Abstract
Kocuria species are found in the environment and on human skin. These micro-organisms are generally considered to be nonpathogenic saprophytes, rarely causing infection. However, the peritoneum has been reported to be a site of Kocuria infection. We reviewed all cases of peritonitis in peritoneal dialysis (PD) patients caused by Kocuria species that were reported in the worldwide literature. In total, 12 episodes of Kocuria species peritonitis have been reported in 9 PD patients. The median age of the patients was 62 years (range: 8 – 78 years). In the reported episodes, 4 different Kocuria species were isolated, with K. varians being the predominant species (41.7%). The most common initial symptom was abdominal pain (83.3%), followed by turbid effluent (75%) and fever (33.3%). Intraperitoneal first-generation cephalosporins and glycopeptides were the most-used antibiotics, with first-generation cephalosporins being more often preferred as first-line therapy. The median duration of treatment was 14 days, and in 2 episodes, the Tenckhoff catheter was removed. Although Kocuria peritonitis in PD patients is rare, it should be promptly treated because relapses can occur, especially with K. varians episodes.
Keywords: Kocuria, peritonitis, review
Kocuria species are gram-positive, coagulase-negative, coccoid Actinobacteria that belong to the family Micrococcaceae (1). Organisms of the genus Kocuria can be found as part of the normal skin and oral flora of humans, but are ubiquitous in the environment, inhabiting soil and several other ecologic niches (2). Only sporadic reports about Kocuria species are found in the literature dealing with infections. In most of them, patients were compromised because of serious underlying conditions, including hematologic malignancies, solid tumors, or metabolic disorders (3). Bacteremia, cholecystitis, and peritonitis are some of the infections that can be caused by these rare pathogens (3,4). The isolation and identification of Kocuria species by standard microbiologic methods has been problematic because of the similarity of this organism to other commonly reported pathogens (2).
We recently treated an 8-year-old immunocompetent girl who experienced peritonitis attributable to K. rosea while receiving automated peritoneal dialysis (PD) treatment because of dysplastic kidneys, and who experienced a favorable outcome (5). Here, we review all cases recorded in the worldwide literature of peritonitis attributable to Kocuria species in PD patients.
Methods
The Medline and Google Scholar databases were searched using the keywords “peritonitis,” “peritoneal dialysis,” and “Kocuria” in any available language. All articles found were reviewed, and a master spreadsheet was constructed. In addition, the reference list for each article was examined to verify that all published cases had been collected for the review.
A predefined set of criteria, based on the definition of peritonitis in PD patients, were used to screen the publications for eligibility for the review. Specifically, cloudy effluent in conjunction with laboratory confirmation of an increased effluent cell count exceeding 100/mm3, with at least half being polymorphonuclear neutrophils, and a positive culture are the criteria establishing a diagnosis of bacterial peritonitis in PD patients (6,7).
When a peritonitis episode occurs within 4 weeks of completion of therapy for a prior episode, but involving a different organism, that episode can be characterized as recurrent peritonitis. When an episode occurs within 4 weeks of completion of therapy for a prior episode and involves the same organism or is classified as sterile, that episode is called relapsing peritonitis. In addition, “repeat peritonitis” is characterized as an episode that occurs more than 4 weeks after completion of therapy for an earlier episode with the same organism. Finally, refractory peritonitis is the failure of the effluent to clear after 5 days of appropriate antibiotic treatment (6,7).
Results
In PD patients, 12 cases of peritonitis attributable to Kocuria species have been recorded in the Medline and Google Scholar databases to date (Tables 1 and 2). One patient experienced 3 episodes of relapsing peritonitis attributable to K. varians; another had 2 episodes of relapsing peritonitis attributable to the same Kocuria species. In total, 9 PD patients with end-stage renal disease were reported to experience peritonitis caused by Kocuria species (4,5,8–13). In all the Kocuria peritonitis episodes, no ultrafiltration problems were reported.
TABLE 1.
