Abstract
Long-term peritoneal dialysis (PD) can lead to fibrotic changes in the peritoneum, characterized by loss of mesothelial cells (MCs) and thickening of the submesothelial area with an accumulation of collagen and myofibroblasts. The origin of myofibroblasts is a central question in peritoneal fibrosis that remains unanswered at present. Numerous clinical and experimental studies have suggested that MCs, through epithelial-mesenchymal transition (EMT), contribute to the pool of peritoneal myofibroblasts. However, recent work has placed significant doubts on the paradigm of EMT in organ fibrogenesis (in the kidney particularly), highlighting the need to reconsider the role of EMT in the generation of myofibroblasts in peritoneal fibrosis. In particular, selective cell isolation and lineage-tracing experiments have suggested the existence of progenitor cells in the peritoneum, which are able to switch to fibroblast-like cells when stimulated by the local environment. These findings highlight the plastic nature of MCs and its contribution to peritoneal fibrogenesis. In this review, we summarize the key findings and caveats of EMT in organ fibrogenesis, with a focus on PD-related peritoneal fibrosis, and discuss the potential of peritoneal MCs as a source of myofibroblasts.
Keywords: Mesothelial cell, epithelial-mesenchymal transition, progenitor cell, fibrosis, peritoneal dialysis
Peritoneal dialysis (PD) is an effective alternative form of renal replacement therapy that is currently used by about 11% of dialysis patients with end-stage renal disease (ESRD) (1). Peritoneal dialysis holds extensive therapeutic advantages for appropriately selected patients when compared with hemodialysis, including early survival benefit, the convenience of home therapy, and lower healthcare costs, which are particularly attractive to patients who are new to renal replacement therapy (2,3). However, one of the most important challenges in the PD community is the long-term preservation of peritoneal membrane integrity, central to the very technique. Cumulative evidence from both laboratory and clinical studies has highlighted that continuous exposure to PD therapeutics, recurrent episodes of peritonitis and various factors other than those related to the PD technique (e.g. uremic inflammation and primary kidney disease per se) lead to damage of the peritoneal structure and final dialysis failure (4). To improve the outcome of PD therapy and favorably expand its clinical application, there is a pressing need to understand the mechanisms that drive peritoneal membrane damage during PD.
Peritoneal fibrosis is the most consistent change observed in the peritoneal tissues of patients who undergo long-term PD therapy (5). The pathogenesis of peritoneal fibrosis is characterized by the loss of mesothelial cells (MCs), angiogenesis, and progressive submesothelial thickening with an increasing presence of myofibroblasts (6). It has been well demonstrated that active (myo)fibroblasts, stimulated by a variety of fibrogenic cytokines from the injured environment (predominantly by transforming growth factor-β1, TGFβ-1), are the dominant collagen-producing cells during organ fibrosis (7,8), which generate an excessive amount of extracellular matrix (ECM) and lead to the destruction of the normal tissue architecture (9). Accordingly, selective depletion of fibroblasts has prevented peritoneal fibrogenesis in transgenic animals (10). Furthermore, pathological fibroblasts contribute to the recruitment of mononuclear leukocytes in the progression of inflammation by expressing a variety of cytokines (8,11). Given the multipotential of fibroblasts in both healthy and diseased conditions, it is important to address the central question of the origin of the accumulating fibroblasts and the mechanism involved in this scenario so that early intervention targets can be identified to slow down the progression of peritoneal fibrosis.
Recent studies indicate that the sources of active fibroblasts are multiple and context-dependent (12–14) and might include local interstitial fibroblasts, bone marrow-derived-circulating fibrocytes, pericytes (or perivascular fibroblasts), endothelial cells, local mesenchymal progenitor cells, and, last but not least, the pathological epithelium itself via a process called epithelial-mesenchymal transition (EMT). The topic of EMT in fibrosis is highly controversial as carefully executed studies have demonstrated conflicting results. At the same time, emerging studies from independent groups have indicated the existence of progenitor cells in the mesothelium, which may give rise to the active fibroblasts during fibrogenesis. In this review, we will analyze the potential of MC transitioning to fibroblasts via EMT and the possibility of fibrogenesis from resident mesothelial progenitor cells in PD-related peritoneal fibrosis.
Characteristics of Peritoneal Mesothelial Cells
Mesothelial cells are epithelial-like cells, resting on a thin basement membrane that lines the entire surface of the serosal cavities (pleural, pericardial, and peritoneal), including the internal organs (visceral mesothelium) and the body wall (parietal mesothelium) (15,16). The layer of cells, termed “mesothelium,” is recognized as a dynamic cellular membrane that provides a protective, non-adhesive surface on the vertebrate thoracic and abdominal cavities and internal organs. In the peritoneum, MCs predominantly adopt an elongated, flattened, and squamous morphology (polygonal cobblestone-like). Peritoneal MCs are supported by submesothelial connective tissue containing blood vessels, lymphatics, resident inflammatory cells, and fibroblast-like cells (17). The boundaries between MCs consist of delicate junctional complexes, including tight junctions, adherens junctions, gap junctions, and desmosomes, which are crucial for the establishment of cell surface polarity and the maintenance of a semi-permeable diffusion barrier (15). The luminal surface of peritoneal MCs is characterized by abundant microvilli and cilia, reflecting a biosynthetically active state in these cells (18).
Mesothelial cells play a critical role in maintaining the integrity and functional properties of the peritoneum. By secreting a variety of mediators in response to external signals, MCs serve as multi-functional regulators of peritoneal homeostasis, including immune surveillance, tissue repair, angiogenesis, inflammatory response and the control of fluid and solute transport (6,17,19,20). For example, during PD-related peritonitis or upon exposure to bacterial products, peritoneal MCs are able to recognize the bacterial pathogens through toll-like receptors, resulting in the activation of nuclear factor κB (NF-κB) signaling pathways and subsequent secretion of numerous inflammatory cytokines, including interleukin 6 (IL-6), interleukin 8 (IL-8), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 2 (MIP-2) (21–24). These dynamic properties of MCs are compromised in the bio-incompatible setting of PD, leading to serious damage in the structural and functional integrity of the peritoneum. Therefore, a better understanding of MC biology can offer an opportunity to protect the peritoneum from PD-related injury and achieve long-term effective therapy.
During embryonic development, MCs originate from the primitive mesodermal layer, where they share the characteristics of both epithelial and mesenchymal cells (25). The origin of MCs might indicate that these cells possess the potential for transformation between epithelial and mesenchymal phenotypes in adults. A subset of adult MCs with a cuboidal morphology have been identified in the “active zone” of the peritoneum, including in close proximity to parenchymal organs (spleen, liver, and diaphragm), to “milky spots” of the omentum, and to the injured part of the mesothelium (26). These cells, when compared with squamous MCs, stay at a more metabolically activated state, as suggested by a greater abundance of mitochondria, a rough endoplasmic reticulum, and microfilaments (15). These active MCs seem to be prone to acquiring a mesenchymal phenotype and transform into fibroblasts during organ injury and fibrogenesis, as supported by a recent study in liver injury (27). However, it remains obscure how MCs switch between epithelial and mesenchymal phenotypes under diverse stimuli in healthy or diseased conditions. Epithelial-mesenchymal transition and the existence of mesothelial progenitor cells (MPCs) have currently been proposed as the origin of myofibroblasts during peritoneal fibrosis.
