Abstract
♦ Background: The utility of local and systemic interleukin 6 (IL-6) as a prognostic marker in incident peritoneal dialysis (PD) patients remains to be fully defined. The present study aimed to explore the capacity of systemic IL-6 concentrations to predict cardiovascular events (CVEs) and mortality in PD patients, and to evaluate the influence of neutral-pH PD solutions low in glucose degradation products (GDPs) on systemic IL-6.
♦ Methods: The study included 175 incident participants from the balANZ trial with at least one stored serum sample. A composite CVE score was used as the primary clinical outcome measure. Multilevel linear regression and Poisson regression models were fitted to describe, respectively, the trend of serum IL-6 over time and its ability to predict composite CVE.
♦ Results: A significant increase in serum IL-6 from baseline to 24 months was observed in the study population (mean difference: 1.68 pg/mL; p = 0.006). The type of PD solution received by patients exerted no significant effect on serum IL-6 (p = 0.12). Composite CVE was significantly and independently associated with baseline serum IL-6 (incidence rate ratio per picogram per milliliter: 1.06; 95% confidence interval: 1.02 to 1.10; p = 0.003).
♦ Conclusions: Baseline serum IL-6 was a significant independent predictor of composite CVE. Serum IL-6 concentrations increased with increasing PD duration and were not significantly modified with the use of biocompatible fluid over the study period. The present study is the first to link systemic IL-6 concentrations with CVE outcomes in incident PD patients.
Keywords: Biocompatibility, cardiovascular events, glucose degradation products, interleukin 6, mortality
Premature cardiovascular events (CVEs) are a leading cause of mortality in patients with end-stage kidney disease receiving peritoneal dialysis (PD) (1). The excess disease burden is attributed to the presence of nontraditional risk factors such as inflammation, which has been shown to promote proliferation and infiltration of inflammatory cells into the tunica intima of small arteries, leading to development of atherosclerosis and stenosis (2).
Some of the important causes of systemic inflammation in PD include loss of residual renal function (RRF), with lowered clearance of proinflammatory cytokines; increased local inflammation as a consequence of infectious episodes (for example, peritonitis); and repeated exposure to conventional PD solutions, with resultant peritoneal injury (3). The use of PD solutions that are more biocompatible, with a neutral pH and reduced levels of glucose degradation products (GDPs), might lower the systemic inflammatory burden by improving peritoneal membrane morphology (4,5); better preserving cellular host defenses (6), leading to a milder physician-rated severity of peritonitis (7); and better preserving RRF (8).
Interleukin 6 (IL-6), a pleiotropic immunomodulatory cytokine secreted by a variety of cell types, plays a critical role in many innate and acquired inflammatory processes (9). Many of the immune effects of IL-6 are achieved by engagement with its soluble receptor, which increases the spectrum of activity to cells that do not display the cognate IL-6 receptor (9). Dysregulation of IL-6 signaling has been implicated in a variety of chronic disease pathologies and in immune and inflammatory diseases (9). Interleukin 6 is also a critical mediator in regulating hepatic synthesis of acute-phase proteins such as C-reactive protein (CRP). A common G/C polymorphism at position -174 in the promoter region of the IL-6 gene that results in IL-6 overproduction has been associated with an increased risk of CVE in healthy men (10) and all-cause mortality in PD patients (11). It has also been identified as a strong and independent predictor of clinically evident CVE in incident dialysis patients (12). Further, compared with other proinflammatory cytokines (and with CRP), IL-6 is recognized as the best prognostic marker of all-cause mortality in prevalent dialysis patients (13).
The aims of the present study were to describe the trend of serum IL-6 in a well-defined cohort of incident PD patients and to explore the utility of IL-6 as a predictor of CVE and mortality in that patient group. In addition, we sought to assess whether the use of PD solutions that are more biocompatible might affect systemic IL-6 concentrations and CVE outcomes (14).
Methods
Study Population
Data were obtained from the participants in the balANZ trial (14). A detailed description of the study design and methodology has previously been published (15), as have the results of the primary and secondary analyses (7,14,16). The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12606000044527). The study protocol was approved by ethics committees in all participating centers. All patients provided written informed consent before trial participation, including consent to biomarker studies using stored samples. Incident adult PD patients who had both a residual measured glomerular filtration rate (GFR) of 5 mL/min/1.73 m2 or more and a measured daily urine volume of 400 mL or more at enrollment were included in the study. Pregnant or breastfeeding patients, individuals expected to die within 12 months, patients participating in trials targeting RRF in PD, and patients with a significant cancer history in the preceding 5 years, acute infection at enrollment, contraindications to PD, any physical or mental disorder that appreciably hampered compliance to the study protocol, or a known or suspected allergy to the trial products or related products were excluded. Of the 185 participants in the balANZ trial, 175 who had at least one serum sample stored during trial participation were included in the present investigation.
