Abstract
♦ Background and Objective: Colombia is a country of diverse geographic regions, some with mountainous terrain that can make access to urban areas difficult for individuals who live in remote areas. In 2005, a program was initiated to establish remote peritoneal dialysis (PD) centers in Colombia to improve access to PD for patients with end-stage renal disease who face geographic or financial access barriers.
♦ Patients and Methods: The present study was a multi-center cohort observational study of prevalent home PD patients who were at least 18 years of age and were being managed by one of nine established remote PD centers in Colombia over a 2-year period. Data were collected from clinical records, databases, and patient interviews. Patient survival, incidence of peritonitis, and rate of withdrawal from PD therapy were assessed.
♦ Results: A total of 345 patients were eligible for the study. The majority (87.8%) of patients lived on one to two times a minimum monthly salary (equivalent to US$243 – US$486). On average, patients traveled 1.2 hours and 4.3 hours from their home to their remote PD center or an urban reference renal clinic, respectively. The incidence rate of peritonitis was 2.54 episodes per 100 patient-months of therapy. A bivariate analysis showed a significantly higher risk of peritonitis in patients who were living on less than one times a monthly minimum salary (p < 0.05) or who had a dirt, cement, or unfinished wood floor (p < 0.05). The 1-year and 2-year patient survival rates were 92.44% and 81.55%, respectively. The 1-year and 2-year technique survival rates were 97.27% and 89.78%, respectively.
♦ Conclusions: With the support of remote PD centers that mitigate geographic and financial barriers to healthcare, home PD therapy is a safe and appropriate treatment option for patients who live in remote areas in Colombia.
Keywords: Colombia, infection, peritoneal dialysis, remote dialysis center, survival
Since its introduction in 1983, peritoneal dialysis (PD) has become an increasingly important treatment option for patients with end-stage renal disease (ESRD) who reside in Colombia (1). According to the United States Renal Data System 2012 Annual Data Report, 31.3% of patients receiving dialysis in Colombia in 2009 were being treated with PD (2). Similarly, the Dialysis Outcomes in Colombia study, which investigated the survival of patients receiving hemodialysis (HD) compared with those receiving PD, reported that 52.7% of incident patients included in a historical cohort analysis started dialysis treatment using PD (3).
Socioeconomic status, climate, and accessibility to dialysis centers can impact the health outcomes of patients receiving PD. Of the approximately 47 million people living in Colombia, 37.2% have an income that is below the poverty level and 15.8% of the population lives on less than US$2 a day (4). Yet, despite poor socioeconomic conditions, 100% of the population has access to entitlement healthcare, and more than 90% of the population has access to healthcare that covers the cost of treatment for chronic kidney disease (CKD), including dialysis, regardless of the patient’s socioeconomic status (5). Even with these overarching advantages in healthcare benefits, the Colombian healthcare system continues to experience a prevalence of ESRD of 544 patients per million population and an incidence of 145 patients per million population per year (2). In 2010, the PD population in Colombia was 6,165 patients and the HD population in Colombia was 13,385 (6). Dialysis programs in Colombia are totally supported financially by public and private health insurers; the reimbursement for HD and PD is the same. Compounding its challenges with poverty, Colombia is a country of diverse geographic regions that include mountainous terrain that can make access to remote locations difficult and increases travel time to urban areas. In addition, many of the country’s regions have a tropical climate with year-round temperatures above 25°C. Higher temperatures often make life conditions and travel uncomfortable or more difficult for patients.
Dialysis clinics are typically located in large urban areas. Colombia has one of the largest percentages of rural populations among the countries in Latin America, with 31% living outside larger cities and towns (7). Until recently, all dialysis patients who lived in remote rural areas or small towns had to travel to urban renal dialysis clinics for treatment or follow-up care. Traveling from a remote area to an urban center requires a significant investment of time and money. Limited financial resources and the challenges associated with traveling great distances to receive treatment or be seen by a nephrologist often lead to reduced patient adherence to treatment, suboptimal control of the patient’s disease, and an increased risk of withdrawal from dialysis therapy.
Results from previous studies investigating outcomes in patients treated with PD therapy at home and who receive routine care and follow-up at remote centers have reported increased morbidity and mortality in these patients compared with those managed by a renal clinic in an urban setting (8–11). However, initial results from a PD satellite dialysis center program that was developed to provide dialysis to patients who live in remote areas in China suggest that a systematic and supportive program can improve outcomes (12).
In 2005, a program to establish remote PD centers in Colombia was initiated to help overcome geographic and financial access barriers for patients desiring PD therapy. These remote PD centers are located in or adjacent to medical clinics or hospitals in small towns, which were selected based on georeferencing criteria. Each remote PD center has an area for medical consultation, nursing procedures, and a peritoneal exchange room and is staffed by a specialized PD nurse who is available at all times for patient training, nursing care, and home visits. All of the patient training and PD procedures, including catheter placement, are performed at the remote PD center. On average, approximately 30 patients are in attendance at each remote PD center at any given time.
The remote PD centers are overseen and supported by one of 53 larger reference renal clinics located in large urban settings and are part of the Baxter Inc Renal Therapy Services (RTS) network in Colombia. The RTS sees approximately 9,000 dialysis patients throughout the country with 45% of the patients receiving PD. These urban reference renal clinics offer HD, PD, and renal prevention programs and are staffed by a nephrologist, who travels once a month to remote PD centers to evaluate patients. When not visiting, the nephrologist maintains contact with the specialized PD nurse working at the remote PD center by phone and intranet. Patients being treated at the remote PD center also undergo quarterly evaluations from a dietician and social worker who visit the remote PD center. The remote PD centers are fully connected to RTS via the intranet to maintain patients’ electronic medical records. When patients in remote areas require a switch from PD therapy to HD for any reason, they are transferred to the urban reference renal clinic.
Herein, we report on the health outcomes, including patient survival, incidence of peritonitis, and rate of withdrawal from PD therapy, among PD patients managed by established remote PD centers in Colombia.
Materials and Methods
Patients
Included in the analysis were all patients who were 18 years of age or older, had been in the PD program for more than 90 days, and had attended one of the nine remote PD centers that are part of the RTS network but are located away from large urban centers in Colombia.