MCS and EMT in Peritoneal Dialysis
Long-term bio-incompatibility of PD causes structural and functional injury of the peritoneal membrane, evidenced by chronic inflammation, angiogenesis, and progressive peritoneal fibrosis (28,29). The mechanism of peritoneal fibrosis is not completely understood, but it is widely accepted that accumulation of activated fibroblasts plays a pivotal role in this process. Given their nature and the pathological changes during peritoneal impairment, it has been suggested that MCs contribute to the pool of fibroblasts through EMT, i.e., a transition from epithelial to mesenchymal cells.
What is EMT?
Normally, a polarized epithelial cell attaches to the basement membrane via its epithelial tight junction components. Epithelial-mesenchymal transition is a biological process that allows an epithelial cell to undergo multiple biological changes and adopt a mesenchymal cell phenotype, including enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, stem cell properties, immunosuppression, and increased production of ECM components (30–34). Recently, EMT has been classified into 3 different biological subtypes based on the biological context in which it occurs: Type 1 EMT occurs during embryogenesis and organ development; Type 2 EMT occurs when secondary epithelial cells switch into mesenchymal cells during tissue repair and fibrosis; Type 3 EMT occurs when epithelial carcinoma cells turn into migrating and invasive mesenchymal cells (35,36). This conversion between the 2 cell phenotypes is finely regulated by key transcription factors, including Snail, zinc-finger E-box-binding (ZEB), and basic helix-loop-helix (bHLH) transcription factors (34). Changes, including the reprogramming of gene expression during EMT as well as non-transcriptional changes, are initiated and controlled by abundant signaling pathways that respond to extracellular cues. Among these, the TGFβ-1 family plays a predominant role (7,37).
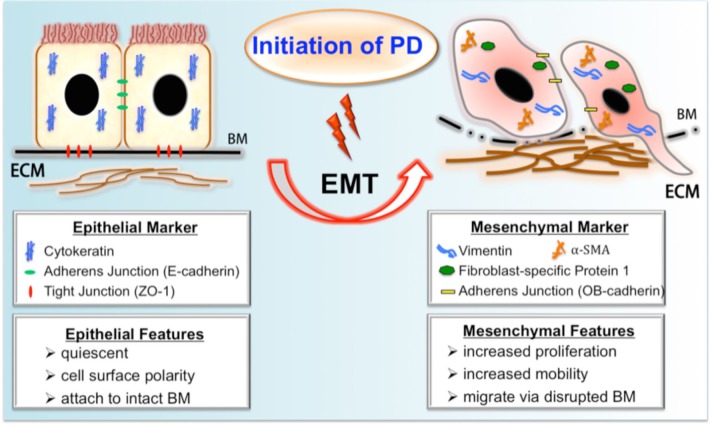
In the context of organ fibrosis, it is believed that EMT begins as part of a repair-associated event that normally generates fibroblasts and other related cells in order to reconstruct tissues following trauma and inflammatory injury and ceases once inflammation is attenuated. However, EMT can pathologically continue to respond to ongoing inflammation, leading eventually to tissue structure destruction (fibrosis). The initiation of EMT includes downregulation of epithelial adherens junction (like E-cadherin) and tight junction components (like zonula occludens-1, ZO-1), and upregulation of mesenchymal junctional proteins (such as N- or OB-cadherin). Thereafter, the main cytoskeletal protein pattern of the epithelium is switched from cytokeratin to vimentin with de novo synthesis of fibroblast-specific protein 1 (FSP1, also known as S100A4) and occasionally smooth muscle actin (α-SMA) (30–38). However, it should be noted that some of these markers are controversial for defining the occurrence of fibrogenic EMT, which will be further discussed in the section Ongoing debate on EMT and kidney fibrosis.
Evidence of EMT in PD-Related Peritoneal Fibrosis
In 2003, Yanez-Mo and colleagues demonstrated that peritoneal MCs in PD patients underwent a transition from an epithelial to a mesenchymal phenotype, indicated by a progressive loss of epithelial morphology and a decrease in the expression of cytokeratins and E-cadherin (39). By comparing detached human MCs from PD effluent with normal MCs from digested omentum, the authors found that peritoneal MCs showed a progressive loss of epithelial phenotype and acquired myofibroblast-like characteristics soon after PD initiation, suggesting the mechanism of EMT in PD, which could be regulated by the transcriptional factor Snail. Although it is easy to access the effluent-derived MCs, one major limitation of this study is that shedding cells do not represent the actual condition of peritoneal MC that remains attached to the peritoneum during PD (40). Prompted by this interesting finding, however, a large number of researchers set to work to establish the potential role of EMT in peritoneal fibrosis. These studies have been summarized in a series of reviews (41–44). A schematic paradigm of MCs undergoing fibrogenic EMT during PD is shown in Figure 1.
FIGURE 1 —
A schematic summary of fibrogenic EMT during PD. Upon exposure to PD, the peritoneal mesothelial cell undergoes profound phenotypic changes of EMT, resulting in the loss of epithelial features and the gain of mesenchymal characteristics. The process of EMT includes downregulation of epithelial adherens junction (like E-cadherin) and tight junction components (like ZO-1) and upregulation of mesenchymal junctional proteins (like OB-cadherin). Thereafter, the main cytoskeletal protein pattern of epithelium is switched from cytokeratin to vimentin with de novo synthesis of FSP-1 and α-SMA. A subset of transitioning cells with increased mobility might migrate into the submesothelial zone through disrupted BM and contribute to the increased deposition of ECM. PD = peritoneal dialysis; EMT = epithelial-mesenchymal transition; BM = basement membrane; ECM = extracellular matrix; ZO-1 = zonula occludens-1; FSP-1 = fibroblast-specific protein 1; α-SMA = α smooth muscle actin.
The supporting evidence for EMT in peritoneal fibrosis can be broadly classified into 3 categories. First, in vitro cultured MCs undergo transdifferentiation when treated with various stimuli to mimic the bio-incompatibility of PD. Among these, TGF-β1 signaling, through Smad-dependent and independent pathways, has been extensively investigated and proves to be determinant in the induction of EMT in vitro. In the context of fibrosis, TGF-β1 exerts its biological effects by activating Smad2 and Smad3, which are negatively regulated by an inhibitory Smad7 (45). Smad proteins, phosphorylated by TGF-β1 stimulation, control the transcription of TGF-β1 responsive genes (45,46), many of which are identified as fibrogenic genes (47) and regulators (48) of EMT in peritoneal fibrosis, including Snail (49,50), collagens (50–54), α-SMA (51,53), fibronectin (53–55), connective tissue growth factor (CTGF) (56), β-catenin (57), monocyte chemoattractant protein-1 (MCP-1) (58), transforming growth factor-activated kinase-1 (TAK1) (59,60), plasminogen activator inhibitor-1 (PAI-1) (53) and matrix metalloproteinase-2 (MMP-2) (50,61). Therefore, TGF-β1-induced mesothelial transition results in a characteristic myofibroblastic phenotype and complex modulation of gene expression, including cytoskeletal organization, cell adhesion, and ECM production (48,62). Besides Smad proteins, emerging studies have illustrated that the cross-talks of non-Smad pathways are also involved in TGF-β1-induced EMT in peritoneal fibrosis, including Akt/mTOR (63), c-Jun N-terminal kinase (JNK) (51,64), Wnt/β-catenin (65), integrin-linked kinase/glycogen synthase kinase-3β (ILK/GSK-3β) (66), extracellular signal-regulated kinase/nuclear factor kappa B (ERK/NF-κB) (67), and mitogen-activated protein kinase (MAPK) (68). The obvious major limitation in this type of study is that cultured MCs can undergo EMT in vitro but an equivalent conclusion can hardly be drawn for in vivo situations. However, it undoubtedly remains an excellent tool for studying the underlying complex signaling and transcriptional pathways of EMT.