Measurements of IL-6
Serum samples were collected at baseline and at the 6-, 12-, 18-, and 24-month visits. Serum was immediately stored at –20°C or –80°C locally and transported frozen to a central storage facility and kept in a –80°C freezer. Samples were thawed once only during the aliquoting process before analysis. Serum IL-6 was measured by an electrochemiluminescence immunoassay technique using the manufacturer’s protocols after samples had been centrifuged at 500g for 5 minutes. The 96-well plates used for measuring IL-6 were analyzed on a Sector Imager 6000 (Meso Scale Discovery, Gaithersburg, MD, USA), whose lower level of detection was 0.7 pg/mL. Samples and standards were analyzed in duplicate with a maximum tolerated coefficient of variation of 20%. No inter-assay coefficient of variation was determined, because all samples from an individual were run at the same time to minimize between-assay variability.
Clinical Outcome Measures
The primary clinical outcome measure was a composite of all episodes of CVE that occurred, including hospitalization for myocardial ischemia or infarction, sustained atrial or ventricular arrhythmia, transient ischemic attack, and ischemic or hemorrhagic cerebrovascular accident diagnosed according to standard clinical criteria; fatal cardiovascular event determined by an attending physician; and peripheral vascular disease, defined as gangrene with or without amputation, ischemic ulceration, or a need for revascularization. A secondary clinical outcome measure was all-cause mortality during the study period.
Statistical Analysis
Results are expressed as frequencies and percentages for categorical variables, mean ± standard deviation for continuous normally distributed variables, and median with interquartile range for continuous non-normally distributed variables. Differences between groups on baseline characteristics were analyzed by chi-square test for categorical data, t-test for continuous normally distributed data, and Mann–Whitney U-test for continuous non-normally distributed data.
The overall trend in serum IL-6 over the follow-up period was analyzed by fitting a multilevel linear regression model to the log-transformed IL-6 data. Baseline IL-6 measurement and categorical time—that is, 6, 12, 18, or 24 months—were included as fixed effects, and random intercepts and slopes were added to allow for repeated measurements over time. To evaluate the differences between the two treatment groups with respect to IL-6, the PD solution type (biocompatible vs control) and the interaction between solution type and time were subsequently added to the model as fixed effects. If the interaction term was not statistically significant, the unconditional effect of PD solution type on serum IL-6 would be retained and the interaction term would be removed from the final model. Because of non-normal distribution, the IL-6 data were log transformed for the purpose of multilevel linear regression modeling.
Although the baseline IL-6 data was missing in only 2.9% of participants, substantial IL-6 data were missing overall (27.8%). As sensitivity analyses, the multilevel linear regression model was applied in the subgroup with complete IL-6 data (that is, in patients with 5 measurements: biocompatible, n = 34; control, n = 41) and the role of the loss of RRF (determined as GFR at the relevant month minus the baseline value) on IL-6 concentration over time was explored as a time-varying covariate.
To determine whether IL-6 predicted composite CVE, a multilevel Poisson regression model was fitted to the CVE counts. The counts of composite CVEs were recorded at 6-month intervals from baseline to 24 months; each participant therefore had up to 4 values for composite CVE. Baseline IL-6, clinically recognized risk factors of CVE (that is, age; ethnicity; body mass index; comorbidities such as diabetes mellitus, cardiovascular disease, dyslipidemia, gout, cerebrovascular disease, peripheral vascular disease; and baseline GFR), and the randomly assigned PD solution type were included as fixed effects in an initial full model. Variables with statistically nonsignificant effects were omitted from the final model. The fit of the final model was checked against the full model using the likelihood ratio test.
Interleukin 6 as a predictor of all-cause mortality was modeled using exact logistic regression. Because of a low event number (n = 17), a multivariable logistic regression model predicting all-cause mortality could not be fitted. Only the results from the univariable analyses of baseline IL-6 (continuous and in tertiles) and the average of IL-6 for each subject are therefore presented. Data were analyzed using the software package Stata/SE 12.0 (StataCorp LP, College Station, TX, USA), with p < 0.05 considered to represent a statistically significant result.