Characteristics of Peritoneal Dialysis (PD) Patients and Kocuria Species Peritonitis Episodes
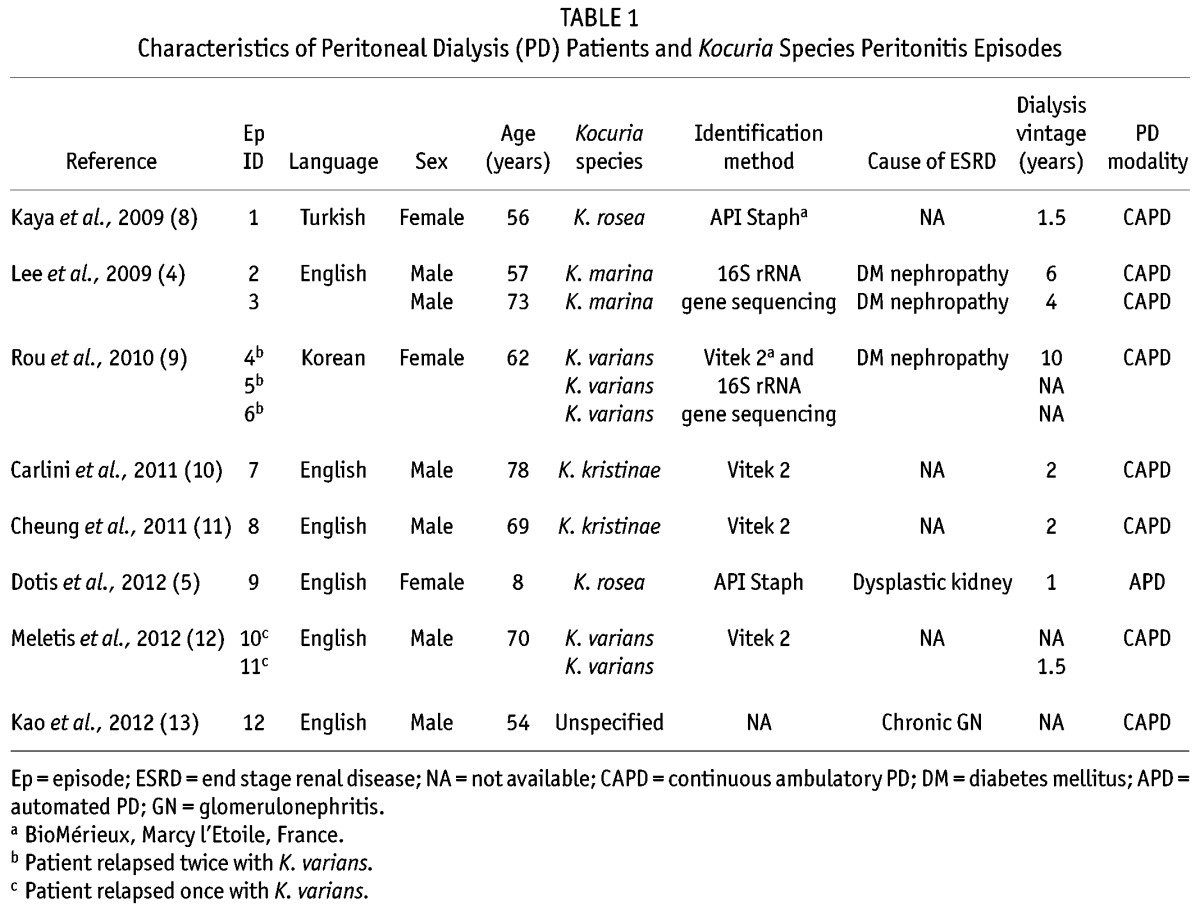
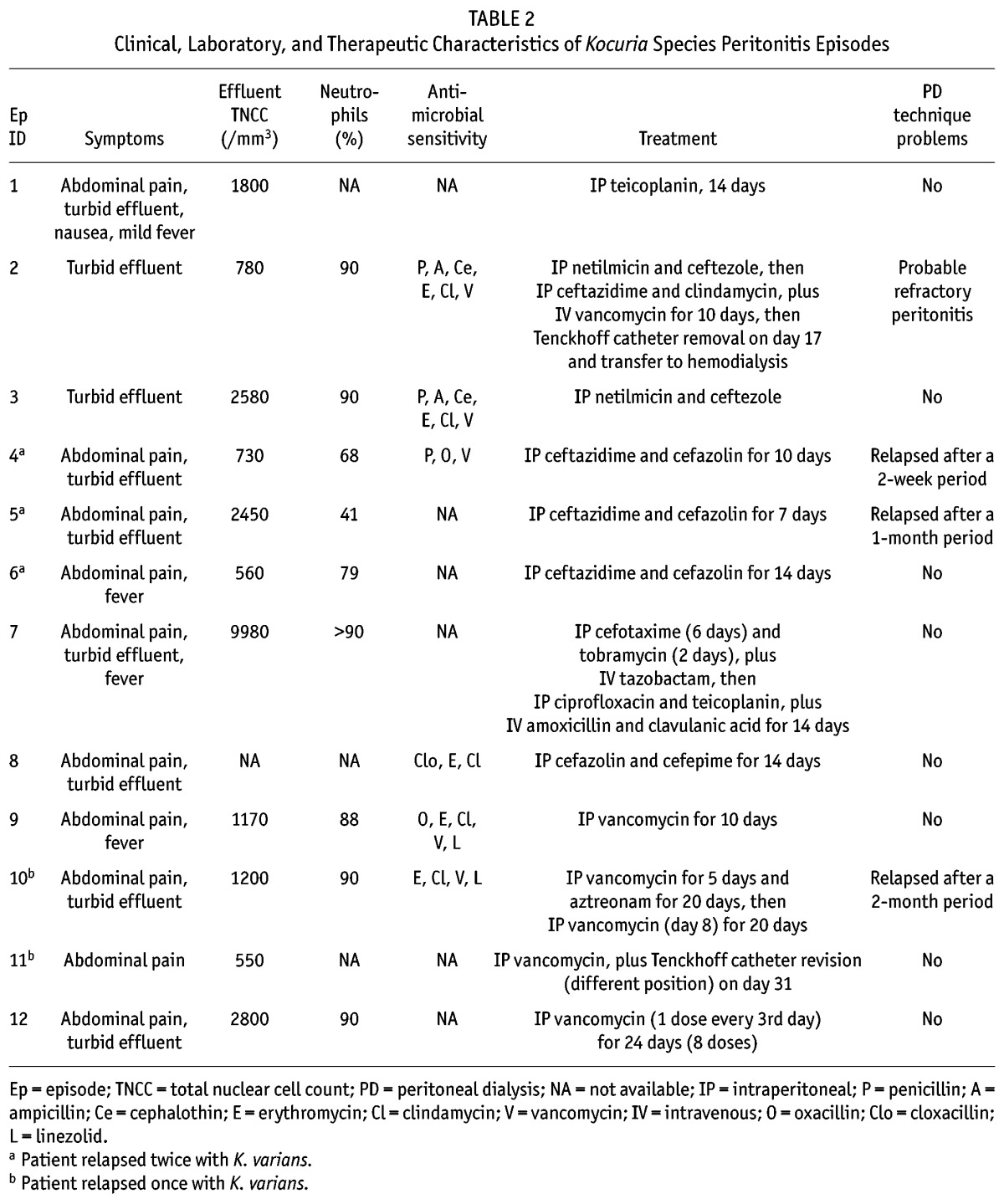
TABLE 2.
Clinical, Laboratory, and Therapeutic Characteristics of Kocuria Species Peritonitis Episodes
The median age of the patients (6 male, 3 female) when their first episode of Kocuria peritonitis occurred was 62 years (range: 8 – 78 years; Table 1). In the reported episodes, 4 different Kocuria species were found, including K. varians in 5 of the 12 episodes (41.7%) and K. rosea, K. marina, and K. kristinae in 2 episodes each (16.7%). In 1 episode, the Kocuria species was unspecified. The preferred identification method was the Vitek 2 system [bioMérieux, Marcy l’Etoile, France (4 of 12 episodes, 33.3%)], followed by the 16S rRNA gene sequencing method (25%) and the API Staph system [bioMérieux (16.7%)]. In 1 case, the Vitek 2 system and 16S rRNA gene sequencing were both used to identify a K. varians peritonitis.
In patients in whom the cause of end-stage renal disease was available, diabetic nephropathy was found to be the most common reason for renal replacement therapy being started. Except for the pediatric patient (who was receiving automated PD), all the other patients were being preferentially treated with continuous ambulatory PD (median dialysis vintage: 2 years; range: 1 – 10 years).
The most common initial symptom was abdominal pain (10 of the 12 episodes, 83.3%), followed by turbid effluent (75%) and fever (33.3%). In the publications reviewed, the mean total effluent white blood cells was 2236 ± 2700/mm3, with a neutrophil percentage of approximately 80%. In episode 7, the standard deviation for white blood cells was exceeded by a massively increased number of nuclear cells (9980/mm3).
Intraperitoneal (IP) first-generation cephalosporins—including cefazolin (4 of 12 episodes, 33.3%) and ceftezole (16.7%)—and glycopeptides—including vancomycin (41.7%) and teicoplanin (16.7%)—were the most-used antibiotics. However, first-generation cephalosporins were more often preferred as first-line IP therapy; glycopeptides were preferred as either first- or second-line IP therapy. The median duration of appropriate treatment was 14 days, ranging between 7 and 20 days, with the exception of episode 12. In that episode, the patient was treated with IP vancomycin given as a single dose every 3 days for a total of 24 days (total of 8 doses given).