The second line of evidence to support EMT in peritoneal fibrosis is the immunohistochemical analysis showing the coexistence of epithelial and mesenchymal hallmarks within MCs during peritoneal fibrosis. In 2005, Margetts and colleagues found that intraperitoneal transfer of active TGF-β1-induced EMT and progressive peritoneal fibrosis in rats was characterized by coexpression of cytokeratin and α-SMA in the submesothelial zone, loss of an intact mesothelial layer, and an increase in gene expression associated with EMT and fibrosis (52). Consistent with this animal work, a clinical study on 35 stable PD patients with parietal peritoneum biopsies showed in situ evidence of EMT in 17% of patients (by submesothelial cytokeratin staining) and loss of the mesothelial layer in 74% of patients, indicating that conversion of epithelial-like MCs to fibroblast-like phenotypes was frequent in the peritoneal membrane during PD therapy (69). Similar changes in the peritoneal membrane, like co-expression of mesothelial markers in stromal spindle-like cells in the submesothelial layer, were observed in a number of subsequent clinical (70–72) and experimental (73–75) studies, positively suggesting a local conversion of MCs to fibroblasts after PD initiation.
Finally, the therapeutic effect of EMT-targeted interventions on peritoneal fibrosis has also been demonstrated. In 2003, Zeisberg and colleagues showed that administration of bone morphogenic protein (BMP)-7, a member of the TGF-β superfamily, reversed TGF-β1-induced EMT in renal tubular epithelial cells and ameliorated kidney fibrosis (76), highlighting the potential of EMT as a target of anti-fibrosis therapy. Similar results were later reported in peritoneal fibrogenesis. Yu (55) and Loureiro (77) found that adenoviral BMP-7 transfection or administration of recombinant BMP-7 reduced the presence of EMT and attenuated peritoneal fibrosis in animal models of PD. In addition, hepatocyte growth factor (HGF) was shown to protect the peritoneal membrane from dialysate-induced damage in a reciprocal manner against TGF-β1 (55,78), which could be achieved by directly blocking TGF-β1 as well (79). Moreover, recent studies showed that TGF-β1 may regulate specific microRNAs (miR) to influence tubular EMT during kidney fibrosis, such as miR-192 (80), miR-21 (81), miR-29 (82), and miR-433 (83), suggesting that specifically targeting microRNAs related to EMT might represent a promising anti-fibrosis therapy (84,85). In peritoneal fibrosis, Zhang and colleagues demonstrated decreased levels of miR589 in effluent-derived MCs of long-term PD patients as well as cultured MCs treated with TGF-β1. Overexpression of miRNA589 partially reversed TGF-β1-induced EMT in MCs, including downregulation of vimentin and upregulation of ZO-1 and E-cadherin (86). Recently, Zhou and colleagues found that miR-30a ameliorated TGF-β1-induced EMT and collagen production in PD-related peritoneal fibrosis by binding the 3′-untranslated region of Snai1 (49). Taken together, these findings highlight the therapeutic effect of targeting EMT on peritoneal fibrogenesis and thus support an occurrence of EMT.
Ongoing Debate on EMT and Kidney Fibrosis
The major challenge in determining the presence of EMT is that it requires detecting a change in cell phenotypes or measuring movement of a cell in real time, which is feasible in vitro but extraordinarily difficult in vivo, particularly in humans. Current lineage-tracing strategy represents the most powerful tool available to confirm EMT in vivo. Briefly, using a site-specific recombinase (Cre) technology (typically Cre-LoxP recombination), gene modification between loxP sites can be targeted to a specific cell type or be triggered by a specific external stimulus (87,88). By choosing cell-specific promoters for the expression of Cre, a target cell could be labeled by subsequent production of a reporter protein, like a fluorescent protein or beta-galactosidase. Therefore, any cell population can be marked when that promoter becomes conditionally activated. If these labeled cells undergo any change (like division, transition, or migration), expression of the reporter will be passed on to all progeny, so that these “colorful” cells can be easily tracked and unambiguously identified. These techniques thus offer sufficient resolution to monitor a group of specific cells in real time and generate reliable data (89). However, recent independent studies with cell fate-mapping showed inconsistent results related to EMT, which raised an intense debate on the role of EMT in organ fibrogenesis, especially in renal fibrosis.
In 2002, Iwano first addressed the hypothesis of EMT in kidney fibrosis by using transgenic reporter mice and bone marrow transplant, demonstrating that a substantial number (about 36%) of renal fibroblasts were derived via EMT from the renal tubular epithelial cells during interstitial fibrogenesis in a mouse model of unilateral ureteral obstruction (UUO) (90). Prompted by this finding, a large number of researchers started to explore the potential role of EMT in tissue fibrogenesis. Using lineage tracing technique, similar findings were obtained in subsequent studies of liver (91), lung (92), intestine (93), and lens epithelium (94). Since then, abundant studies providing experimental and clinical support for the possibility that EMT is a contributor to organ fibrosis have been published and systematically reviewed (95–101). For example, supportive evidence from a large number of in vitro studies (35,36,76,102–107) has conclusively shown that cultured tubular and other epithelial cells could indeed transform into cells with a myofibroblast phenotype when exposed to profibrotic cytokines (typically TGF-β1). This conversion is characterized by loss of cell polarity, downregulation of epithelial markers, and acquisition of mesenchymal markers and features (spindle-shaped and mobile). Epithelialmesenchymal transition was also suggested by substantial evidence from a variety of human kidney diseases (108–112), where the injured epithelium was presented with several signs of mesenchymal plasticity.
However, the hypothesis of EMT in the kidney and liver has been recently challenged, according to some compelling findings in new lineage-tracing studies. Kriz summarized these findings in a recent review and pointed out several “flaws” in previous studies of EMT, with a clear point of view that “unequivocal evidence supporting EMT as an in vivo process in kidney fibrosis is lacking” (113). First, it is stated that none of the biochemical markers is specific enough for the occurrence of EMT. For example, independent studies have shown that Fsp1 staining might be negative in a proportion of interstitial fibroblasts in the healthy (114) or fibrotic kidney (115) and some Fsp1-positive cells in an injured kidney may actually be interstitial mononuclear cells (114,116,117) or endothelial cells of renal microvessels (114,117). Furthermore, it has been suggested by different studies that the expression of α-SMA should not be used as a criterion of EMT because only a subset of fibroblasts express this protein and even for in vitro experiments, various epithelial cell lines show differing sensitivity to α-SMA expression (36,98). In addition, vimentin is more likely to be expressed in the injured and proliferative tubular cells with an epithelial pattern, indicating the tubular regenerating activity rather than the phenotype of fibrogenic EMT (118,119). Second, the limitation of in vitro studies is that although cultured cell lines and primary tubular cells can undergo EMT in vitro, this fact cannot prove the same transition of tubular cells in vivo. Third, the strongest evidence against EMT in the kidney comes from a series of new cell fate-mapping studies where no evidence was found for the expression of (myo) fibroblast markers in the labeled epithelium of the fibrotic kidney. Representatively, using 3 Cre/Lox transgenic lines to genetically label the specific populations of renal cells, Humphreys and colleagues found that the epithelium did not give rise to any cells contributing to fibrosis in mouse models of UUO or ischemic kidney injury (120). Instead, these investigators proposed that (myo)fibroblasts were mostly derived from local pericytes or perivascular fibroblasts (121). Another 2 lineage-tracing studies by Li (117) and Koesters (122) also found no evidence that renal tubular cells transform into mesenchymal cells, more specifically into fibroblasts or myofibroblasts. Using multiple genetically engineered mice to track in-kidney fibrosis, LeBleu and colleagues supported an interesting possibility that the total pool of kidney myofibroblasts is composed of proliferation (50%) from local resident fibroblasts, differentiation (35%) from bone marrow, endothelial-to-mesenchymal transition program (10%) and EMT transition program (5%) (123). Although this study raised the potential of multiple contributors to kidney fibrosis, the role of EMT in the source of myofibroblasts, if any, is supposed to be minimal. In a latest review of kidney fibrosis, Duffield summarized these updated findings and suggested FOXD1-lineage cells, mostly as pericytes and resident fibroblasts, are the major precursor population of pathological myofibroblasts in kidney disease (124), which is casting serious doubts on previous results and is an argument against the role of EMT in renal fibrosis.