Results
Patient Characteristics
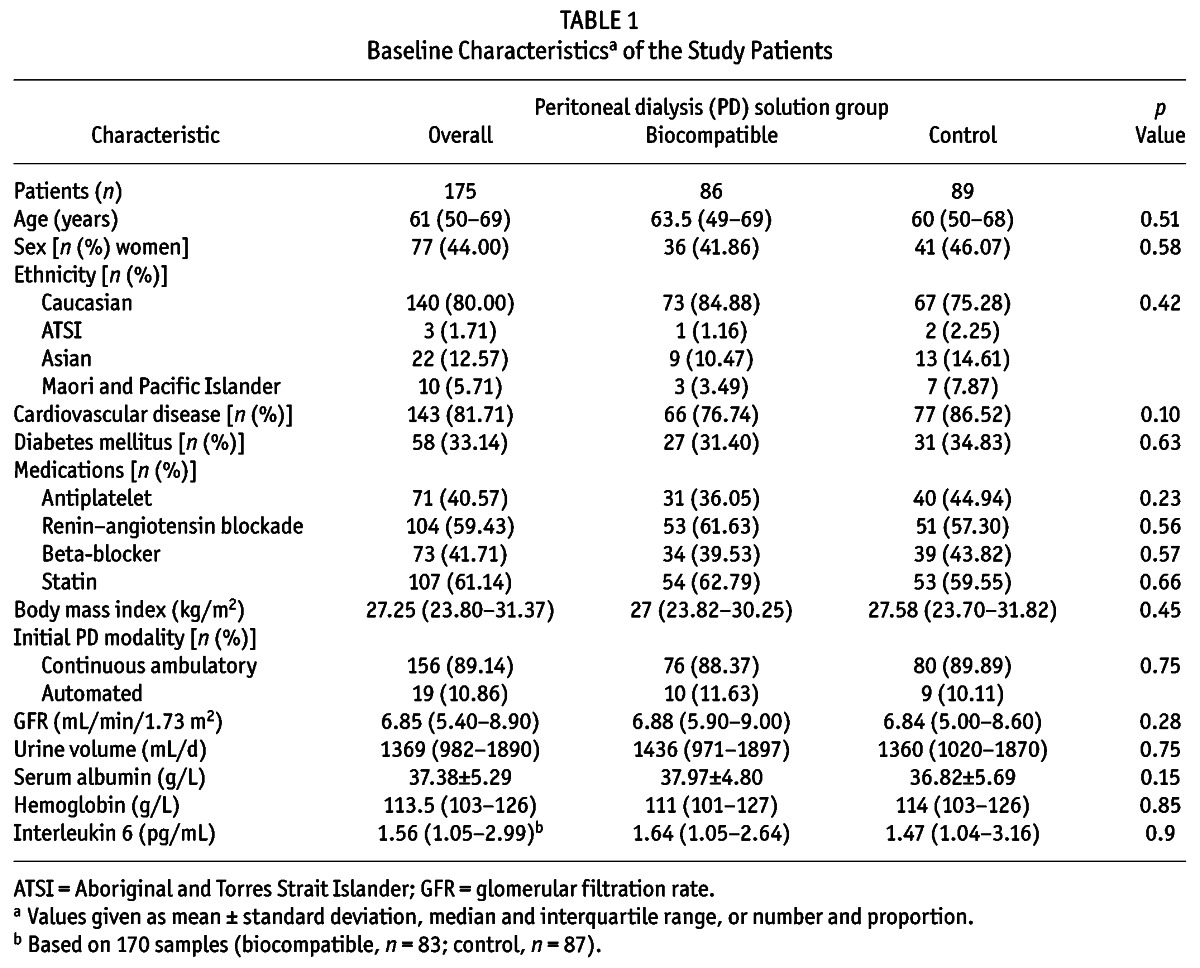
The patients—86 using biocompatible solution (Balance: Fresenius Medical Care, Bad Homburg, Germany) and 89 using standard solution (Stay•Safe: Fresenius Medical Care)—were well matched for all baseline characteristics, including baseline serum IL-6 (Table 1). The baseline characteristics of this subgroup were comparable to those of the original balANZ trial cohort (14).
TABLE 1.
Baseline Characteristicsa of the Study Patients
Trend of Serum IL-6 Concentration
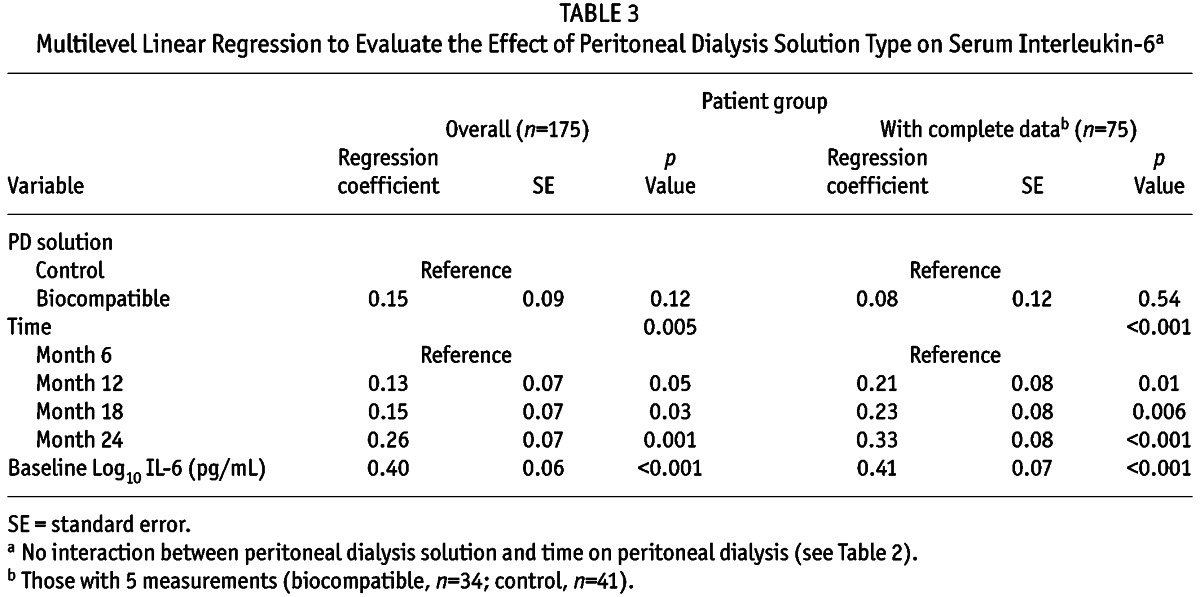
Serum IL-6 concentrations increased with longer PD duration (median values at baseline and 24 months were 1.56 pg/mL and 2.26 pg/mL respectively, Figure 1), and were comparable between the biocompatible and control groups at all time points during the 24-month study period (Table 2, Figure 2). The increment in absolute terms was relatively small, but when a multilevel linear regression model was fitted, statistically significant increases in serum