Abstract
♦ Introduction: It has been reported that klotho deficiency is associated with oxidative stress and inflammation in experimental kidney disease models. Patients with endstage renal disease (ESRD) are particularly characterized by increased oxidative stress and inflammation. However, little is known about the relationship between these features and klotho in patients with ESRD.
♦ Methods: We conducted a single-center, cross-sectional study of 78 patients receiving peritoneal dialysis (PD). Serum concentrations of klotho, high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), and 8-isoprostane were measured by enzyme-linked immunosorbent assay. To define factors independently associated with klotho, we determined Spearman’s correlation coefficients for between co-variates and conducted multiple linear regression analyses.
♦ Results: Patients were classified by median concentration of klotho. In patients with klotho levels > 329.6 pg/mL, serum 8-isoprostane and IL-6 levels were significantly higher than in those with klotho levels < 329.6 pg/mL. In correlation analyses, log 8-isoprostane (γ = –0.310, p = 0.006) and log IL-6 (γ = –0.343, p = 0.002) were inversely correlated with log klotho. After adjustment for age, gender, mean arterial pressure, log intact parathyroid hormone, and log IL-6, log 8-isoprostane was independently associated with log klotho (β = –0.158, p = 0.040). However, the significant relationship between klotho and IL-6 was not seen in an adjusted model.
♦ Conclusions: This study showed that circulating klotho levels were significantly associated with 8-isoprostane levels in patients undergoing PD, suggesting a potential link between klotho deficiency and enhanced oxidative stress in ESRD patients.
Keywords: ESRD, inflammation, klotho, oxidative stress
Cardiovascular disease is the leading cause of death in patients with end-stage renal disease (ESRD) (1,2). ESRD patients have both traditional and nontraditional risk factors for cardiovascular disease including anemia, inflammation, oxidative stress and abnormal calcium phosphorus metabolism (3–5). Oxidative stress and inflammation are proposed to play a pivotal role in the pathophysiology of uremia and development of cardiovascular complications (6). These two features increase atherosclerotic burden, amplifying cardiovascular risk (3,4), and are clearly evident in patients receiving dialysis (3,6–8).
Klotho, an anti-aging gene, is predominantly expressed in the distal convoluted tubules of the kidney, but is also expressed in the brain choroid plexus, pituitary gland, pancreas, and reproductive organs (9). Previous studies showed that klotho is downregulated after kidney injury. Expression of klotho mRNA and protein in the kidney is reduced in experimental models of angiotensin II-induced hypertensive renal damage (10), diabetic nephropathy (11–13), and ischemia-reperfusion acute kidney injury (14). Although findings in humans are lacking, Koh et al. showed that klotho mRNA and protein expression in the kidneys of patients with ESRD and advanced stages of chronic kidney disease (CKD) were significantly lower than in healthy controls (15). Furthermore, we recently showed that circulating klotho levels decrease as CKD stage advances and that levels are inversely correlated with estimated glomerular filtration rate (eGFR) (16).
Klotho has two forms. The membrane form is a cofactor in fibroblast growth factor 23 (FGF23) signaling in the kidney. This complex modulates phosphate homeostasis and vitamin D metabolism (17,18). The soluble form of klotho acts like a hormone, with anti-oxidative stress (19,20) and anti-inflammatory effects (21). These findings and the distinct features of ESRD patients led us to hypothesize that klotho deficiency might contribute to increased oxidative stress and inflammation in ESRD patients. However, no human studies have addressed this issue. Therefore, this study aimed to identify the relationship between klotho, oxidative stress, and inflammation in patients receiving peritoneal dialysis (PD).
Materials and Methods
Patients
This single-center, cross-sectional study examined 135 ESRD patients receiving PD at Yonsei University Severance Hospital, Seoul, Korea. Patients were eligible if they were 20 to 75 years old and had maintained PD > 3 months. Exclusion criteria were history of overt infection during the 3 months prior to the study, history of malignancy or other chronic inflammatory disease (e.g., rheumatoid arthritis or systemic lupus erythematosus), and prior history of kidney transplantation. After exclusions, 78 patients were included (Figure 1). We included healthy controls (n = 30) who underwent a routine health examination, had no history of medical disease and were not taking regular medication.