This was an observational analytical study; therefore, there was low risk to patients. This study was designed to protect the identities and ensure data confidentiality of all patients who participated. The study was also reviewed and approved by a research ethics committee.
Study Design and Assessments
This was a multicenter historical cohort observational study of prevalent PD patients who were closely monitored from January 1, 2008, to December 31, 2009. Using specially developed forms, patient information and data were gathered by physicians and nurses who were responsible for attending to the patients at the remote PD centers.
Information and data were collected from historical clinical records, databases, and patient interviews and included demographics, cause of CKD, comorbidity (assessed using the Charlson comorbidity index) (13), performance status (assessed using the Karnofsky functional scale) (14), socioeconomic variables (income level, average monthly income (measured in terms of minimum monthly salaries in Colombian pesos), educational level, occupation, and type of health insurance coverage), housing (type of dwelling, type of floor in the dialysis area, type of walls in the dialysis area, type of wash basin used), access to public utilities (water, electric power, sewerage, natural gas, and telephone services), and transportation (type of transportation used to get to a dialysis center, time required to get to a remote PD center, and time required to get to an urban reference renal clinic). The date of admission into the PD program, date of entry into the study, number of months in the study, and the number of months of therapy patients had received at the time the study was closed were also recorded.
Outcome measures for this analysis included patient survival, rate of withdrawal from PD therapy (technique survival), the cause of withdrawal from PD therapy, and rates of peritonitis.
Statistical Analysis
SPSS statistical software package, version 18 (IBM SPSS, Armonk, New York) was used to perform statistical data analysis. A statistical description was prepared of all the variables, including calculations of the central trend measures and dispersion for quantitative variables and determinations of absolute frequencies and ratios for qualitative variables. Additionally, an estimate was made of the rate of peritonitis associated with PD, reported as one episode per x number of patient-months at risk, and the percentage of patients who withdrew from PD therapy due to causes related to dialysis. Subsequently, a bivariate analysis was performed using comparisons of ratios and chi-square association tests of independent variables and the occurrence of peritonitis associated with PD. The functions of survival were assessed for two outcomes of the cohort (mortality and withdrawal from PD) by plotting the Kaplan-Meier curve. For patient and technique survival rate analyses, the date of admission to the PD program was taken as the date of entry to the cohort. A 95% confidence interval (CI) was used for the estimators, and a 0.05 level of significance (α) was used for the two-tailed hypothesis tests.
Baxter RTS Colombia employees were involved in the collection, analysis, and interpretation of the data. All of the authors, some of whom were Baxter RTS and Baxter Latin America employees, approved the final manuscript for submission. The authors maintained autonomy and developed the manuscript without input from other Baxter employees.
Results
Treatment and Duration of Follow-Up
A total of 345 patients were eligible for the study; 198 (57.4%) received continuous ambulatory PD therapy and 147 (42.6%) received automated PD therapy. Patients were managed at one of nine remote PD centers with a median of 34 patients at each location (Figure 1). Patients were monitored for an average of 15.6 months (median, 16.6 months; range, 1 to 24 months) and 224 (64.9%) patients were monitored for more than a year. Patients had received PD therapy for a median of 26 months (interquartile range, 12 to 46.5 months) as of the closing date of the study.
Figure 1 —
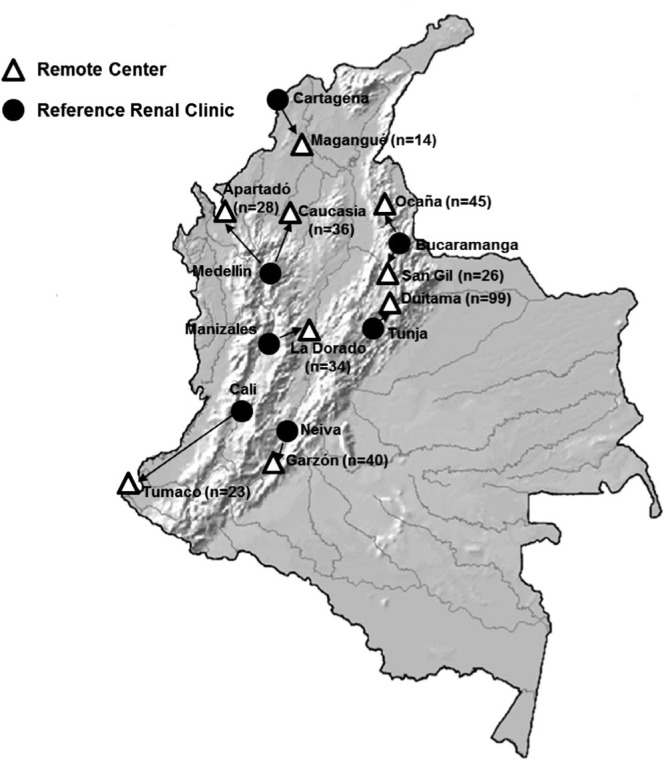
Distribution of home peritoneal dialysis patients receiving routine care and follow-up at remote peritoneal dialysis centers and associated urban renal clinics.