IL-6 were observed over time (p = 0.006). Because there was no significant interactive effect of PD solution type and PD duration on serum IL-6 (p = 0.12, supplemental Table 1), solution type was analyzed as an unconditional effect. The unconditional effect of PD solution type on serum IL-6 was not statistically significant (p = 0.12, Table 3). However, serum IL-6 concentrations were associated with longer PD duration (p = 0.005) and with baseline serum IL-6 concentration (p < 0.001, Table 3). The sensitivity analysis performed in the subgroup in whom a full set of IL-6 data were available (n = 75) produced a similar pattern of results (Table 3). The rate of RRF loss had no significant effect on serum IL-6 over time (p = 0.27).
Figure 1 —
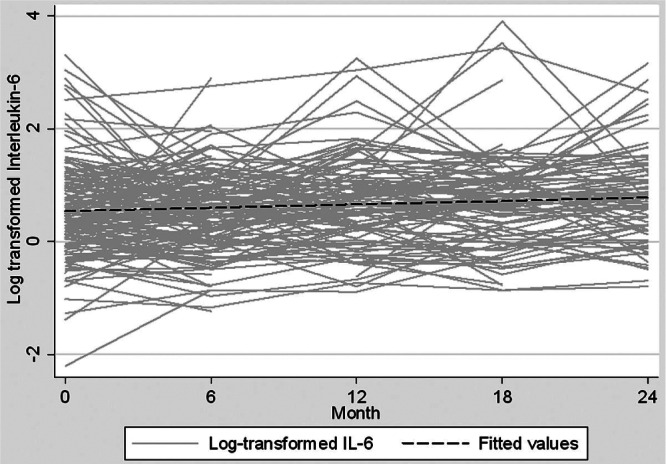
Overall trend of log-transformed serum interleukin-6 (IL-6) over time in incident peritoneal dialysis patients.
TABLE 2.
Trend in Serum Interleukin 6 (IL-6)a
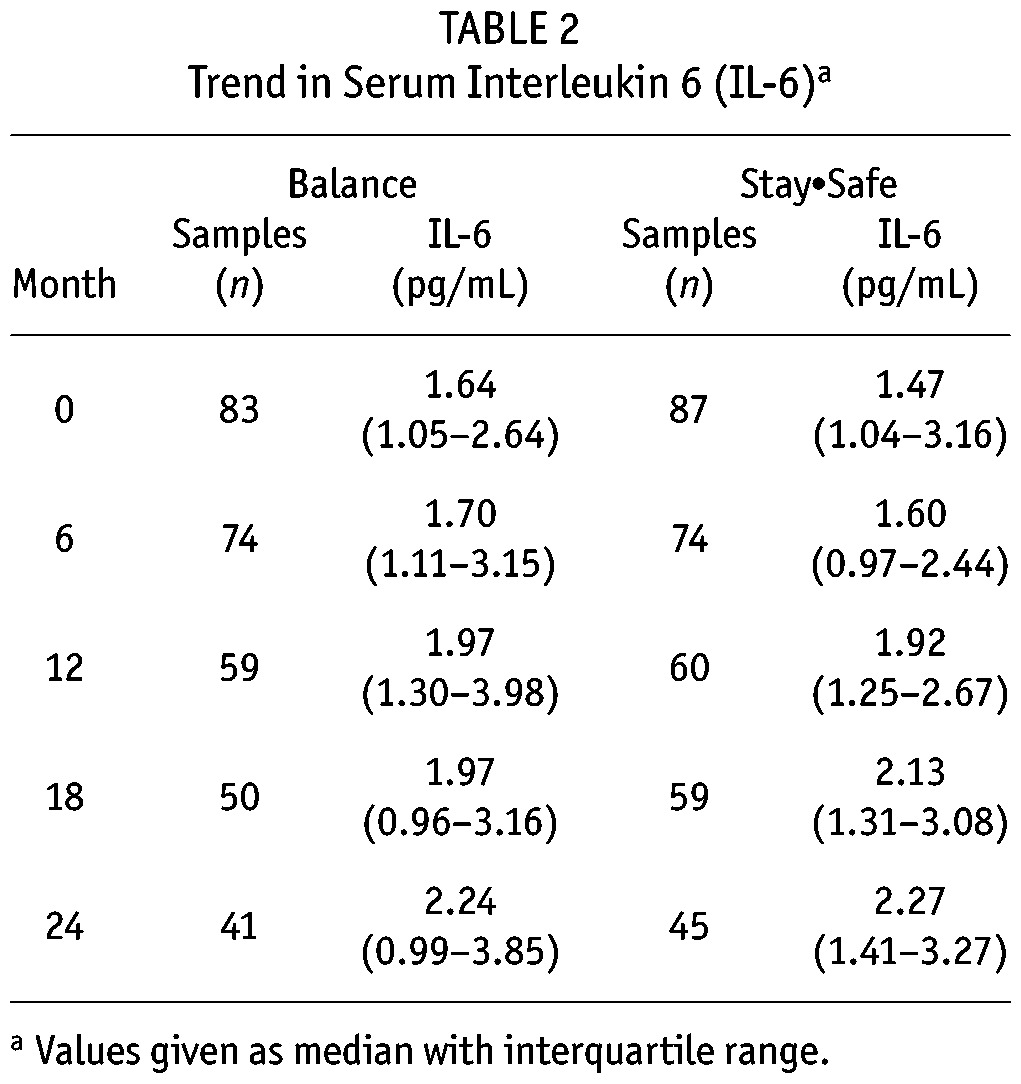
Figure 2 —
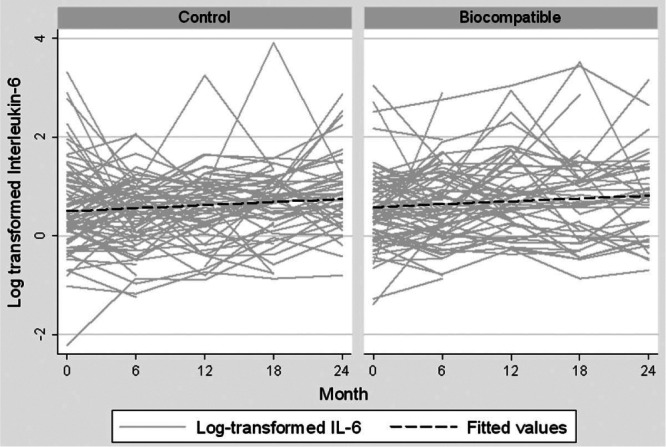