Figure 1 —
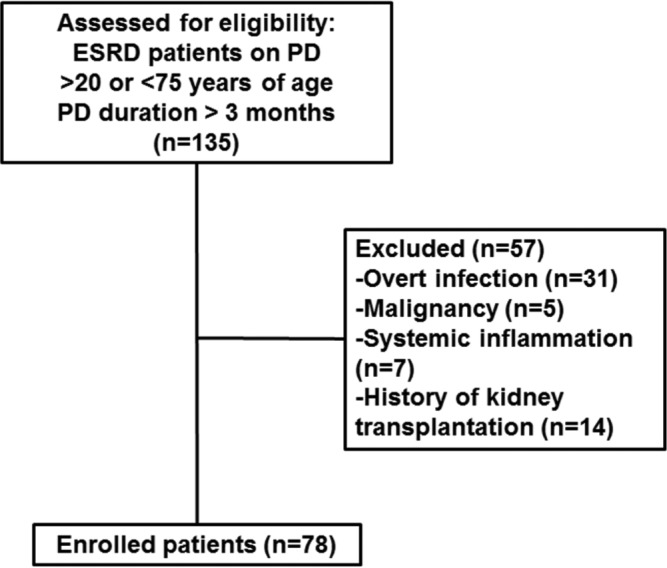
Flow diagram of patient recruitment and exclusion. ESRD = end-stage renal disease; PD = peritoneal dialysis.
This study protocol was approved by the Institutional Review Boards at our institution and all patients provided their written informed consent to participate in the study.
Data Collection
Demographic and clinical data were recorded at study entry. After an overnight fast, venous samples were drawn to assess the following: hemoglobin, blood urea nitrogen, creatinine, albumin, calcium, phosphorus, intact parathyroid hormone (iPTH), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride. The preceding overnight dwell was regulated to 1.5% dextrose dialysate to standardize glucose load. Body weight and blood pressure were measured in the morning on the same day when the first dialysate of the day was drained out.
Serum Soluble Klotho and Inflammatory and Oxidative Stress Markers
For measurement of klotho and inflammatory and oxidative stress markers, blood samples were immediately centrifuged at 1500 x g, 4°C for 10 min, and supernatants were stored in aliquots at –80°C. Serum soluble klotho was measured by enzyme-linked immunosorbent assay (ELISA) kit (Immuno Biological Laboratories Co., Ltd, Tokyo, Japan) as previously reported (22). Means ± standard deviation (SD) were 6.5 ± 3.3% for interassay and 3.6 ± 1.5% for intra-assay coefficients of variability. Interleukin-6 (IL-6) serum concentration was determined by ELISA (R&D Systems Europe, Abingdon, Oxon, UK) and 8-isoprostane, an oxidative stress marker, was measured in duplicate using a specific enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA).
Dialysis Adequacy, Residual Renal Function and Glucose Exposure
Urea kinetic studies were conducted using a 24-hour collection of dialysate and urine at study enrollment. Dialysis adequacy was determined by measuring total weekly urea clearance (Kt/Vurea) using the Watson equation (23), and residual renal function (RRF) was determined as the average of urea and creatinine clearance from a 24-hour urine collection. Estimated average glucose exposure was calculated by multiplying the instilled dialysate volume by the respective glucose concentration.
Statistical Analysis
Statistical analysis used SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as mean ± SD. The Kolmogorov-Smirnov test was used to analyze distribution normality of measured parameters. Data with a skewed distribution (klotho, 8-isoprostane, IL-6 and iPTH), were expressed as median with interquartile range or log transformations. For normally distributed data, variables were compared between two groups using Student t-test and chi-square test. Pearson’s correlation tests were used to evaluate relationships between klotho and clinical and laboratory parameters. For skewed data, the Mann-Whitney U-test was conducted to compare variables between two groups, and Spearman’s correlation coefficient was determined to identify relationships between covariates. To define independent factors associated with log-transformed klotho (log klotho), multiple linear regression analyses were conducted using variables such as age, gender and other variables that were significantly associated with log klotho in correlation analyses. P < 0.05 was considered statistically significant.