Patient Characteristics and Standard of Living
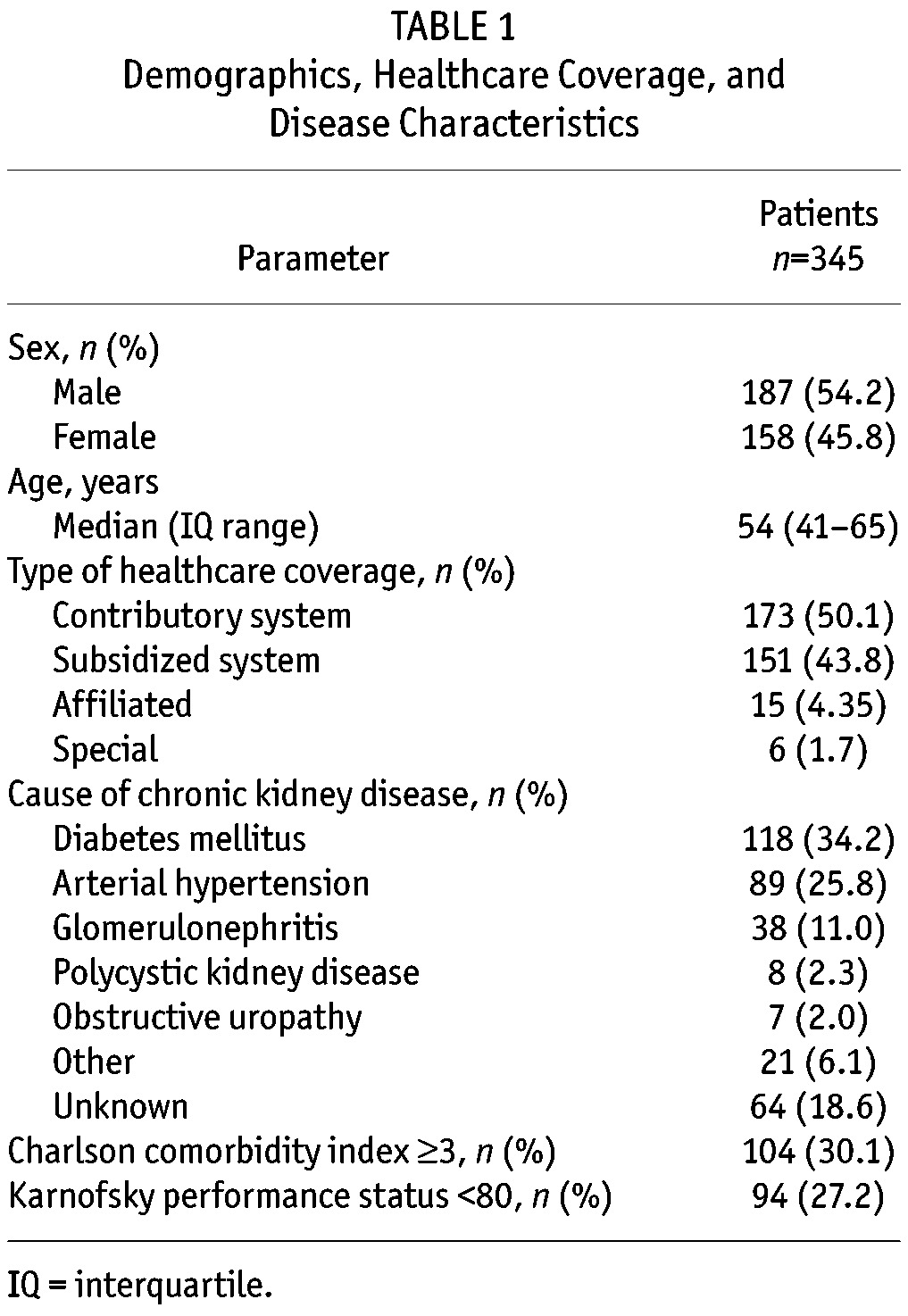
A summary of baseline demographics, healthcare coverage, and disease characteristics is shown in Table 1. The median age was 54 years and approximately half of the patients were male. All the patients had some type of healthcare coverage. The most common cause of CKD was diabetes mellitus, followed by arterial hypertension, and glomerulonephritis. The majority (72.8%) of patients had a Karnofsky performance status score of ≥ 80 (normal activity with some difficulty, some signs and symptoms).
TABLE 1.
Demographics, Healthcare Coverage, and Disease Characteristics
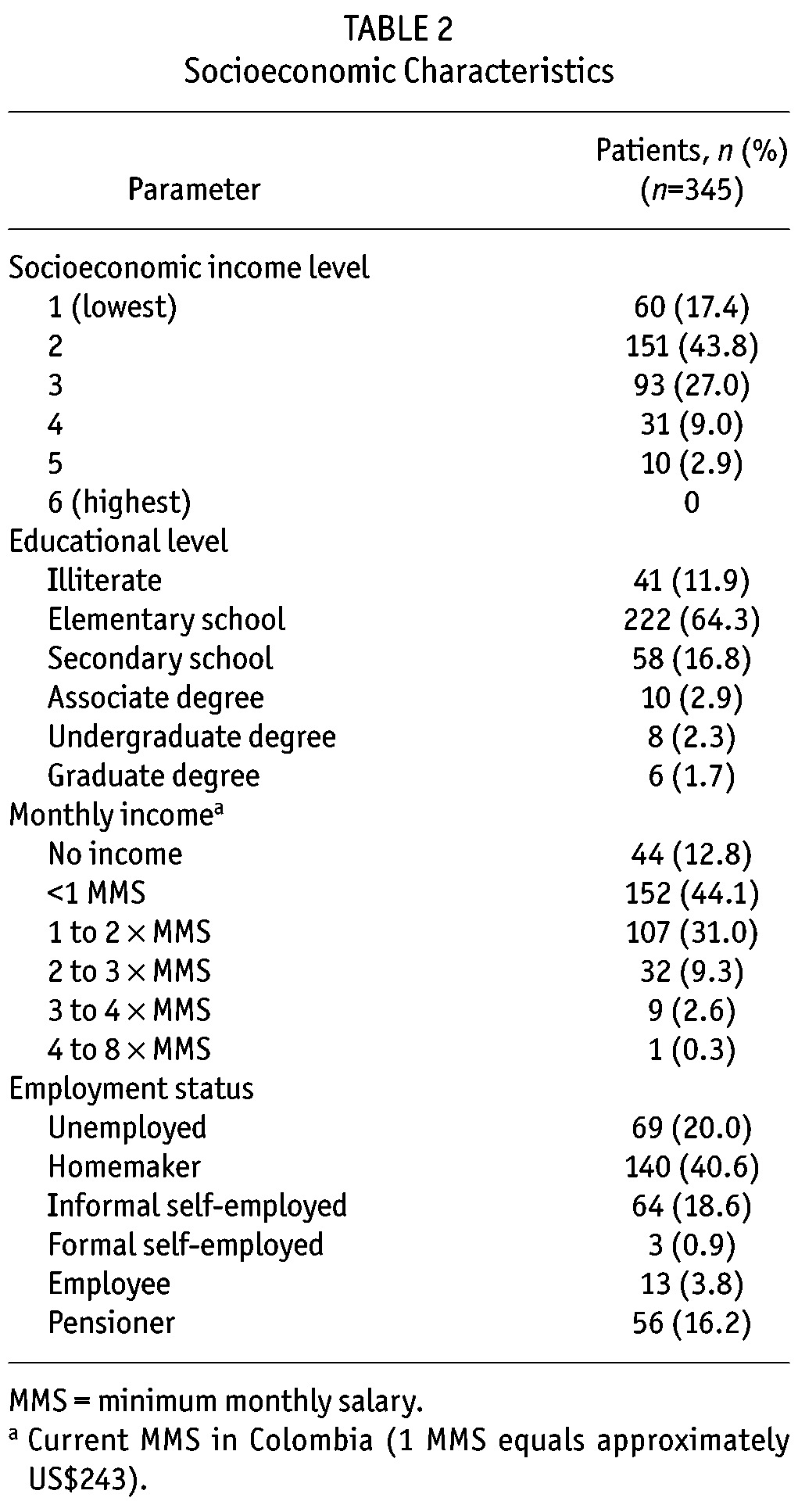
Socioeconomic Characteristics: The socioeconomic characteristics of the patient cohort are summarized in Table 2. The majority (88.1%) of patients had an income level in the lower half of the socioeconomic range. Most (93%) of the patients had completed only elementary or secondary school or were illiterate; few patients had received an undergraduate or graduate college degree. Most of the patients were homemakers or were engaged in some type of self-employment. More than half (56.8%) of the patients lived on less than one times minimum monthly salary (equivalent to US$243 USD); and 87.9% lived with two times a minimum monthly salary or less.