Trend of log-transformed serum interleukin-6 over time, by type of peritoneal dialysis solutions received (biocompatible, n = 86; control, n = 89).
TABLE 3.
Multilevel Linear Regression to Evaluate the Effect of Peritoneal Dialysis Solution Type on Serum Interleukin-6a
IL-6 as a Predictor of CVES
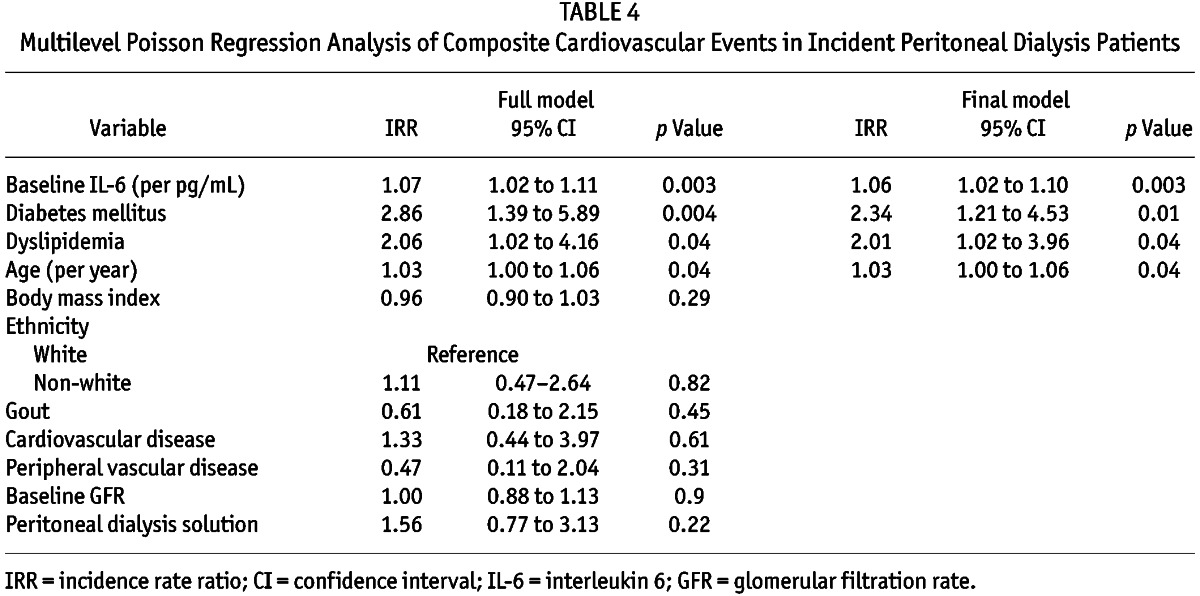
Of the 52 CVEs observed in 38 patients (biocompatible group, n = 19; control group, n = 19), 20 (38.5%), 11 (21.2%), 13 (25%), and 8 (15.4%) were recorded at 6, 12, 18, and 24 months respectively. Multivariable multilevel Poisson regression analysis observed that the composite CVE was significantly and independently associated with baseline serum IL-6 [incidence rate ratio (IRR) per picogram per milliliter: 1.06; 95% confidence interval (CI): 1.02 to 1.10; p = 0.003], older age (IRR per year: 1.03; 95% CI: 1.00 to 1.06; p = 0.04), and presence of dyslipidemia (IRR: 2.01; 95% CI: 1.02 to 3.96; p = 0.04) and diabetes mellitus (IRR: 2.34; 95% CI: 1.21 to 4.53; p = 0.01; Table 4).