Challenge to EMT in Peritoneal Fibrosis
Similar questions have also been raised about the view of EMT in peritoneal fibrosis. Through comprehensive genetic lineage analysis, Chen and colleagues showed the first in vivo evidence that submesothelial fibroblasts were the major precursors of myofibroblast during peritoneal fibrogenesis, and the surviving MCs in different peritoneal injuries serve as the principal cells for mesothelium repair, arguing against the transition of MCs to myofibroblast by EMT during peritoneal fibrosis (125). In this study, about 83% of cytokeratin-positive MCs were genetically labeled by the expression of red fluorescence protein (RFP), using the Wilms tumor-1 homolog (Wt1)-CreERT2 mice. These marked MCs were found not to contribute, at least not significantly, to the source of myofibroblasts in different mouse models of peritoneal fibrosis. To determine the primary source of α-SMA (+) myofibroblasts in peritoneal fibrosis, they further generated transgenic reporter mice in which the expression of green fluorescence protein (GFP) and RFP was under the regulation of the Col1a1 and Col1a2 promoters, respectively. By comparing the proportion of cells with coexpression of GFP and RFP before and after the peritoneal injury, they demonstrated that submesothelial fibroblasts, rather than other cell sources, were the major precursors of collagen-producing myofibroblasts in the injured peritoneum. This study, using robust cell-fate mapping strategy, revealed the biological behaviors of MCs in the course of peritoneal injury and fibrosis. Moreover, Chen’s study also demonstrated that pharmacologic inhibition of platelet-derived growth factor (PDGF) receptor in submesothelial fibroblasts resulted in significant attenuation of peritoneal fibrosis, providing new insights into the novel therapeutics against peritoneal fibrosis. It will be necessary to reproduce these findings in more clinically relevant animal models in the future.
However, it is worth noting that the potential role of EMT in peritoneal fibrosis cannot be totally excluded by Chen’s experiment, due to the technical limitations of genetic labeling in this study. As stated, part of the submesothelial cells were also positively labeled for the expression of Wt1-RFP (about 24% of all RFP+ cells in the peritoneum). At the same time, only approximately 65% of submesothelial fibroblasts could be well tracked according to their conditional labeling strategies. Thus, the results are insufficient to determine whether or not some of the MCs might have moved across the basement membrane into the submesothelial area (via EMT) during peritoneal fibrosis, based only on the statistical analysis of RFP (+) cells. Furthermore, a transgenic model of Wt1-CreERT2 is not specific enough to track the transition of peritoneal MCs. Using similar lineage tracing strategy, it has been demonstrated in other studies that Wt1 (+) precursor cells might give rise to MCs, submesothelial cells, hepatic stellate cells and perivascular mesenchymal cells during liver development and injury, where Wt1 (+) MCs could actually migrate inward from the peritoneum (liver surface) to generate fibroblasts via EMT (27,126). Finally, the peritoneal MCs in Col1a1-GFPtg mice were found to significantly express Col1a1-GFP after different peritoneal injuries, although no detectable migration or expression of α-SMA was observed in those cytokeratin (+) cells in Chen’s experiment. The results actually indicated a fibrotic phenotype transition in the injured MCs because the production of collagens has been regarded as a defining hallmark for a fibrogenic cell (121,124,127). This phenomenon has been reported in the other study as well, i.e. that mobilization is not necessary for peritoneal MCs to undergo a mesenchymal transition induced by PDGF-B, which was thus called “non-invasive EMT” (128). Therefore, we suppose that activated MCs with a fibrogenic phenotype can play a critical role in regulating peritoneal fibrosis through production of collagens and growth factors (see Characteristics of peritoneal mesothelial cells section), even if they do not transform directly into submesothelial myofibroblasts.
Conversion of Mesothelial Progenitor Cell to Myofibroblast Cell
Given the characteristics of MCs and their embryological origin (refer to Characteristics of peritoneal mesothelial cells), early studies also highlighted the existence of mesothelial progenitor cells and their potential role in tissue repair and fibrosis (25). The rigorous definition of tissue progenitor cell or tissue stem cell remains lacking due to the differences among cell populations derived from various sources of tissues (129,130). Generally, it is defined as a group of cells possessing the hallmark stem cell characteristics of self-renewal and capable of successively giving rise to more differentiated cells. Using immunohistochemical techniques, Bolen and colleagues identified a population of multipotential submesothelial cells possessing the capability of differentiating toward a mesothelial cell phenotype and contributing to the repair of injured mesothelium (131,132). These findings suggest that a group of progenitor cells might exist in the submesothelial area and differentiate into MCs in the context of tissue repair. An intriguing question raised by these early studies is whether the coelomic mesothelium retains the ability to transform into other specific cell lineage, like fibroblasts. By examining a cell line derived from rat epicardial MCs, Wada and colleagues demonstrated that these cells retain the ability to form a polarized epithelium and, more importantly, to generate smooth muscle cells in response to specific growth factors (133). Similarly, it has been reported that a subset of epicardial MCs could differentiate into various cell types in vitro, including endothelium, smooth muscle cell, perivascular, and cardiac interstitial fibroblasts (134–136). Taken together, these previous studies indicated the presence of MPCs, which can differentiate into various specific cell types upon stimuli.
Recently, a series of studies using genetic lineage-tracing technique identified epicardium as the main source of diverse cardiac fibroblasts, myocardium, endothelium, and smooth muscle during heart development (137,138). These findings further raised the question of whether the epicardial MCs in adults could reproduce their embryonic program and acquire the multipotential characteristics of stem cells under pathological conditions. In 2007, Smart and colleagues demonstrated that thymosin beta-4, an actin-binding peptide, can restore the pluripotency of quiescent adult epicardium and trigger its differentiation into fibroblasts, smooth muscle cells, and endothelial cells, suggesting adult epicardial cells as a viable source of vascular progenitors upon stimulation (139). Following this study, they further identified in mice that adult epicardium contains a viable source of resident progenitor cells that could give rise to differentiated cardiomyocytes after ischemic injury (140), demonstrating the multipotential capability of epicardial MPCs in adult animals. Increasing interest has been raised since then concerning the potential of adult epicardium in tissue regeneration during myocardial injury (141–144). Subsequent studies positively support that epicardium in adults, when stimulated (typically by thymosin beta-4), is capable of reactivating its embryonic potential with substantial effects on generation of fibroblast, endothelial cell, and smooth muscle cell (145–147). These findings on epicardium support the intriguing possibility that mesothelium may represent a progenitor pool in adults that could be activated during tissue repair. More recently, Rinkevich and colleagues, using a genetic lineage tracing approach, demonstrated that both embryonic and adult mesothelium themselves have plasticity that allows them to become fibroblasts and smooth muscle cells (named FSMCs) (148). They first revealed a robust generation of FSMCs from both cultured and transplanted mesothelium tissue. To investigate a pure population of MCs, they generated transgenic mice to lineage trace mesothelial progenitor cells, by targeting Mesothelin (MSLN), a surface marker expressed on MCs. By isolation, tracing, and quantification of these Mesothelin-positive precursors, it has been shown that embryonic and adult mesothelium represents a common lineage to trunk FSMCs and trunk vasculature.