Results
Baseline Demographic, Clinical and Laboratory Findings
Circulating klotho levels were significantly lower in patients receiving PD than in healthy controls (329.6 (215.8–464.8) vs 717.8 (490.3–1080.2) pg/mL, p < 0.001), whereas serum concentrations of inflammatory and oxidative stress markers were significantly higher in PD patients compared with in healthy controls (Table 1). Baseline characteristics of study patients are presented in Table 2. Mean age was 48.9 ± 11.0 years, 38 patients (48.7%) were male, and mean PD duration was 76.7 ± 50.6 months. A total of 93.6% and 17.9% of patients had hypertension and diabetes mellitus, respectively. In addition, 16.7% of patients had cardiovascular disease defined as a history of coronary artery disease, peripheral artery occlusive disease, or cerebrovascular disease. Average body mass index (BMI) was 22.8 ± 3.0 kg/m2, mean arterial pressure (MAP) was 101.3 ± 10.6 mmHg and over 70% of the patients were using renin-angiotensin system blockades. In addition, 23 of 78 patients (29.5%) used one daily exchange of a long-dwell 2.0 L icodextrin PD solution (Extraneal, Baxter Health Corporation, Singapore). Median values were 329.6 (215.8–464.8) pg/mL for klotho, 308.9 (197.5–487.7) ng/mL for 8-isoprostane, and 7.3 (4.8–8.5) pg/mL for IL-6.
TABLE 1.
Serum Concentrations of Klotho, IL-6, and 8-isoprostane in Healthy Controls and Patients with PD
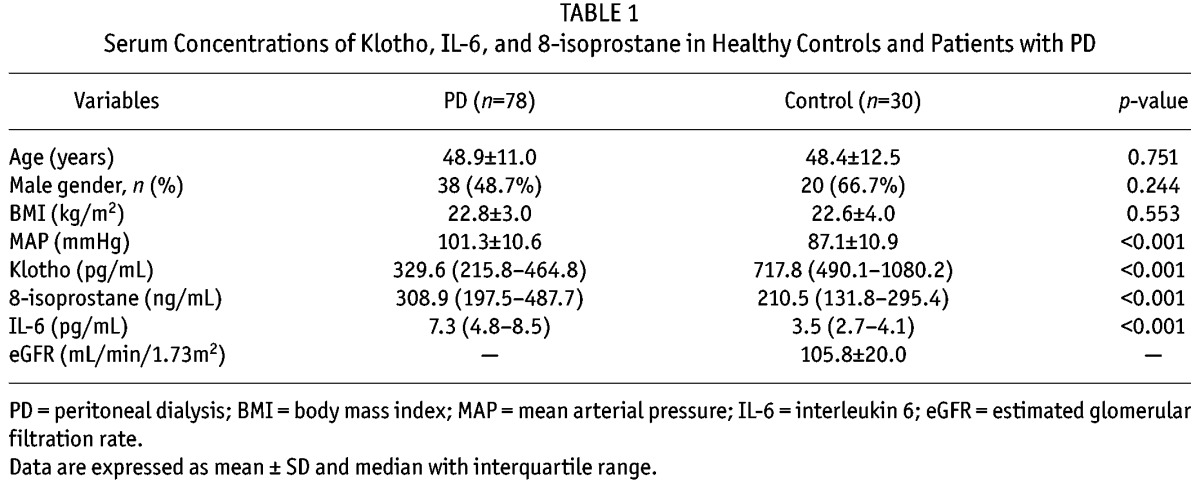
TABLE 2.