TABLE 2.
Socioeconomic Characteristics
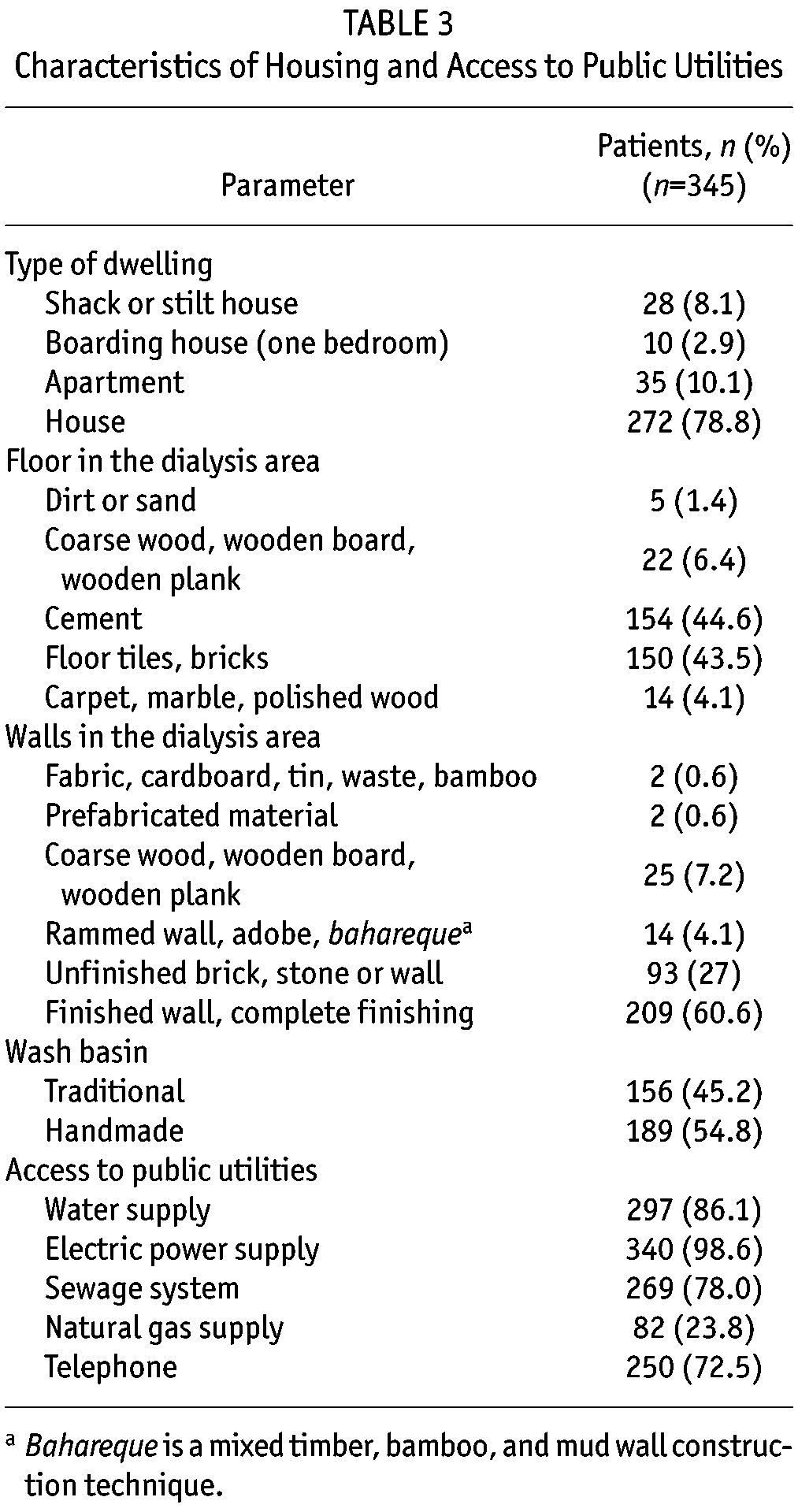
Housing and Access to Public Utilities: Two hundred thirty-nine (69.3%) patients lived in a small town and 106 (30.7%) patients lived in a rural or farm-like setting. Characteristics of the patients’ housing and their access to public utilities are shown in Table 3. Most of the patients lived in a house or apartment. For most patients, the floor of the room where dialysis was performed was made of cement, floor tiles, or brick. Similarly, for a majority of patients, dialysis area walls were finished or unfinished brick or stone. Approximately half of the patients used a traditional wash basin and the other half used a hand-made adapted wash basin. Most patients had access to electric power, water, sewage facilities, and telephone utilities. However, just 23.8% had access to a natural gas utility.
TABLE 3.