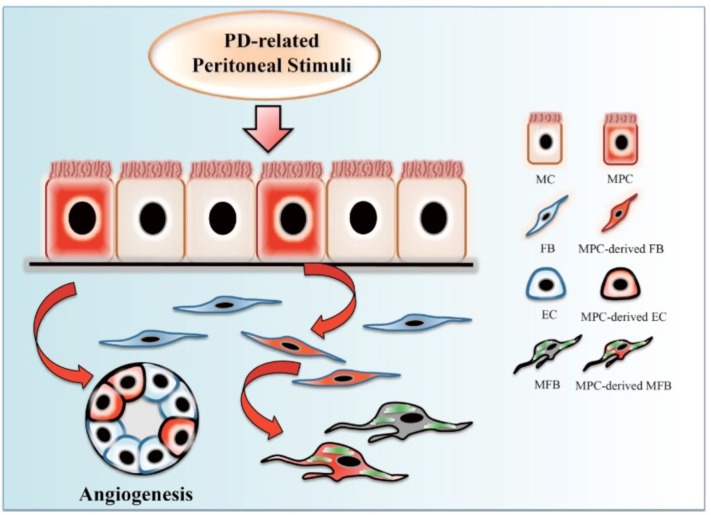
Although a direct identification of MPCs in adult peritoneum has yet to be established, current studies of lineagetracing assay suggest that a subpopulation of peritoneal MPCs might exist and possess the ability to differentiate into fibroblast-like cells upon various stimuli. These findings provide crucial insight into the cell behavior of MCs in both homeostatic and pathological conditions. Potential differentiation of MPCs during PD is shown in Figure 2. Lineage-tracing experiments with specific reporter genes in MPCs must be conducted in animal models of peritoneal fibrosis in order to mark and monitor these cells in real time during the course of fibrogenesis.
FIGURE 2 —
Potential differentiation of mesothelial progenitor cell during PD. A subpopulation of mesothelial progenitor cells rest in the peritoneum, possessing the capability of multipotential differentiation. These cells, when triggered by PD-related stimuli, can differentiate toward interstitial (myo)fibroblasts and endothelial cells, contributing to a pool of fibroblasts and angiogenesis during peritoneal dialysis. PD = peritoneal dialysis; MC = mesothelial cell; MPC = mesothelial progenitor cell; FB = fibroblast; EC = endothelial cell; MFB = myofibroblast.
Concluding Remarks
The arguments for and against the role of EMT in organ fibrosis are numerous (32,34–36,95,97,98,113,149). Most evidence for EMT found in PD patients or animal models can be summarized as morphological changes of MCs with concomitant loss of epithelial cells but with the acquisition of mesenchymal markers, evidenced through immunohistochemical analysis. However, few of those reported biomarkers are sensitive or specific enough to determine a real process of EMT in peritoneal fibrosis. Furthermore, it should be pointed out that MCs might progressively lose their normal phenotype under pathological conditions due to decomposition. It is not surprising that they could also lose some characteristics of the junction proteins at the same time, such as epithelial adherens junction and tight junction constituents. In addition, transient images (snapshot) by staining at a given time only reflect the static condition. Even if markers can be more specific, double-labeling approaches may also lead to confusing results based on the coexistence of epithelial and mesenchymal features in the submesothelial area, which could reflect an abnormal status of MCs, instead of a switch to a mesenchymal cell.
Recent findings indicate the existence of progenitor cells in mesothelium, breaking a new path to further explore the bioactivity of MCs in peritoneal fibrosis. Although significant, current evidence is not sufficient to demonstrate how mesothelial progenitor cells differentiate into fibroblast-like cells and the mechanisms that may be involved in regulating this process. A series of important questions should be addressed carefully to reveal the whole story. First of all, how to determine a MPC in adult peritoneum remains a key question that should be answered by means of a dynamic process, instead of a static snapshot, regarding aspects of aging or silence of MCs in PD patients. A solution to this issue will also help to identify and distinguish the processes of differentiation (progenitors) and de-differentiation (EMT) during peritoneal fibrosis, both of which seem to be supported by an abundance of studies. Moreover, a careful evaluation of how complex influences from PD therapeutics may affect the final differentiation of mesothelial progenitor cells is necessary, as progenitor cells may progress down various differentiation pathways according to biological and mechanical stimuli, like growth factors, cytokines, cellular crosstalks, pH, osmolarity, and so on.
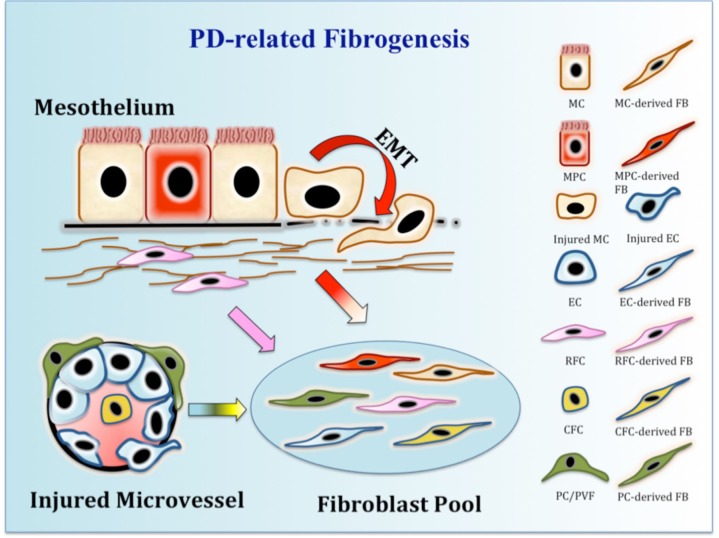
In conclusion, we are still at the first step in a long process to understand the biological role of MCs in peritoneal fibrosis. In considering the origin of fibroblasts during peritoneal fibrogenesis, it is necessary to define the fundamental concepts in question and further evaluate the limitation of published approaches used to address this problem. The transition of MCs to myofibroblasts in peritoneal fibrosis may be a matter of degree rather than a black and white question. Last but not least, other origins of myofibroblasts, from resident fibroblasts, endothelial cells, or circulating fibrocytes, should likewise be fully considered (Figure 3).