In 2 episodes, the Tenckhoff catheter was removed. In one episode, caused by K. marina, the cause for removal was a patient response that remained unsatisfactory despite appropriate IP treatment, suggesting a probable refractory peritonitis (4). In the other episode, Tenckhoff catheter removal was a result of relapsing K. varians peritonitis and catheter colonization (12).
Discussion
Named after a Slovakian microbiologist, Miroslav Kocur, the genus Kocuria was split from the genus Micrococcus, the members of which had been known over the years by the vernacular “micrococci,” which overlaps the genus Arthrobacter. In a re-evaluation of this complex cluster of Arthrobacter and Micrococcus, a new genus Kocuria was proposed (1). Based on phylogenetic and chemotaxonomic analysis, the genus Micrococcus was divided into the genera Kocuria, Micrococcus, Kytococcus, Nesterenkonia, and Dermacoccus. A new rearrangement separates them into two families Micrococcaceae and Dermacoccaceae, both belonging to the suborder Micrococcineae. To date, 19 Kocuria species have been described (1,2,14).
Peritonitis caused by Kocuria species requires laboratory testing for diagnosis, and isolation of the organism from peritoneal effluent samples constitutes the confirmatory test for the disease. However, correct identification of Kocuria species by commercial systems is sometimes problematic, because systems such as Vitek 2 and API Staph do not include all Kocuria species in their database (4). For that reason, 16S rRNA gene sequencing can confirm and establish the identification of the various Kocuria species; unfortunately, however, this technique is not available in all microbiology laboratories. When 16S rRNA gene sequencing is unavailable, double confirmation with both Vitek 2 and API Staph can be a reasonable option.
Infections in humans caused by Kocuria species have included bacteremia related to a central venous catheter, cholecystitis, brain abscess, endocarditis, synovitis, periarticular bursitis, and of course, peritonitis in PD patients. Catheter-related bacteremia and PD-related peritonitis have been hypothesized to be related to an ability of Kocuria to produce biofilm, but that potential was not established until recently. A study using both culture-dependent and -independent methods, including 16S rRNA gene clone libraries and pyrosequencing, recently showed that K. varians can be found in the flora of endotracheal tube biofilm (15). That finding accords with the known recommendation to remove venous catheters in the presence of bacteremia caused by certain Kocuria species (including K. kristinae, K. rhizophila, and K. rosea), which probably indicates biofilm formation (16–18). The ability of K. varians to form biofilm can partly explain the increased frequency of relapsing peritonitis in the cases involving that species compared with other Kocuria species. Nevertheless, further evaluation of the ability of the various Kocuria species to produce biofilm on certain medical devices, such as Tenckhoff catheters, is needed. A suggestion to remove the Tenckhoff catheter in peritonitis caused by Kocuria species cannot yet be established, but some evidence suggests removal in cases of K. varians. However, the ability of the other Kocuria species to produce biofilm is unknown, and considering the fact that the Tenckhoff catheter was removed in only 2 reported cases involving refractory and relapsing peritonitis, any recommendation for removal is currently problematic.
Current empiric regimens for peritonitis caused by gram-positive pathogens in PD patients most commonly recommend IP use of a first-generation cephalosporin, such as cefazolin or ceftezole. However, because of a high rate of methicillin-resistant organisms, many programs often use IP glycopeptides such as vancomycin or teicoplanin for gram-positive coverage. The reported Kocuria-species peritonitis episodes were caused by pathogens susceptible to both first-generation cephalosporins and glycopeptides; no resistance to those antibiotic categories seems yet to have appeared. However, in the full range of infections caused by Kocuria species, variable susceptibility to beta-lactam drugs has been observed. Notably, no cases of resistance to glycopeptides, streptogramins, fusidic acid, rifampicin, or linezolid have been reported so far (2).
Our study found that the median duration of IP treatment was 14 days, which accords with guidelines in PD patients for the treatment of peritonitis caused by common and sensitive gram-positive pathogens (6,7). Nevertheless, the patient who experienced 3 relapsing peritonitis episodes with K. varians received IP cefazolin for 10 days for the first episode, IP cefazolin for 7 days after peritonitis relapse, and IP cefazolin for 14 days after the second relapse, which is the formal suggested regimen (4). Recommendations for avoidance of Kocuria-species peritonitis follows the general consensus guidelines for the prevention of catheter-related infections and peritonitis in pediatric patients receiving PD (7).
This review is a first attempt to collect and review all published Kocuria peritonitis cases in PD patients; it could potentially be a useful tool for future studies. However, taking in account that this is a retrospective multi-source case review study, limitations in the available data might affect the ability to draw specific conclusions, a problem that is compounded by the relatively small number of reported cases.