Characteristics of Housing and Access to Public Utilities
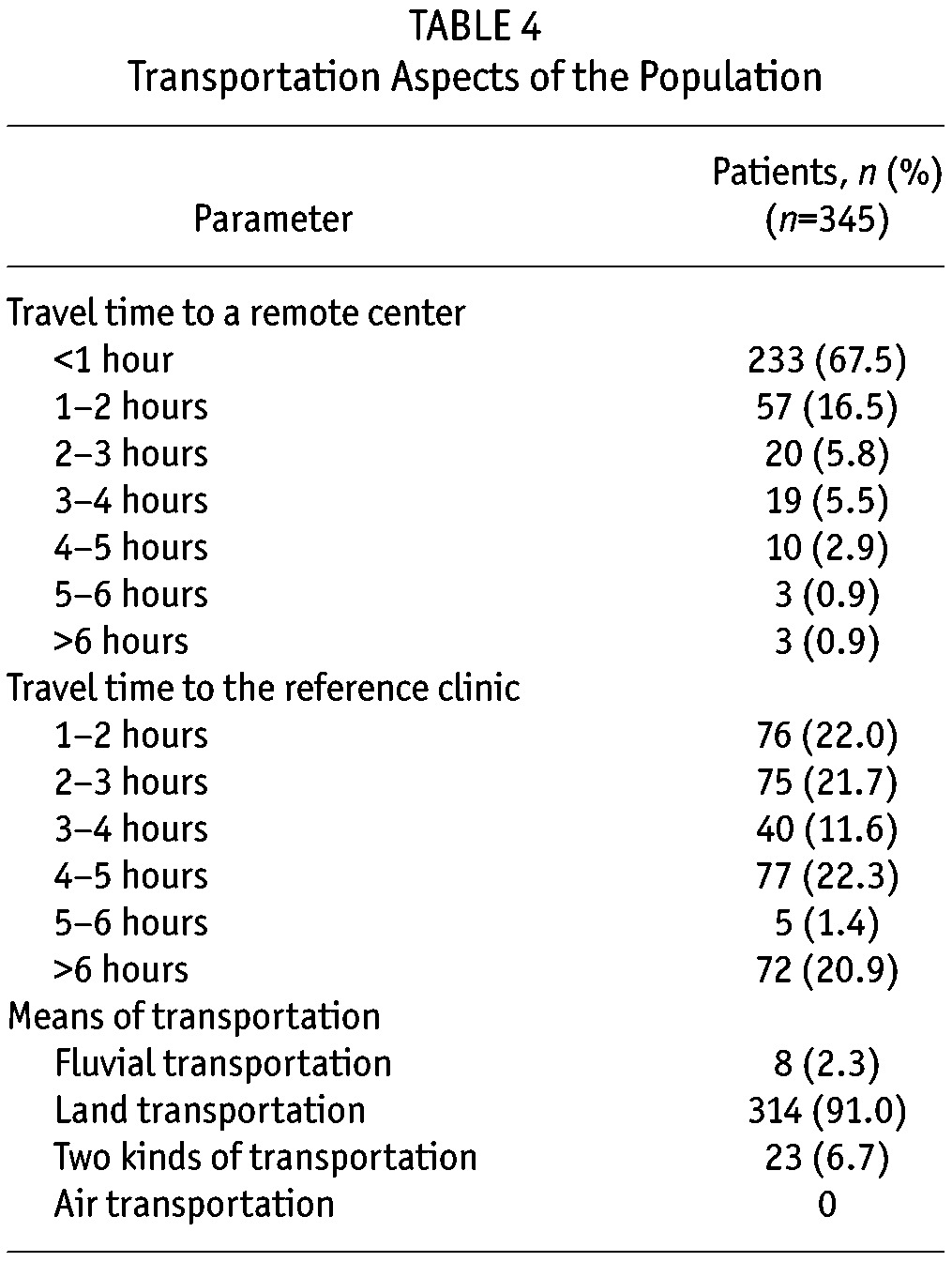
Aspects of Transportation: A detailed analysis of the time required to travel to a remote PD center or to an urban reference renal clinic for routine care and follow-up is provided in Table 4. The time for patients to travel from their homes to an urban reference renal clinic was, on average, 4.3 hours. In contrast, patients could travel from their homes to their remote PD center in 1.2 hours, on average. The majority (91%) of patients used land transportation to travel to either site; a few patients traveled by river or used two modes of transportation.
TABLE 4.
Transportation Aspects of the Population
Outcome Measures
Episodes of Peritonitis: A total of 137 episodes of peritonitis were reported during 5,380 patient-months of therapy. The incidence rate of peritonitis was 2.54 episodes per 100 patient-months (95% CI: 2.11, 2.97), which is equivalent to one episode of peritonitis for every 39.3 patient-months of risk.
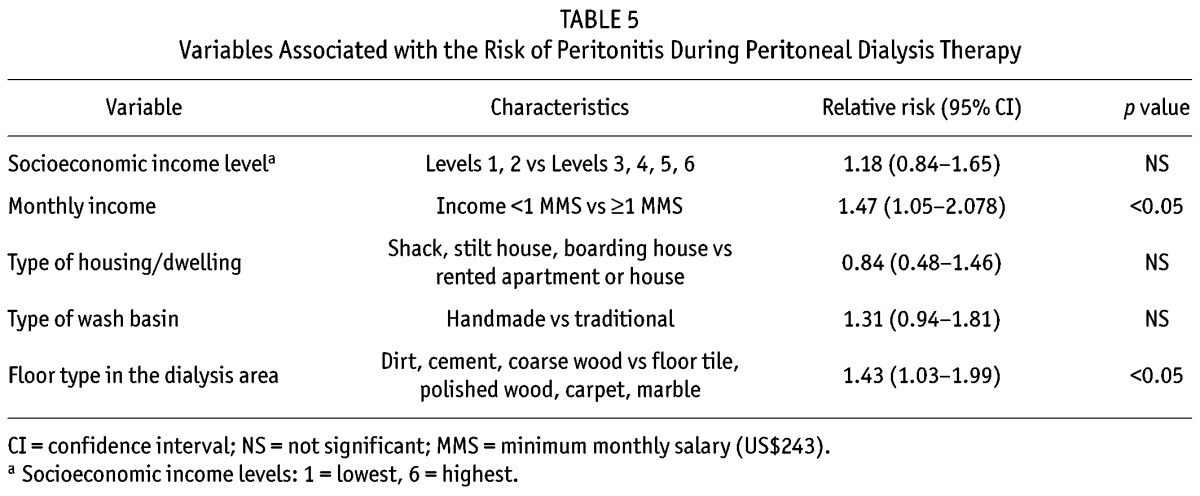
Based on a bivariate analysis, the risk of peritonitis was significantly higher in patients who were living on an income of less than one times the monthly minimum salary compared with one or more times the monthly minimum salary (p < 0.05) or who had a floor in their dialysis area made of dirt, cement, or unfinished wood compared with a floor made of floor tile, polished wood, carpet, or marble (p < 0.05) (Table 5). The risk of peritonitis was not associated with socioeconomic income level, type of house, or type of wash basin.