FIGURE 3 —
A schematic paradigm of multiple contributors to the origin of fibroblasts in PD-related peritoneal fibrosis. Upon exposure to PD fibrogenic stimuli, injured MC and EC undergo EMT and EndMT, respectively, transitioning into a subset of fibroblasts. A subpopulation of MPCs in peritoneum start differentiating toward fibroblasts and migrate into the submesothelial area. Local resident fibrocytes proliferate in response to stimuli, together with circulating fibrocytes that infiltrate into the interstitium through the microvascular system. In addition, pericytes (or perivascular fibrocytes) around injured microvessels proliferate and transform into interstitial fibroblasts. PD = peritoneal dialysis; EMT = epithelial-mesenchymal transition; MC = mesothelial cell; FB = fibroblast; MPC = mesothelial progenitor cell; EC = endothelial cell; RFC = resident fibrocyte; CFC = circulating fibrocyte; PC/PVF = pericyte/perivascular fibrocyte; EndMT = endothelial-to-mesenchymal transition.
Disclosures
The authors have declared that no conflict of interests exists.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81300566) to Dr. Guochun Chen.
REFERENCES
- 1. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bargman JM. Advances in peritoneal dialysis: a review. Semin Dial 2012; 25:545–9. [DOI] [PubMed] [Google Scholar]
- 3. Davies SJ. Peritoneal dialysis—current status and future challenges. Nat Rev Nephrol 2013; 9:399–408. [DOI] [PubMed] [Google Scholar]
- 4. Pletinck A, Vanholder R, Veys N, Van Biesen W. Protecting the peritoneal membrane: factors beyond peritoneal dialysis solutions. Nat Rev Nephrol 2012; 8:542–50. [DOI] [PubMed] [Google Scholar]
- 5. Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int 2003; 23:530–41. [PubMed] [Google Scholar]
- 6. Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 2010; 21:1077–85. [DOI] [PubMed] [Google Scholar]
- 7. Massague J. TGF-beta signalling in context. Nat Rev Mol Cell Biol 2012; 13:616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18:1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 2009; 5:319–28. [DOI] [PubMed] [Google Scholar]
- 10. Okada H, Inoue T, Kanno Y, Kobayashi T, Watanabe Y, Ban S, et al. Selective depletion of fibroblasts preserves morphology and the functional integrity of peritoneum in transgenic mice with peritoneal fibrosing syndrome. Kidney Int 2003; 64:1722–32. [DOI] [PubMed] [Google Scholar]
- 11. Cieslik KA, Trial J, Crawford JR, Taffet GE, Entman ML. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J Mol Cell Cardiol 2014; 70C:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 2010; 225:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballhause TM, Soldati R, Mertens PR. Sources of myofibroblasts in kidney fibrosis: all answers are correct, however to different extent! Int Urol Nephrol 2014; 46:659–64. [DOI] [PubMed] [Google Scholar]
- 14. Kokubo S, Sakai N, Furuichi K, Toyama T, Kitajima S, Okumura T, et al. Activation of p38 mitogen-activated protein kinase promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int 2012; 32:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mutsaers SE, Wilkosz S. Structure and function of mesothelial cells. Cancer Treat Res 2007; 134:1–19. [DOI] [PubMed] [Google Scholar]
- 16. Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol 2004; 36:9–16. [DOI] [PubMed] [Google Scholar]
- 17. Yung S, Chan TM. Mesothelial cells. Perit Dial Int 2007; 27(Suppl 2):S110–5. [PubMed] [Google Scholar]
- 18. Bird SD. Mesothelial primary cilia of peritoneal and other serosal surfaces. Cell Biol Int 2004; 28:151–9. [DOI] [PubMed] [Google Scholar]
- 19. Yung S, Chan TM. Intrinsic cells: mesothelial cells—central players in regulating inflammation and resolution. Perit Dial Int 2009; 29(Suppl 2):S21–7. [PubMed] [Google Scholar]
- 20. Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int 2009; 29:605–17. [PubMed] [Google Scholar]
- 21. Topley N, Jorres A, Luttmann W, Petersen MM, Lang MJ, Thierauch KH, et al. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int 1993; 43:226–33. [DOI] [PubMed] [Google Scholar]
- 22. Kato S, Yuzawa Y, Tsuboi N, Maruyama S, Morita Y, Matsuguchi T, et al. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J Am Soc Nephrol 2004; 15:1289–99. [PubMed] [Google Scholar]
- 23. Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, et al. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol 2007; 179:514–21. [DOI] [PubMed] [Google Scholar]
- 24. Lai KN, Tang SC, Leung JC. Mediators of inflammation and fibrosis. Perit Dial Int 2007; 27(Suppl 2):S65–71. [PubMed] [Google Scholar]
- 25. Herrick SE, Mutsaers SE. Mesothelial progenitor cells and their potential in tissue engineering. Int J Biochem Cell Biol 2004; 36:621–42. [DOI] [PubMed] [Google Scholar]
- 26. Mutsaers SE. Mesothelial cells: their structure, function and role in serosal repair. Respirology 2002; 7:171–91. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA 2013; 110:2324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Combet S, Ferrier ML, Van Landschoot M, Stoenoiu M, Moulin P, Miyata T, et al. Chronic uremia induces permeability changes, increased nitric oxide synthase expression, and structural modifications in the peritoneum. J Am Soc Nephrol 2001; 12:2146–57. [DOI] [PubMed] [Google Scholar]
- 29. Margetts PJ, Kolb M, Yu L, Hoff CM, Holmes CJ, Anthony DC, et al. Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am J Pathol 2002; 160:2285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112:1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest 2009; 119:1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871–90. [DOI] [PubMed] [Google Scholar]
- 33. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013; 342:1234850. [DOI] [PubMed] [Google Scholar]
- 34. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009; 19:156–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013; 19:1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 2003; 348:403–13. [DOI] [PubMed] [Google Scholar]
- 40. Cho JH, Do JY, Oh EJ, Ryu HM, Park SY, Kim SO, et al. Are ex vivo mesothelial cells representative of the in vivo transition from epithelial-to-mesenchymal cells in peritoneal membrane? Nephrol Dial Transplant 2012; 27:1768–79. [DOI] [PubMed] [Google Scholar]
- 41. Aguilera A, Yanez-Mo M, Selgas R, Sanchez-Madrid F, Lopez-Cabrera M. Epithelial-to-mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr Opin Investig Drugs 2005; 6:262–8. [PubMed] [Google Scholar]
- 42. Selgas R, Bajo A, Jimenez-Heffernan JA, Sanchez-Tomero JA, Del Peso G, Aguilera A, et al. Epithelial-to-mesenchymal transition of the mesothelial cell—its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant 2006; 21(Suppl 2):ii2–7. [DOI] [PubMed] [Google Scholar]
- 43. Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, Jimenez-Heffernan JA, et al. Epithelial-to-mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18:2004–13. [DOI] [PubMed] [Google Scholar]
- 44. Kim YL. Update on mechanisms of ultrafiltration failure. Perit Dial Int 2009; 29(Suppl 2):S123–7. [PubMed] [Google Scholar]
- 45. Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol 2012; 32:236–43. [DOI] [PubMed] [Google Scholar]
- 46. Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 2013; 25:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 2001; 276:17058–62. [DOI] [PubMed] [Google Scholar]
- 48. Margetts PJ, Oh KH, Kolb M. Transforming growth factor-beta: importance in long-term peritoneal membrane changes. Perit Dial Int 2005; 25(Suppl 3):S15–7. [PubMed] [Google Scholar]
- 49. Zhou Q, Yang M, Lan H, Yu X. miR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am J Pathol 2013; 183:808–19. [DOI] [PubMed] [Google Scholar]