Baseline Characteristics and Comparison between Groups by Median Klotho Level
Demographic, Clinical and Laboratory Parameters by Median Klotho Level
Patients were stratified into two groups using median klotho level (329.6 pg/mL) and demographic, clinical, and laboratory parameters were compared between the two groups (Table 1). Compared to patients with klotho > 329.6 pg/mL (L group), patients with klotho > 329.6 pg/mL (H group) had significantly lower MAP (98.9 ± 9.4 vs 103.7 ± 11.4 mmHg, p = 0.042), log 8-isoprostane (2.4 ± 0.4 vs 2.6 ± 0.3, p = 0.023), log IL-6 (0.7 ± 0.1 vs 0.9 ± 0.2, p = 0.044), and serum uric acid levels (6.3 ± 1.0 vs 6.9 ± 1.4 mg/dL, p = 0.019). However, iPTH was significantly higher in the H group than the L group (253.1 vs 189.1 pg/mL, p = 0.037). No significant differences were observed between the two groups in age, gender, BMI, dialysis duration, medication use, comorbid conditions, peritonitis episodes, dialysis adequacy and RRF, or other serum biomarkers.
Factors Associated with Klotho Levels
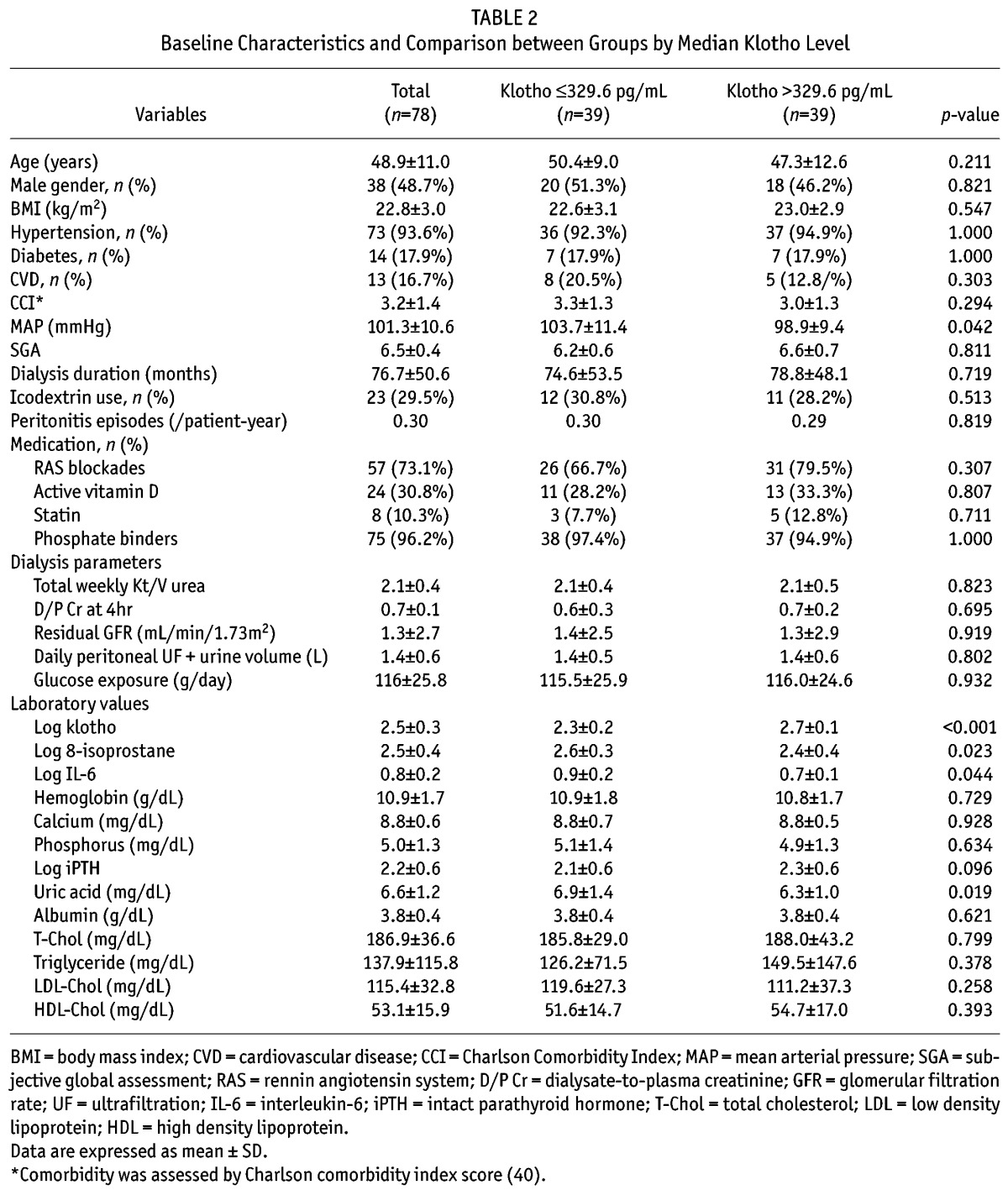
Bivariate correlation analyses were conducted to determine relationships between klotho levels and other variables (Table 3 and Figure 2). Log klotho was inversely correlated with age (γ = –0.229, p = 0.044), log 8-isoprostane (γ = –0.310, p = 0.006), log IL-6 (γ = –0.343, p = 0.002), and MAP (γ = –0.257, p = 0.023), but positively correlated with log iPTH (γ = 0.246, p = 0.030). However, serum calcium and phosphorus levels and RRF were not associated with log klotho. Log IL-6 was