TABLE 5.
Variables Associated with the Risk of Peritonitis During Peritoneal Dialysis Therapy
Patient Survival: A total of 42 (40.8%) patients died during 448.3 patient-years of treatment, which is equivalent to a rate of 9.4 deaths per 100 patient-years (95% CI: 6.8, 12.5). Cause of death included cardiovascular (28 patients), unknown (five patients), peritoneal access-related infection (four patients), infection not related to peritoneal access (three patients), metabolic syndrome (one patient), and functional declination syndrome (one patient). A Kaplan-Meier curve depicting the rate of patient survival during two years of follow-up is shown in Figure 2. The 1-year patient survival rate was 92.44% and the 2-year patient survival rate was 81.55%.
Figure 2 —
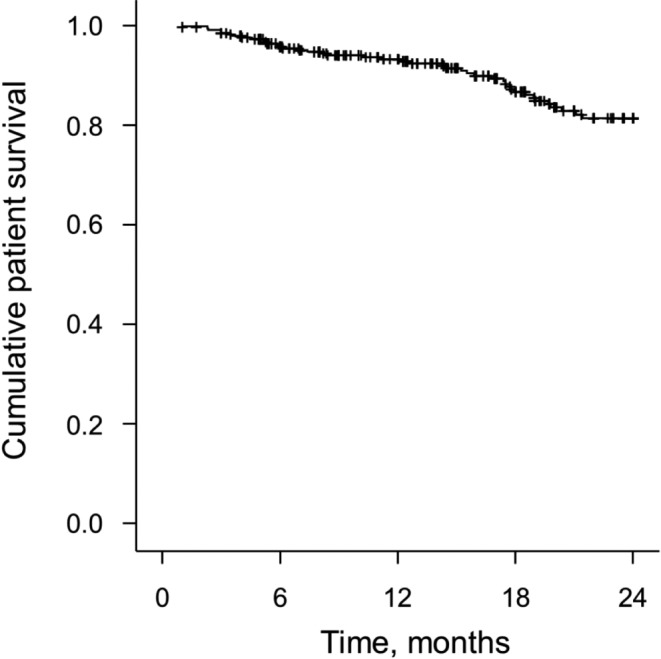
Kaplan-Meier curve depicting rate of patient survival.
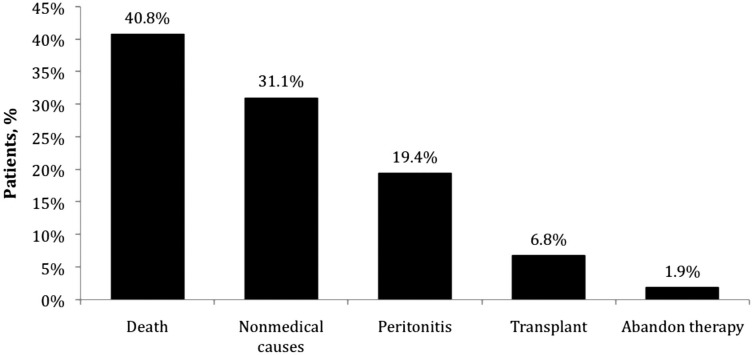
Withdrawal from Peritoneal Dialysis: During a total of 448.3 patient-years of treatment, 103 of the 345 patients withdrew from PD therapy, which is equivalent to a rate of 22.9 withdrawals per 100 patient-years (95% CI: 18.4, 27.4). Figure 3 summarizes the causes of patient withdrawal from PD therapy. The most common reason for patient withdrawal from PD therapy was death. Thirty-two (31.1%) patients withdrew from PD therapy for nonmedical reasons including 30 (29.1%) who withdrew because of a change in insurance and two (1.9%) who withdrew due to recovery of renal function. A total of 20 (19.4%) patients withdrew from PD therapy due to peritonitis. Therefore, the rate of withdrawal for reasons other than mortality was 13.6 withdrawals per 100 patient-years (95% CI: 10.3, 16.9). A Kaplan-Meier curve depicting the rate of patient withdrawal from PD therapy during two years of follow-up is shown in Figure 4. The 1-year and 2-year technique survival rates were 97.27% and 89.78%, respectively.
Figure 3 —
Causes of withdrawal from peritoneal dialysis (PD) therapy.
Figure 4 —
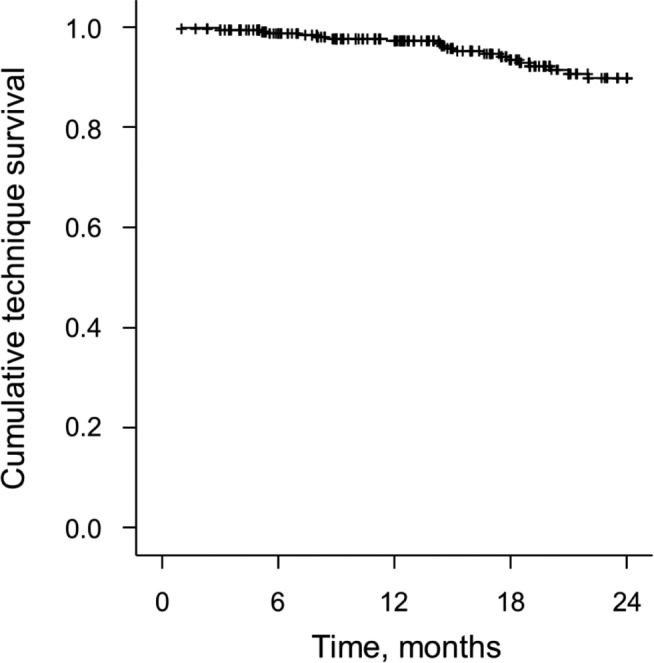
Kaplan-Meier curve depicting rate of patient withdrawal from peritoneal dialysis (technique survival).