- 50. Hirahara I, Ishibashi Y, Kaname S, Kusano E, Fujita T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant 2009; 24:437–47. [DOI] [PubMed] [Google Scholar]
- 51. Liu Q, Mao H, Nie J, Chen W, Yang Q, Dong X, et al. Transforming growth factor {beta}1 induces epithelial-mesenchymal transition by activating the JNK-Smad3 pathway in rat peritoneal mesothelial cells. Perit Dial Int 2008; 28(Suppl 3):S88–95. [PubMed] [Google Scholar]
- 52. Margetts PJ, Bonniaud P, Liu L, Hoff CM, Holmes CJ, West-Mays JA, et al. Transient overexpression of TGF-{beta}1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol 2005; 16:425–36. [DOI] [PubMed] [Google Scholar]
- 53. Lei P, Jiang Z, Zhu H, Li X, Su N, Yu X. Poly (ADP-ribose) polymerase-1 in high glucose-induced epithelial-mesenchymal transition during peritoneal fibrosis. Int J Mol Med 2012; 29:472–8. [DOI] [PubMed] [Google Scholar]
- 54. Bajo MA, Perez-Lozano ML, Albar-Vizcaino P, del Peso G, Castro MJ, Gonzalez-Mateo G, et al. Low-GDP peritoneal dialysis fluid (‘balance’) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol Dial Transplant 2011; 26:282–91. [DOI] [PubMed] [Google Scholar]
- 55. Yu MA, Shin KS, Kim JH, Kim YI, Chung SS, Park SH, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol 2009; 20:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao L, Sun L, Liu FY, Peng YM, Duan SB. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-beta1 in cultured human peritoneal mesothelial cells. Ther Apher Dial 2010; 14:27–34. [DOI] [PubMed] [Google Scholar]
- 57. Ito T, Yorioka N, Yamamoto M, Kataoka K, Yamakido M. Effect of glucose on intercellular junctions of cultured human peritoneal mesothelial cells. J Am Soc Nephrol 2000; 11:1969–79. [DOI] [PubMed] [Google Scholar]
- 58. Lee SH, Kang HY, Kim KS, Nam BY, Paeng J, Kim S, et al. The monocyte chemoattractant protein-1 (MCP-1)/CCR2 system is involved in peritoneal dialysis-related epithelial-mesenchymal transition of peritoneal mesothelial cells. Lab Invest 2012; 92:1698–711. [DOI] [PubMed] [Google Scholar]
- 59. Strippoli R, Benedicto I, Perez Lozano ML, Pellinen T, Sandoval P, Lopez-Cabrera M, et al. Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial-to-mesenchymal transition of mesothelial cells. PLoS One 2012; 7:e31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strippoli R, Benedicto I, Foronda M, Perez-Lozano ML, Sanchez-Perales S, Lopez-Cabrera M, et al. p38 maintains E-cadherin expression by modulating TAK1-NF-kappa b during epithelial-to-mesenchymal transition. J Cell Sci 2010; 123:4321–31. [DOI] [PubMed] [Google Scholar]
- 61. Ertilav M, Timur O, Hur E, Bozkurt D, Nar H, Kologlu T, et al. What does the dialysate level of matrix metalloproteinase 2 tell us? Adv Perit Dial 2011; 27:6–10. [PubMed] [Google Scholar]
- 62. Yang AH, Chen JY, Lin JK. Myofibroblastic conversion of mesothelial cells. Kidney Int 2003; 63:1530–9. [DOI] [PubMed] [Google Scholar]
- 63. Patel P, Sekiguchi Y, Oh KH, Patterson SE, Kolb MR, Margetts PJ. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int 2010; 77:319–28. [DOI] [PubMed] [Google Scholar]
- 64. Liu Q, Zhang Y, Mao H, Chen W, Luo N, Zhou Q, et al. A crosstalk between the Smad and JNK signaling in the TGF-β-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. PLoS One 2012; 7:e32009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang F, Liu H, Liu F, Peng Y, Chen M, Liu Y, et al. New insights into the pathogenesis and treatment of peritoneal fibrosis: a potential role of Wnt/β-catenin induced epithelial-to-mesenchymal transition and stem cells for therapy. Med Hypotheses 2013; 81:97–100. [DOI] [PubMed] [Google Scholar]
- 66. Luo L, Liu H, Dong Z, Sun L, Peng Y, Liu F. Small interfering RNA targeting ILK inhibits EMT in human peritoneal mesothelial cells through phosphorylation of GSK3β. Mol Med Rep 2014; 10:137–44. [DOI] [PubMed] [Google Scholar]
- 67. Strippoli R, Benedicto I, Perez Lozano ML, Cerezo A, Lopez-Cabrera M, del Pozo MA. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model Mech 2008; 1:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jang YH, Shin HS, Sun Choi H, Ryu ES, Jin Kim M, Ki Min S, et al. Effects of dexamethasone on the TGF-β1-induced epithelial-to-mesenchymal transition in human peritoneal mesothelial cells. Lab Invest 2013; 93:194–206. [DOI] [PubMed] [Google Scholar]
- 69. Del Peso G, Jimenez-Heffernan JA, Bajo MA, Aroeira LS, Aguilera A, Fernandez-Perpen A, et al. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int (Suppl) 2008:S26–33. [DOI] [PubMed] [Google Scholar]
- 70. Jimenez-Heffernan JA, Aguilera A, Aroeira LS, Lara-Pezzi E, Bajo MA, del Peso G, et al. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch 2004; 444:247–56. [DOI] [PubMed] [Google Scholar]
- 71. Do JY, Kim YL, Park JW, Cho KH, Kim TW, Yoon KW, et al. The effect of low glucose degradation product dialysis solution on epithelial-to-mesenchymal transition in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 2005; 25(Suppl 3):S22–5. [PubMed] [Google Scholar]
- 72. Aroeira LS, Aguilera A, Selgas R, Ramirez-Huesca M, Perez-Lozano ML, Cirugeda A, et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am J Kidney Dis 2005; 46:938–48. [DOI] [PubMed] [Google Scholar]
- 73. Oh EJ, Ryu HM, Choi SY, Yook JM, Kim CD, Park SH, et al. Impact of low glucose degradation product bicarbonate/lactate-buffered dialysis solution on the epithelial-mesenchymal transition of peritoneum. Am J Nephrol 2010; 31:58–67. [DOI] [PubMed] [Google Scholar]
- 74. Pletinck A, Consoli C, Van Landschoot M, Steppan S, Topley N, Passlick-Deetjen J, et al. Salt intake induces epithelial-to-mesenchymal transition of the peritoneal membrane in rats. Nephrol Dial Transplant 2010; 25:1688–96. [DOI] [PubMed] [Google Scholar]
- 75. Sandoval P, Loureiro J, Gonzalez-Mateo G, Perez-Lozano ML, Maldonado-Rodriguez A, Sanchez-Tomero JA, et al. PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Lab Invest 2010; 90:1517–32. [DOI] [PubMed] [Google Scholar]
- 76. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9:964–8. [DOI] [PubMed] [Google Scholar]
- 77. Loureiro J, Schilte M, Aguilera A, Albar-Vizcaino P, Ramirez-Huesca M, Perez-Lozano ML, et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol Dial Transplant 2010; 25:1098–108. [DOI] [PubMed] [Google Scholar]
- 78. Nakamura S, Niwa T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J Am Soc Nephrol 2005; 16:144–50. [DOI] [PubMed] [Google Scholar]
- 79. Loureiro J, Aguilera A, Selgas R, Sandoval P, Albar-Vizcaino P, Perez-Lozano ML, et al. Blocking TGF-beta1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 2011; 22:1682–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 2010; 21:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 2012; 4:121ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 2011; 22:1462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int 2013; 84:1129–44. [DOI] [PubMed] [Google Scholar]
- 84. Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 2011; 7:286–94. [DOI] [PubMed] [Google Scholar]
- 85. Patel V, Noureddine L. MicroRNAs and fibrosis. Curr Opin Nephrol Hypertens 2012; 21:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang K, Zhang H, Zhou X, Tang WB, Xiao L, Liu YH, et al. miRNA589 regulates epithelial-mesenchymal transition in human peritoneal mesothelial cells. J Biomed Biotechnol 2012; 2012:673096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo F, Gopaul DN, van Duyne GD. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature 1997; 389:40–6. [DOI] [PubMed] [Google Scholar]
- 88. Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis 2000; 26:99–109. [PubMed] [Google Scholar]