TABLE 4.
Multilevel Poisson Regression Analysis of Composite Cardiovascular Events in Incident Peritoneal Dialysis Patients
IL-6 as a Predictor of All-Cause Mortality
On univariable exact logistic regression analysis, baseline serum IL-6 was not significantly associated with all-cause mortality (odds ratio: 1.07; 95% CI: 0.99 to 1.15; p = 0.06). In contrast, however, when baseline IL-6 was categorized in tertiles, incremental increases in mortality risk were observed [tertile 1: 1 (5.9%); tertile 2: 7 (41.2%); tertile 3: 9 (52.9%)], accompanied by a statistically significant association with the highest tertile of IL-6 (odds ratio: 10.72; 95% CI: 1.31 to 87.75; p = 0.03; supplemental Table 2). Similarly, when IL-6 was examined as an average value for each subject, a significant association with mortality risk was observed (odds ratio: 1.15; 95% CI: 1.03 to 1.34; p = 0.009).
Discussion
The present investigation is the first to examine the utility of IL-6 as a predictor of composite CVE in incident PD patients, and one of the largest studies examining longitudinal trends in serum IL-6. A small but statistically significant increase in serum IL-6 with longer time on PD was observed, but neither the PD solution type used (biocompatible vs standard), nor the rate of RRF loss had an effect on serum IL-6 concentration. Higher serum IL-6 was predictive of composite CVEs and possibly a greater risk of all-cause mortality, especially in patients with the highest serum IL-6 concentrations.
Reports about the impact of PD duration on systemic IL-6 concentrations are conflicting. In a single-center retrospective observational study of incident PD patients receiving treatment using conventional PD solutions (n = 31), Pecoits–Filho and colleagues (17) observed a significant increase in plasma IL-6 from baseline to 1 year (median: 3.7 pg/mL vs 6.5 pg/mL; p < 0.05). The recently completed GLOBAL fluid study (18) also described longer PD duration as a significant predictor of random plasma IL-6 concentrations in prevalent, but not in incident, PD patients (coefficient: 0.02 per year vs –0.2 per year; p = 0.04 vs p = 0.4). Results from the GLOBAL study were strengthened by long follow-up (up to 8 years) involving a large number of patients (n = 959). However, no data were reported pertaining to the longitudinal trend of systemic IL-6 concentration or to a detailed examination of the impact of biocompatible PD solution use on systemic inflammatory burden. In contrast, a prospective observational study (n = 109) reported a lack of significant change in serum IL-6 over 12 months (19). Although the reasons for such discrepant findings are unclear, some of the differences might stem from variations in study design, differences in assay techniques and samples used for the measurement of IL-6 (serum vs plasma), and the duration over which the changes were measured. As in the report by Pecoits–Filho and colleagues (17), observations in the present investigation included a statistically significant increase in systemic IL-6 over a 24-month period, regardless of whether standard or biocompatible PD solutions were administered. A sensitivity analysis confirmed the robustness of the results.