positively correlated with log 8-isoprostane (γ = 0.305, p = 0.007). To further identify factors that were independently associated with klotho, multiple linear regression analysis was conducted. Age, log 8-isoprostane, log IL-6, MAP and log iPTH, which were significantly correlated with klotho in bivariate correlation analyses, were used to construct multiple linear regression models. In model 1, log 8-isoprostane (β ± standard error (SE), –0.190 ± 0.071; p = 0.010) was significantly associated with log klotho after adjusting for age, gender, MAP, and log iPTH (Table 4). When log IL-6 was used instead of log 8-isoprostane in model 2, log IL-6 (β ± SE, –0.372 ± 0.185; p = 0.048) was also significantly associated with log klotho. In model 3, both log 8-isoprostane and log IL-6 were used, and only log 8-isoprostane remained independently correlated with log klotho (β ± SE, –0.158 ± 0.075; p = 0.040), while the significant relationship between log IL-6 and log klotho disappeared (Table 4). We determined variance inflation factor to check potential multicollinearity and found no variables with variance inflation factor > 10, suggesting that multicollinearity was not present among variables.
TABLE 3.
Correlations between Klotho and Other Variables
Figure 2 —
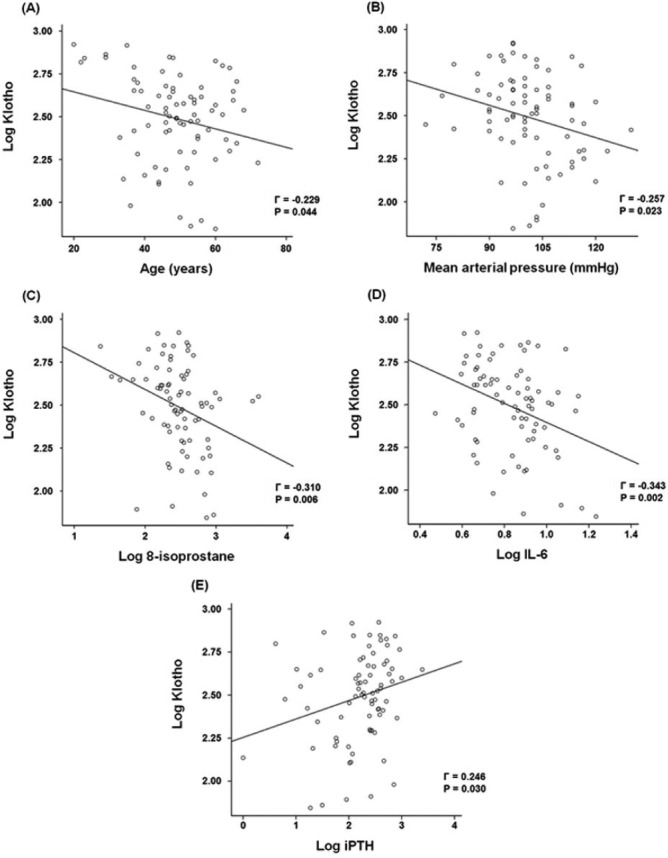
Correlation between klotho and other variables. Log klotho was inversely correlated with age (A), MAP (B), log 8-isoprostane (C) and log IL-6 (D), but was positively correlated to log iPTH (E). IL-6 = interleukin-6; iPTH = intact parathyroid hormone.