- 89. Humphreys BD, DiRocco DP. Lineage-tracing methods and the kidney. Kidney Int 2014; 86:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002; 110:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 2007; 282:23337–47. [DOI] [PubMed] [Google Scholar]
- 92. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006; 103:13180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial-to-mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 2010; 285:20202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol 2004; 164:651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res 2012; 347:103–16. [DOI] [PubMed] [Google Scholar]
- 96. Hills CE, Squires PE. The role of TGF-β and epithelial-to-mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev 2011; 22:131–9. [DOI] [PubMed] [Google Scholar]
- 97. Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis—evidence for and against. Int J Exp Pathol 2011; 92:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int 2011; 80:41–50. [DOI] [PubMed] [Google Scholar]
- 99. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010; 21:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 2006; 290:F20–34. [DOI] [PubMed] [Google Scholar]
- 101. Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 2006; 17:19–27. [DOI] [PubMed] [Google Scholar]
- 102. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995; 130:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, et al. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest 2007; 117:482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 2003; 162:1937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McMorrow T, Gaffney MM, Slattery C, Campbell E, Ryan MP. Cyclosporine A induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. Nephrol Dial Transplant 2005; 20:2215–25. [DOI] [PubMed] [Google Scholar]
- 106. Ivanova L, Butt MJ, Matsell DG. Mesenchymal transition in kidney collecting duct epithelial cells. Am J Physiol Renal Physiol 2008; 294:F1238–48. [DOI] [PubMed] [Google Scholar]
- 107. Qi W, Twigg S, Chen X, Polhill TS, Poronnik P, Gilbert RE, et al. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol 2005; 288:F800–9. [DOI] [PubMed] [Google Scholar]
- 108. Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, et al. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis 2001; 38:761–9. [DOI] [PubMed] [Google Scholar]
- 109. Rastaldi MP, Ferrario F, Giardino L, Dell’Antonio G, Grillo C, Grillo P, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 2002; 62:137–46. [DOI] [PubMed] [Google Scholar]
- 110. Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, et al. Glomerular epithelial-mesenchymal transdifferentiation in pauciimmune crescentic glomerulonephritis. Nephrol Dial Transplant 2003; 18:1777–84. [DOI] [PubMed] [Google Scholar]
- 111. Carvajal G, Droguett A, Burgos ME, Aros C, Ardiles L, Flores C, et al. Gremlin: a novel mediator of epithelial mesenchymal transition and fibrosis in chronic allograft nephropathy. Transplant Proc 2008; 40:734–9. [DOI] [PubMed] [Google Scholar]
- 112. Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 2007; 71:846–54. [DOI] [PubMed] [Google Scholar]
- 113. Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 2011; 121:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/s100a4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol 2005; 123:335–46. [DOI] [PubMed] [Google Scholar]
- 115. Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol 2008; 130:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, et al. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int 2005; 68:2621–8. [DOI] [PubMed] [Google Scholar]
- 117. Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol 2010; 176:1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Grone HJ, Weber K, Grone E, Helmchen U, Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol 1987; 129:1–8. [PMC free article] [PubMed] [Google Scholar]
- 119. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 1994; 93:2175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010; 176:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 2008; 173:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 2010; 177:632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 2013; 19:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 2014; 124:2299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen YT, Chang YT, Pan SY, Chou YH, Chang FC, Yeh PY, et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J Am Soc Nephrol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011; 53:983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 2011; 178:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Patel P, West-Mays J, Kolb M, Rodrigues JC, Hoff CM, Margetts PJ. Platelet derived growth factor B and epithelial mesenchymal transition of peritoneal mesothelial cells. Matrix Biol 2010; 29:97–106. [DOI] [PubMed] [Google Scholar]
- 129. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell 2012; 10:709–16. [DOI] [PubMed] [Google Scholar]
- 130. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 2013; 31:285–316. [DOI] [PubMed] [Google Scholar]
- 131. Bolen JW, Hammar SP, McNutt MA. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol 1986; 10:34–47. [DOI] [PubMed] [Google Scholar]
- 132. Bolen JW, Hammar SP, McNutt MA. Serosal tissue: reactive tissue as a model for understanding mesotheliomas. Ultrastruct Pathol 1987; 11:251–62. [DOI] [PubMed] [Google Scholar]
- 133. Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res 2003; 92:525–31. [DOI] [PubMed] [Google Scholar]
- 134. Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol 2002; 46:1005–13. [PubMed] [Google Scholar]
- 135. Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol 2004; 276:43–57. [DOI] [PubMed] [Google Scholar]
- 136. van Tuyn J, Atsma DE, Winter EM, van der Velde-van Dijke I, Pijnappels DA, Bax NA, et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 2007; 25:271–8. [DOI] [PubMed] [Google Scholar]
- 137. Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008; 454:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008; 454:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007; 445:177–82. [DOI] [PubMed] [Google Scholar]
- 140. Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011; 474:640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gittenberger-de Groot AC, Winter EM, Poelmann RE. Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia. J Cell Mol Med 2010; 14:1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Limana F, Capogrossi MC, Germani A. The epicardium in cardiac repair: from the stem cell view. Pharmacol Ther 2011; 129:82–96. [DOI] [PubMed] [Google Scholar]
- 143. Gittenberger-de Groot AC, Winter EM, Bartelings MM, Goumans MJ, DeRuiter MC, Poelmann RE. The arterial and cardiac epicardium in development, disease and repair. Differentiation 2012; 84:41–53. [DOI] [PubMed] [Google Scholar]
- 144. Smart N, Dube KN, Riley PR. Epicardial progenitor cells in cardiac regeneration and neovascularisation. Vascul Pharmacol 2013; 58:164–73. [DOI] [PubMed] [Google Scholar]
- 145. Riley PR, Smart N. Thymosin beta4 induces epicardium-derived neovascularization in the adult heart. Biochem Soc Trans 2009; 37:1218–20. [DOI] [PubMed] [Google Scholar]
- 146. Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Riegler J, et al. Myocardial regeneration: expanding the repertoire of thymosin β4 in the ischemic heart. Ann N Y Acad Sci 2012; 1269:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen S, Shimoda M, Chen J, Grayburn PA. Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery. Gene Ther 2013; 20:225–33. [DOI] [PubMed] [Google Scholar]
- 148. Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr., Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 2012; 14:1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol 2010; 21:1247–53. [DOI] [PubMed] [Google Scholar]