To date, the specific causes of an increase in systemic inflammatory burden with longer time on PD remain to be determined. The use of biocompatible PD solutions (with neutral pH and lower GDP content) or glucose-sparing regimens have at least the theoretical capacity to lower the inflammatory burden in PD patients by reducing the extent of peritoneal injury and GDP-mediated nephrotoxicity that leads to RRF decline. Szeto and colleagues (20) were the first to present data showing an improvement in systemic inflammation levels, as evidenced by lower serum CRP measurements, in patients (n = 50) using biocompatible PD solutions (p = 0.03). However, since then, several randomized controlled trials comparing the effects of biocompatible and standard PD solutions have measured systemic IL-6 (21–24), and have not shown any differences between patients in the two solution groups. Failure to find a difference in systemic IL-6 associated with the use of biocompatible PD solution might have been a consequence of the relatively short follow-up (<12 months) (21); the cross-over study design, with its risk of carry-over effects (21); inclusion of biocompatible PD solutions with higher GDP content (23,24); or small sample sizes (22). Equally, it might be a real effect, emphasizing the fact that local (peritoneal) and systemic inflammation are uncoupled (18). Despite the longer follow-up (24 months), parallel-group study design, and evaluation of the biocompatible PD solution with the lowest GDP content on the market, the present investigation also observed comparable systemic IL-6 levels in both the biocompatible and the control group. The lack of difference might have been a consequence of a substantial drop-out rate of 50.9% by month 24 (n = 89), which could have increased the risk of a type 2 statistical error. Nevertheless, in spite of biologic plausibility, the small effect size observed in our study is consistent with the possibility that the type of PD solution used has no major effect on the systemic inflammatory burden. Those findings are consistent with recent data from the GLOBAL incident and prevalent cohorts, in which biocompatible solution use was not associated with systemic IL-6 concentrations (18). Furthermore, the rate of RRF loss made no significant impact on serum IL-6. Although those results contradict findings from others who have reported an association between lower GFR and higher IL-6 in patients with chronic kidney disease (25), it is supported by findings from the GLOBAL study (18), in which urine volume had no significant effect on systemic IL-6 concentrations (p = 0.3).
Nonetheless, baseline serum IL-6 was a significant and independent predictor of composite CVE (IRR: 1.06; 95% CI: 1.02 to 1.10; p = 0.003) in incident PD patients. Previously, IL-6 was identified to be an important predictor of poor prognosis as part of a composite cardiovascular risk factor (13,26) and on its own (13) in prevalent dialysis populations. In fact, on its own and compared with a combination of inflammatory markers (CRP, IL-1β, IL-6, IL-18, tumor necrosis factor α), IL-6 concentration has, based on receiver operating characteristic curve analysis, been described as the most influential predictor of poor prognosis: 0.59 ± 0.04 versus 0.59 ± 0.04 (13).
The present study is the first to suggest a link between systemic IL-6 and CVEs in incident PD patients. The findings from our investigation are perhaps not unexpected, given that inflammation is a well-established nontraditional risk factor for CVE, and IL-6 is a sensitive marker indicating the level of inflammation. Other observed significant predictors of composite CVE include the presence of dyslipidemia (IRR: 2.01; 95% CI: 1.02 to 3.96; p = 0.04), which was previously shown to be associated with a higher risk of mortality in CAPD patients (27). Subgroup analysis of the SHARP trial (28) pointed out a statistically nonsignificant trend toward a reduction in atherosclerotic events with the use of simvastatin and ezetimibe in PD patients (risk ratio: 0.70; 95% CI: 0.75 to 1.08). It therefore remains uncertain whether treatment of dyslipidemia leads to a decrease in CVEs in PD patients.
As mentioned earlier, the small number of events in the present study meant that exploration of the relationship between IL-6 and all-cause mortality was restricted to univariable analysis. A trend toward increased mortality risk was observed when IL-6 was examined as a continuous variable, and a statistically significant result was noted in the highest tertile of IL-6 when incremental risk was assessed. Although those findings are far from definitive evidence, they suggest the presence of a relationship between IL-6 and mortality that is in keeping with the recent analysis from GLOBAL, in which systemic IL-6 was an independent predictor of survival in both the incident and the prevalent cohorts of PD patients (18).
Our study is strengthened by its sample size in comprehensively described cohorts who were participants of a well-controlled randomized trial. It is one of the largest studies of its type, with the longest follow-up, and the first to examine IL-6 as a predictor of poor CVE in incident PD patients.
The conclusions that can be drawn from the present study are challenged by several limitations. First, the balANZ trial was a randomized controlled trial whose primary outcome measure was RRF decline; it was not a biomarker study. The study design therefore did not account for potential biologic and pre-analytical sources of variation in IL-6 that could have affected the measured results. For example, plasma IL-6 has been reported to be influenced by an individual’s age, weight, comorbidities, most recent meal composition, and activity level (29). Second, even though ours is one of the largest studies to date, the sample size was relatively small, with fairly low event numbers in the context of substantial missing data for serum IL-6. Those factors could have weakened the statistical power and placed restrictions on the ability to examine IL-6 as a time-varying covariate in predicting composite CVEs. Finally, because of a lack of black patients in the current cohort, an extrapolation of results to that population should be made with caution.
Conclusions
Baseline serum IL-6 was an independent predictor of composite CVE. Serum IL-6 increased with longer time on PD and did not significantly differ between patients receiving biocompatible PD solutions and those receiving standard PD solutions. The role of IL-6 in predicting mortality risk could not be conclusively established, although the data are strongly suggestive of such an effect. Future studies should aim to evaluate the sources of IL-6 and to validate the use of this marker as a potential monitoring tool for effective therapeutic intervention, with resultant improvement in patient prognosis.