Discussion
This report describes patient characteristics, standard of living, and outcomes of a historical patient cohort undergoing PD therapy at home and receiving routine care and follow-up at remote PD centers that are part of the RTS network in Colombia. Patients in this cohort were in the lowest socioeconomic income levels or considered to be living at or below the poverty level. The majority of the patients had only a basic level of education and 11.9% of the patients were illiterate. Most of the patients lived in a house with a hard nonporous floor and wall surfaces in the area where they perform their home PD. However, some patients did not have access to public utilities.
It could be hypothesized that the unfavorable socioeconomic status of the patient cohort in this study would disqualify them from treatment with PD therapy at home. A lower education level has been suggested to predict technique failure in a patient cohort receiving PD therapy in Canada (15). However, in a prospective cohort study of nearly 2,000 incident PD patents conducted in Brazil, economic status was not a significant predictor of patient survival or technique failure (16). In another historical cohort study of incident patients in Colombia, mortality rate was higher among patients receiving HD than among patients treated with PD, even though the patients receiving PD were poorer (3).
Other factors that may influence PD patient outcomes are the locality of their home and the distance of their home to a renal clinic. Patients with CKD who live in remote areas are less likely to receive recommended care and are more likely to experience adverse health outcomes (17). Thompson et al. (10) reported that HD patients who lived more than 160 km from a dialysis unit had an increased risk of death compared with those patients living closest to the center. Interestingly, although patients living in remote areas were more likely to live in a rural area, half of the patients in that study who lived more than 160 km from the renal center resided in an urban setting, suggesting that distance rather than rural or urban status had a greater effect on risk of mortality in that cohort of patients in the United States. Higher rates of mortality have also been reported in China for ESRD patients who live in rural areas compared with those living in urban areas (18). Importantly, none of the patients living in rural areas in that study were receiving PD therapy.
As with HD, studies have demonstrated an increased rate of mortality in PD patients who live more than 50 to 80 km from a renal clinic (11,19). PD patients living in remote rural areas have also been reported to have a higher risk of switching to HD, although this did not appear to be the case for patients living in small rural towns (20). In contrast, results from a retrospective cohort study in Canada reported that the distance to a treatment center and residence in a rural area did not have an effect on either mortality or technique failure in patients receiving PD (15). In addition, although Tonelli et al. (11) reported an increased risk of mortality with increasing distance from a renal clinic, they also reported a lower risk of technique failure for patients living in a remote area that was similar among patients receiving automated PD and those receiving continuous ambulatory PD.
As a departure from other cohort studies, our study focused only on patients who lived in remote areas in Colombia and were being supported by a remote PD center. These remote PD centers were established to overcome socioeconomic or geographic barriers to treatment. A similar program established in China reported a decrease in the PD therapy dropout rate, improved patient survival, and improved technique survival for those patients receiving PD (12). All of the patients in our study lived in rural areas or small towns with 78% and 45% of the patients living ≥ 2 hours or ≥ 4 hours away from an urban reference renal clinic, respectively. Despite the distance from an urban reference renal clinic, the patient survival rate was 92.44% at one year and 81.55% at two years of follow-up, and the mortality rate was 9.4 deaths per 100 patient-years. These results compare favorably with a 70% 2-year survival rate reported for a prospective cohort of incident PD patients in Brazil that had approximately 30% of its patients living farther than 50 km from the clinic (16), and to the 14.3 to 15.0 deaths per 100 patient-years reported for patients in Canada who lived farther than 50 km away from the closest nephrologist (11).
Similarly, technique survival rates in our study compare favorably or are similar to those reported by other groups. In this study, the 1- and 2-year technique survival rates were 97.27% and 89.78%, respectively. In comparison, the 1-year technique survival rate for patients in the satellite PD program in China was 93% (12) and the 2-year technique survival rate for patients in the Brazilian cohort was 73% (16). Notably, Tonelli et al. (11) reported that, as the distance from a patient’s home to an attending nephrologist increased, the likelihood of technique failure actually decreased.
An increase in the rates of complications including peritonitis and infections has been reported for patients receiving PD therapy in remote areas (9). A peritonitis rate of 0.77 episodes per patient-year (equivalent to one episode for every 15.58 patient-months) was reported for patients living 100 km or farther from a PD center in Australia (8). In comparison, the rate of peritonitis in the current study was one episode for every 39.3 patient-months of risk. In addition, we report peritonitis rates that are lower than those recommended by the International Society for Peritoneal Dialysis (one episode for every 18 patient-months) (21). These findings suggest that in spite of living in remote areas under perhaps less than ideal conditions for treatment, home PD therapy in remote areas in Colombia is safe when supported by a remote PD center with additional assistance provided by an urban reference renal clinic.
A bivariate analysis of the current patient cohort revealed a significant association between a patient’s monthly income and floor type in his or her dialysis area and the risk of peritonitis (p < 0.05). These two variables are most representative of a family’s overall well-being and the hygiene conditions at the home. The potential relationships between a patient’s monthly income, floor type in the dialysis area, or any other variables and patient survival or technique survival were not explored because this study lacked the necessary statistical power for such an analysis. As noted previously, a recent study did not find a relationship between a family’s monthly income and patient survival or PD technique dropout rate (16).
Like all observational cohort studies, this study is associated with limitations and potential bias. One potential patient selection bias may have over-estimated the outcomes of PD therapy, as only patients with stable disease are likely to receive care at remote PD centers, while patients with uncontrolled disease tend to receive routine care and follow-up at a large urban center or are switched to HD. The status of a patient’s disease may also influence the way therapeutic teams make treatment recommendations to their patients.