Summary
Our study addresses the demographics; frequencies and species panel; and the clinical, laboratory, and therapeutic characteristics of peritonitis caused by Kocuria species in PD patients. More such cases are expected to occur in upcoming years because of major advances in the diagnosis of these infections. These organisms are usually considered to be contaminants, but they can cause severe disease and symptomatic peritonitis. The effect on PD patients of peritonitis caused by Kocuria species seems to be minimal, but in most cases in which treatment failed, a relapsing peritonitis was caused by K. varians. Therefore, although Kocuria peritonitis in PD patients is rare, it should be promptly treated.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Stackebrandt E, Koch C, Gvozdiak O, Schumann P. Taxonomic dissection of the genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int J Syst Bacteriol 1995; 45:682–92. [DOI] [PubMed] [Google Scholar]
- 2. Savini V, Catavitello C, Masciarelli G, Astolfi D, Balbinot A, Bianco A, et al. Drug sensitivity and clinical impact of members of the genus Kocuria. J Med Microbiol 2010; 59:1395–402. [DOI] [PubMed] [Google Scholar]
- 3. Dunn R, Bares S, David MZ. Central venous catheter-related bacteremia caused by Kocuria kristinae: case report and review of the literature. Ann Clin Microbiol Antimicrob 2011; 10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JY, Kim SH, Jeong HS, Oh SH, Kim HR, Kim YH, et al. Two cases of peritonitis caused by Kocuria marina in patients undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol 2009; 47:3376–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dotis J, Printza N, Papachristou F. Peritonitis attributable to Kocuria rosea in a pediatric peritoneal dialysis patient. Perit Dial Int 2012; 32:577–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. on behalf of the International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 7. Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, et al. Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 2012; 32(Suppl 2):S32–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaya KE, Kurtoglu Y, Cesur S, Bulut C, Kinikli S, Irmak H, et al. Peritonitis due to Kocuria rosea in a continuous ambulatory peritoneal dialysis case [Turkish]. Mikrobiyol Bul 2009; 43:335–7. [PubMed] [Google Scholar]
- 9. Rou WS, Lee HK, Kwak YG, Han SY, Han KH. Relapsing Kocuria varians peritonitis in a continuous ambulatory peritoneal dialysis patient. Korean J Nephrol 2010; 29:535–8. [Google Scholar]
- 10. Carlini A, Mattei R, Lucarotti I, Bartelloni A, Rosati A. Kocuria kristinae: an unusual cause of acute peritoneal dialysis-related infection. Perit Dial Int 2011; 31:105–7. [DOI] [PubMed] [Google Scholar]
- 11. Cheung C, Cheng N, Chau C, Li CS. An unusual organism for CAPD related peritonitis: Kocuria kristinae. Perit Dial Int 2011; 31:107–8. [DOI] [PubMed] [Google Scholar]
- 12. Meletis G, Gogou V, Palamouti M, Spiropoulos P, Xanthopoulou K, Tantou P, et al. Catheter-related relapsing peritonitis due to Kocuria varians in a patient undergoing continuous ambulatory peritoneal dialysis. Nefrologia 2012; 32:541–2. [DOI] [PubMed] [Google Scholar]
- 13. Kao CC, Chiang CK, Huang JW. Micrococcus species-related peritonitis in patients receiving peritoneal dialysis. Int Urol Nephrol 2012;:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Takarada H, Sekine M, Kosugi H, Matsuo Y, Fujisawa T, Omata S, et al. Complete genome sequence of the soil actinomycete Kocuria rhizophila. J Bacteriol 2008; 190:4139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vandecandelaere I, Matthijs N, Van Nieuwerburgh F, Deforce D, Vosters P, De Bus L, et al. Assessment of microbial diversity in biofilms recovered from endotracheal tubes using culture dependent and independent approaches. PLoS One 2012; 7:e38401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altuntas F, Yildiz O, Eser B, Gündogan K, Sumerkan B, Cetin M. Catheter-related bacteremia due to Kocuria rosea in a patient undergoing peripheral blood stem cell transplantation. BMC Infect Dis 2004; 4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai CC, Wang JY, Lin SH, Tan CK, Wang CY, Liao CH, et al. Catheter-related bacteraemia and infective endocarditis caused by Kocuria species. Clin Microbiol Infect 2011; 17:190–2. [DOI] [PubMed] [Google Scholar]
- 18. Moissenet D, Becker K, Mérens A, Ferroni A, Dubern B, Vu-Thien H. Persistent bloodstream infection with Kocuria rhizophila related to a damaged central catheter. J Clin Microbiol 2012; 50:1495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