Disclosures
DWJ is a consultant for Baxter Healthcare Pty Ltd. and has previously received research funds from that company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care and has previously been a consultant to Gambro Pty Ltd. He is an International Society of Peritoneal Dialysis Councilor and a current recipient of a Queensland Government Health Research Fellowship. YC is a current recipient of an Australian Postgraduate Award and recipient of a 2012 Jacquot Research Entry Scholarship. CMH has received research grants from Baxter Healthcare Pty Ltd. and Gambro Pty Ltd., and has been a consultant to Fresenius Medical Care. MC is an employee of Fresenius Medical Care. NT, DAV, and EMP have no financial conflicts of interest.
Supplementary Material
Acknowledgments
The invaluable assistance provided by Dr. Sabine Lange is gratefully acknowledged. This biomarker substudy of the balANZ trial was funded by the Princess Alexandra Hospital Research Foundation Small Project Grant 2013.
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol 2009; 4:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker AE, de Boer OJ, van Der Wal AC. The role of inflammation and infection in coronary artery disease. Annu Rev Med 2001; 52:289–97. [DOI] [PubMed] [Google Scholar]
- 3. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9. [DOI] [PubMed] [Google Scholar]
- 4. Ayuzawa N, Ishibashi Y, Takazawa Y, Kume H, Fujita T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: recent clinical practice in Japan. Perit Dial Int 2012; 32:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65. [DOI] [PubMed] [Google Scholar]
- 6. Mortier S, Lameire NH, De Vriese AS. The effects of peritoneal dialysis solutions on peritoneal host defense. Perit Dial Int 2004; 24:123–38. [PubMed] [Google Scholar]
- 7. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. The effects of biocompatible compared with standard peritoneal dialysis solutions on peritonitis microbiology, treatment, and outcomes: the balANZ trial. Perit Dial Int 2012; 32:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Justo P, Sanz AB, Egido J, Ortiz A. 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005; 54:2424–9. [DOI] [PubMed] [Google Scholar]
- 9. Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001; 14:705–14. [DOI] [PubMed] [Google Scholar]
- 10. Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin-6-174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J 2001; 22:2243–52. [DOI] [PubMed] [Google Scholar]
- 11. Verduijn M, Marechal C, Coester AM, Sampimon DE, Boeschoten EW, Dekker FW, et al. The -174G/C variant of IL6 as risk factor for mortality and technique failure in a large cohort of peritoneal dialysis patients. Nephrol Dial Transplant 2012; 27:3516–23. [DOI] [PubMed] [Google Scholar]
- 12. Liu Y, Berthier-Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, et al. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol 2006; 17:863–70. [DOI] [PubMed] [Google Scholar]
- 13. Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 2006; 17(Suppl 3):S169–73. [DOI] [PubMed] [Google Scholar]
- 14. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson DW, Clarke M, Wilson V, Woods F, Brown FG. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol 2010; 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: the balANZ trial. Nephrol Dial Transplant 2012; 27:4445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pecoits-Filho R, Carvalho MJ, Stenvinkel P, Lindholm B, Heimbürger O. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int 2006; 26:53–63. [PubMed] [Google Scholar]
- 18. Lambie M, Chess J, Donovan KL, Kim YL, Do JY, Lee HB, et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 2013; 24:2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho JH, Hur IK, Kim CD, Park SH, Ryu HM, Yook JM, et al. Impact of systemic and local peritoneal inflammation on peritoneal solute transport rate in new peritoneal dialysis patients: a 1-year prospective study. Nephrol Dial Transplant 2010; 25:1964–73. [DOI] [PubMed] [Google Scholar]
- 20. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9. [DOI] [PubMed] [Google Scholar]
- 21. Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 2009; 29:647–55. [PubMed] [Google Scholar]
- 22. Kim S, Oh KH, Oh J, Kim SJ, Chung W, Song YR, et al. Biocompatible peritoneal dialysis solution preserves residual renal function. Am J Nephrol 2012; 36:305–16. [DOI] [PubMed] [Google Scholar]
- 23. Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis 2012; 60:966–75. [DOI] [PubMed] [Google Scholar]
- 24. Lai KN, Lam MF, Leung JC, Chan LY, Lam CW, Chan IH, et al. A study of the clinical and biochemical profile of peritoneal dialysis fluid low in glucose degradation products. Perit Dial Int 2012; 32:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003; 41:1212–18. [DOI] [PubMed] [Google Scholar]
- 26. Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Long-term mortality and cardiovascular risk stratification of peritoneal dialysis patients using a combination of inflammation and calcification markers. Nephrol Dial Transplant 2009; 24:3826–33. [DOI] [PubMed] [Google Scholar]
- 27. Little J, Phillips L, Russell L, Griffiths A, Russell GI, Davies SJ. Longitudinal lipid profiles on CAPD: their relationship to weight gain, comorbidity, and dialysis factors. J Am Soc Nephrol 1998; 9:1931–9. [DOI] [PubMed] [Google Scholar]
- 28. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picotte M, Campbell CG, Thorland WG. Day-to-day variation in plasma interleukin-6 concentrations in older adults. Cytokine 2009; 47:162–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


