase) activity is neutralized by a BLI, that strain would appear susceptible, as wild-type K. pneumoniae and P. aeruginosa are each imipenem susceptible. Using a dosing regimen of 8 mg/liter and a clinical breakpoint of 2 mg/liter (the EUCAST S breakpoint), the %fT>MIC is 34.2%, and this value correlates with the static effects observed in animal models described by others. For each strain, an MK7655 regimen that resulted in a static effect at clinically achievable concentrations was found. Considering that the number of approvals for new antibacterial agents are still dropping and the existing β-lactamase inhibitors are poorly active against class C and D enzymes and not active against class B enzymes, MK7655 is a promising β-lactamase inhibiter and will restore the activity of imipenem against carbapenemases; thus, it warrants clinical evaluation.
ACKNOWLEDGMENTS
This study was performed with an unrestricted grant from Merck Inc. USA.
J. W. Mouton received research grants and/or was a consultant for Merck Inc. USA, Pfizer, Polyphor, Cubist, Astra-Zeneca, Basilea, and Roche.
REFERENCES
- 1.Babouee B, Widmer AF, Dubuis O, Ciardo D, Droz S, Betsch BY, Garzoni C, Fuhrer U, Battegay M, Frei R, Goldenberger D. 2011. Emergence of four cases of KPC-2 and KPC-3-carrying Klebsiella pneumoniae introduced to Switzerland, 2009–10. Euro Surveill 16:pii=19817 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19817. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball P, Geddes A, Rolinson G. 1997. Amoxycillin clavulanate: an assessment after 15 years of clinical application. J Chemother 9:167–198. doi: 10.1179/joc.1997.9.3.167. [DOI] [PubMed] [Google Scholar]
- 5.Rolinson GN. 1982. The history and background of Augmentin. S Afr Med J 62:3A–4A. [PubMed] [Google Scholar]
- 6.Sorgel F, Kinzig M. 1993. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother 31(Suppl A):39–60. [DOI] [PubMed] [Google Scholar]
- 7.Rotschafer JC, Ostergaard BE. 1995. Combination beta-lactam and beta-lactamase-inhibitor products: antimicrobial activity and efficiency of enzyme inhibition. Am J Health Syst Pharm 52:S15–S22. [DOI] [PubMed] [Google Scholar]
- 8.Young K, Raghoobar SL, Hairston NN, Painter RE, Racine F, Dorso KL, Park Y-W, Ogawa AM, Wisniewski D, Hermes J, Blizzard TA, Hammond ML, Motyl MR. 2010. In vitro activity of the class A and C β-lactamase inhibitor MK-7655, p 2139 In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Boston, MA, 12–15 September, 2010. [Google Scholar]
- 9.Badal R, Young K, Motyl MR, Hoban D, Hackel M, Biedenbach D, Bouchillon SK. 2013. Impact of MK-7655 on imipenem MICs of a large collection of Enterobacteriaceae and Pseudomonas aeruginosa isolates, p 1163 In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Denver, CO, 10–13 September 2013. [Google Scholar]
- 10.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 11.Mouton JW, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, Macgowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 12.Drusano GL, Preston SL, Hardalo C, Hare R, Banfield C, Andes D, Vesga O, Craig WA. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother 45:13–22. doi: 10.1128/AAC.45.1.13-22.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Standards Organization. 2006. ISO 20776-1. Clinical laboratory testing and in vitro diagnostic test systems—susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility testing devices—part 1. International Standards Organization, Geneva, Switzerland. [Google Scholar]
- 14.Tang W, Dingley K, Colwell L, Blizzard T, Young K, Motyl MR. 2010. MK-7655 human dose projection based on its pharmacokinetics in preclinical species, p 1560 In Abstr 50th Intersci Conf Antimicrob Agents Chemother, Boston, MA, 12 to 15 September 2010. [Google Scholar]
- 15.Butterton JR, Jumes P, Calder N, Rizk ML, Nefliu M, Sun P, Schwartz M, Mangin E, Warrington S, Stoch A, Wagner JA. 2010. A phase I study evaluating the safety, tolerability, and pharmacokinetics of an intravenous beta-lactamase inhibitor in healthy male volunteers, p 1967 In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Boston, MA, 12–15 September, 2010. [Google Scholar]