TABLE 4.
Multiple Linear Regression Analysis for Klotho
Discussion
In this study, we investigated the relationship between klotho, oxidative stress, and inflammation in patients treated with PD. We showed that circulating klotho levels were lower in patients with PD than in healthy controls and were negatively correlated with 8-isoprostane and IL-6 levels. However, only 8-isoprostane showed a significant association with klotho level in all analyses, suggesting a potential link between klotho deficiency and enhanced oxidative stress in ESRD patients.
Klotho is a recently discovered anti-aging gene (9). Mutation of the klotho gene accelerates aging and shortens lifespan (9,24). The phenotype of mice with a defective klotho gene includes atherosclerosis, osteoporosis, hypercalcemia, hyperphosphatemia, vascular calcification, skin atrophy, and pulmonary emphysema, resembling human premature-aging syndrome. Overexpression of klotho reverses these phenotypes (19). The klotho-FGF23 complex induces phosphorus and vitamin D homeostasis, and the soluble form of klotho is reported to suppress oxidative stress. Klotho has an anti-oxidative stress effect that is mediated by inhibition of insulin/insulin-like growth factor-1 signaling, which facilitates nuclear translocation of forkhead box O, leading to upregulation of antioxidant enzymes such as catalase and mitochondrial manganese-superoxide dismutase (19,25). Yamamoto et al. showed that urinary 8-hydroxydeoxyguanosine levels are lower in mice overexpressing klotho after paraquat injection, supporting the anti-oxidative stress activity of klotho (20). Our findings are consistent with these experimental results. Uremia has a key role in oxidative stress development (26–28). In this study, we showed that 8-isoprostane, a reliable marker of oxidative stress in patients with uremia (29,30), was correlated with klotho levels. Our results provide clinical evidence of a significant relationship between decreased circulating klotho levels and increased oxidative stress in ESRD patients.
Inflammation is another well-characterized feature of ESRD patients (1,26,31,32), and experimental evidence suggests that klotho modulates inflammation. Zhao et al., using a db/db mouse model of diabetes, showed that klotho negatively regulates the production of NFκB-linked inflammatory cytokines by inhibiting phosphorylation of RelA Ser 536 (12). In addition, Liu et al. reported that klotho suppresses the retinoic-acidinducible gene-1, which decreases expression of IL-6 and IL-8 both in vitro and in vivo (21). These findings suggest that klotho is an anti-inflammatory modulator. In this study, we showed that log IL-6 was inversely correlated with log klotho. However, independent association between IL-6 and klotho levels was observed only in a multivariable model when 8-isoprostane was excluded (Table 4, Model 2). It is possible that strong interaction between klotho and oxidative stress might mitigate the association with inflammation. Because log klotho was significantly associated with both log 8-isoprostane and log IL-6, and log IL-6 was also significantly correlated with log 8-isoprostane (Table 3), we checked interactions between klotho, 8-isoprostane, and IL-6 using multiple regression analysis and found that variance inflation factor of each variable was < 10, suggesting that association between klotho and oxidative stress is not affected by inflammation. However, our cross-sectional study could not fully explain the pathogenic link between klotho, oxidative stress, and inflammation. Further studies are required to clarify the relationships.
We previously showed that circulating klotho levels are inversely correlated with phosphorus and iPTH levels in patients with CKD prior to dialysis (31). However, the results of this study were not consistent with our earlier findings. This discrepancy can be partly explained by the dysregulated relationship between klotho, phosphorus, and PTH in ESRD patients. This study included patients on long-term dialysis, many of whom were anuric. Atrophied kidney tubules in this late stage of CKD do not have the same physiological response as tubules in earlier stages of CKD. In addition, more of the patients with PD in this study were treated with active vitamin D and phosphorus binders than patients in our previous study (16). These factors might make the relationship between relevant parameters more complicated and difficult to analyze. The dysregulated relationship between klotho, phosphorus, and PTH was further supported by the lack of significance we observed in relationships between calcium, phosphorus, and iPTH levels (Table 3), while these parameters are significantly interrelated in patients with earlier stages of CKD (33).
In our previous work (34), circulating klotho levels were inversely associated with GFR in CKD patients prior to dialysis, suggesting that klotho is largely affected by kidney function. There have been some conflicting data on the relationship between serum soluble klotho level and RRF in ESRD patients. Akimoto et al. (35) reported that urinary klotho level rather than serum klotho level was significantly related to RRF. In addition, it was also reported that serum klotho level was not associated with RRF, but was related to residual diuresis volume in a recently published paper (36). In this study, we investigated the association between log klotho and RRF in these patients and found that log klotho level was not related to RRF (γ = 0.118, p = 0.307). However, this should be interpreted with caution because 55 (71.4%) patients exhibited residual GFR < 1 mL/min/1.73 m2; thus the relationship between klotho and RRF could not be accurately assessed in our study.
This study had several limitations. First, the singlecenter, cross-sectional study design had inherent biases. The cross-sectional design did not allow causality to be determined between klotho and oxidative stress. Although klotho is reported to attenuate oxidative stress, recent studies showed that oxidative stress itself downregulates klotho expression (37,38). Klotho and oxidative stress might work together in a vicious cycle of reduction in klotho levels and aggravation of oxidative stress. Second, we did not measure FGF23 levels. In patients with CKD prior to dialysis, circulating klotho levels are inversely correlated with FGF23 levels (16,39). However, whether this relationship between klotho and FGF23 occurs in ESRD patients is unknown. A recent study showed that serum klotho levels were not correlated with FGF23 levels in patients with hemodialysis, suggesting that klotho-FGF23 relationship is altered in ESRD patients. Finally, patients with hemodialysis were not included in this study. Therefore, our findings cannot be extrapolated to the entire ESRD population. However, uremia per se is associated with increased oxidative stress activity (26–28). In addition, both hemodialysis and PD further enhance oxidative stress and contribute to a decrease in antioxidant capacity (3,6).
In conclusion, this study showed that circulating klotho levels were independently associated with oxidative stress in ESRD patients undergoing PD. Significant association of klotho with inflammation was attenuated after adjustment for oxidative stress, suggesting that klotho is more strongly correlated to oxidative stress than inflammation. However, our findings suggest hypotheses based on many previous experimental studies. Further studies with larger sample sizes are required to clarify the relationship between klotho, oxidative stress, and inflammation in ESRD patients.
Disclosures
All authors declared that there are no competing interests.
Acknowledgments
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2012; and by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (H10C2020).
REFERENCES
- 1. Port FK. Morbidity and mortality in dialysis patients. Kidney Int 1994; 46:1728–37. [DOI] [PubMed] [Google Scholar]
- 2. Patient mortality and survival. United states renal data system. Am J Kidney Dis 1998; 32:S69–80. [DOI] [PubMed] [Google Scholar]
- 3. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005; 45:S1–153. [PubMed] [Google Scholar]
- 4. Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet 2000; 356:147–52. [DOI] [PubMed] [Google Scholar]
- 5. Goicoechea M, de Vinuesa SG, Gomez-Campdera F, Luno J. Predictive cardiovascular risk factors in patients with chronic kidney disease (CKD). Kidney Int Suppl 2005:S35–8. [DOI] [PubMed] [Google Scholar]
- 6. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002; 62:1524–38. [DOI] [PubMed] [Google Scholar]
- 7. Sundl I, Roob JM, Meinitzer A, Tiran B, Khoschsorur G, Haditsch B, et al. Antioxidant status of patients on peritoneal dialysis: Associations with inflammation and glycoxidative stress. Perit Dial Int 2009; 29:89–101. [PubMed] [Google Scholar]
- 8. Guo CH, Wang CL, Chen PC, Yang TC. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in patients undergoing either hemodialysis or peritoneal dialysis. Perit Dial Int 2011; 31:583–91. [DOI] [PubMed] [Google Scholar]
- 9. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390:45–51. [DOI] [PubMed] [Google Scholar]
- 10. Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, et al. In vivo klotho gene transfer ameliorates angiotensin iiinduced renal damage. Hypertension 2002; 39:838–43. [DOI] [PubMed] [Google Scholar]
- 11. Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, et al. Downregulation of the klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 1998; 249:865–71. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 2011; 60:1907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, et al. Decreased renal alpha-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 2012; 81:539–47. [DOI] [PubMed] [Google Scholar]
- 14. Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 2005; 20:2636–45. [DOI] [PubMed] [Google Scholar]
- 15. Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 2001; 280:1015–20. [DOI] [PubMed] [Google Scholar]
- 16. Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis 2013. [DOI] [PubMed] [Google Scholar]
- 17. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006; 281:6120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens 2006; 15:437–41. [DOI] [PubMed] [Google Scholar]
- 19. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone klotho. Science 2005; 309:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005; 280:38029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011; 13:254–62. [DOI] [PubMed] [Google Scholar]
- 22. Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-klotho measurement: Age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun 2010; 398:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 1980; 33:27–39. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev 2009; 8:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem 2008; 389:233–41. [DOI] [PubMed] [Google Scholar]
- 26. Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 2008:S4–9. [DOI] [PubMed] [Google Scholar]
- 27. Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens 2003; 12:593–8. [DOI] [PubMed] [Google Scholar]
- 28. Vaziri ND. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol 2004; 24:469–73. [DOI] [PubMed] [Google Scholar]
- 29. Lim PS, Chang YM, Thien LM, Wang NP, Yang CC, Chen TT, et al. 8-iso-prostaglandin F2alpha as a useful clinical biomarker of oxidative stress in ESRD patients. Blood Purif 2002; 20:537–42. [DOI] [PubMed] [Google Scholar]
- 30. Sakamoto H, Corcoran TB, Laffey JG, Shorten GD. Isoprostanes—markers of ischaemia reperfusion injury. Eur J Anaesthesiol 2002; 19:550–9. [DOI] [PubMed] [Google Scholar]
- 31. Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant 2012; 27(Suppl 4):iv6–10. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen-Khoa T, Massy ZA, De Bandt JP, Kebede M, Salama L, Lambrey G, et al. Oxidative stress and haemodialysis: Role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant 2001; 16:335–40. [DOI] [PubMed] [Google Scholar]
- 33. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–8. [DOI] [PubMed] [Google Scholar]
- 34. Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis 2013; 61:899–909. [DOI] [PubMed] [Google Scholar]
- 35. Akimoto T, Shiizaki K, Sugase T, Watanabe Y, Yoshizawa H, Otani N, et al. The relationship between the soluble klotho protein and the residual renal function among peritoneal dialysis patients. Clin Exp Nephrol 2012; 16:442–7. [DOI] [PubMed] [Google Scholar]
- 36. Golembiewska E, Safranow K, Kabat-Koperska J, Myslak M, Ciechanowski K. Serum soluble klotho protein level is associated with residual diuresis in incident peritoneal dialysis patients. Acta Biochim Pol 2013; 60:191–4. [PubMed] [Google Scholar]
- 37. Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101:e67–74. [DOI] [PubMed] [Google Scholar]
- 38. Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R. Iron chelation and a free radical scavenger suppress angiotensin ii-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett 2003; 551:58–62. [DOI] [PubMed] [Google Scholar]
- 39. Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, et al. Serum levels of soluble secreted alphaklotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 2012; 16:722–9. [DOI] [PubMed] [Google Scholar]
- 40. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–51. [DOI] [PubMed] [Google Scholar]