Although PD therapy is associated with positive clinical outcomes and has recently been shown, among patients who started dialysis treatment after 2000, to be associated with a risk of death that is similar to that experienced by patients undergoing HD (22), many have suggested that PD therapy is underutilized (23–25). The outcome results of this study may have been influenced by the marked improvements and maturation of care practices in the field of PD therapy (26). Even so, our findings suggest that a remote PD program can safely provide effective PD support to patients who live in remote areas under less than favorable conditions. While no quality-of-life measures were assessed in this study, it would be interesting to determine if the closeness of the patient’s home to a remote PD center has a positive effect on the patient’s overall quality of life. Future studies are needed to fully characterize the effectiveness of long-term PD therapy using a remote PD center program.
Conclusions
Most of the PD patients in this study who performed PD at home and received routine care and follow-up at remote PD centers in Colombia reported a low socioeconomic income level and some had poor access to basic public utilities. Monthly income and the type of floor in the area where a patient’s dialysis was performed were factors associated with a risk of peritonitis. Results from this study demonstrate that remote PD centers can provide safe and favorable patient and technique survival outcomes. With the support of a remote PD center program, home PD therapy is an appropriate treatment option for patients who live in remote areas. Such a PD program can mitigate a patient’s financial and healthcare inequities and provide the additional benefit of reducing travel time.
Disclosures
This study was supported by Baxter Renal Therapy Services Colombia. Funding to support the preparation of this manuscript was provided by Baxter RTS Colombia, Bogotá, Colombia. The authors verify that they have met all of the journal’s requirements for authorship. The authors received no financial compensation for the development of this manuscript.
Gilma Hernández has received grant/research support from Baxter and served as a statistical consultant for this study. Carlos Trillos, MD, and Catalina Latorre, MD, have no potential conflicts to report. Mauricio Sanabria, Mauricio Uribe, Martha Devia, Kindar Astudillo, and Astrid Bernal are full-time employees of Baxter RTS Colombia. Angela Rivera is a full-time employee of Baxter Latin America.
Acknowledgments
The authors thank all the local investigators and PD nursing teams who participated in the study, including Alvaro Ordoñez and Nancy Salazar (RTS San Gil), Alvaro Ordoñez and Erika Jacome (RTS Ocaña), Theo Marinez and Natali Valderrama (RTS Garzón), Mauricio Uribe and Yorledys Moron (RTS Apartadó), Mauricio Uribe and Lidis Durango (RTS Caucasia), Cesar Restrepo and Gloria Barragan (RTS La Dorada), Fabio Rojas and Fabiola Gutierrez (RTS Duitama), Oscar Guerrero and Jazmin Arias (RTS Tumaco), and Fredy Balocco and Claudia Benitez (RTS Magangué).
The authors thank Lamara D. Shrode, PhD, CMPP, of The JB Ashtin Group, Inc., for assistance in preparing this manuscript for publication. The authors take full responsibility for all content.
REFERENCES
- 1. Riella MC, Locatelli AJ. History of peritoneal dialysis in Latin America. Perit Dial Int 2007; 27:322–7. [PubMed] [Google Scholar]
- 2. U S Renal Data System. Chapter 12: International Comparisons. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012: 341–52. [Google Scholar]
- 3. Sanabria M, Muñoz J, Trillos C, Hernández G, Latorre C, Díaz CS, et al. Dialysis outcomes in Colombia (DOC) study: a comparison of patient survival on peritoneal dialysis vs hemodialysis in Colombia. Kidney Int Suppl 2008; (108):S165–72. [DOI] [PubMed] [Google Scholar]
- 4. The World Bank. Data. Countries and Economics. Colombia: Available at: http://data.worldbank.org/indicator/SI.POV.2DAY. Accessed September 14, 2012. [Google Scholar]
- 5. Guerrero R, Gallego AI, Becerril-Montekio V, Vásquez J. [The health system of Colombia]. Salud Publica Mex 2011; 53(Suppl 2):S144–55. [PubMed] [Google Scholar]
- 6. Ospina ML, Montaño JI, Acuña L. Enfermedad Renal Crónica en Colombia 2010. Capítulo 4. Enfermedad Renal Crónica Terminal en Colombia. Bogotá, Colombia: Cuenta de Alto Costo; 2011. Available at: http://www.cuentadeltocosto.org. [Google Scholar]
- 7. Pérez Correa E, Pérez Martínez M. [The rural sector in Colombia and its current crisis]. Cuadernos de Desarrollo Rural 2002; 48:35–58. [Google Scholar]
- 8. Cho Y, Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville N, et al. The effects of living distantly from peritoneal dialysis units on peritonitis risk, microbiology, treatment and outcomes: a multi-centre registry study. BMC Nephrol 2012; 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology (Carlton) 2005; 10:192–7. [DOI] [PubMed] [Google Scholar]
- 10. Thompson S, Gill J, Wang X, Padwal R, Pelletier R, Bello A, et al. Higher mortality among remote compared to rural or urban dwelling hemodialysis patients in the United States. Kidney Int 2012; 82:352–9. [DOI] [PubMed] [Google Scholar]
- 11. Tonelli M, Hemmelgarn B, Culleton B, Klarenbach S, Gill JS, Wiebe N, et al. Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int 2007; 72:1023–8. [DOI] [PubMed] [Google Scholar]
- 12. Jiang Z, Yu X. Advancing the use and quality of peritoneal dialysis by developing a peritoneal dialysis satellite center program. Perit Dial Int 2011; 31:121–6. [DOI] [PubMed] [Google Scholar]
- 13. Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 2003; 42:125–32. [DOI] [PubMed] [Google Scholar]
- 14. Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C, ed. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949: 196. [Google Scholar]
- 15. Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int 2011; 31:565–73. [DOI] [PubMed] [Google Scholar]
- 16. de Andrade Bastos K, Qureshi AR, Lopes AA, Fernandes N, Barbosa LM, Pecoits-Filho R, et al. Family income and survival in Brazilian peritoneal dialysis multicenter study patients (BRAZPD): time to revisit a myth? Clin J Am Soc Nephrol 2010; 6:1676–83. [DOI] [PubMed] [Google Scholar]
- 17. Bello AK, Hemmelgarn B, Lin M, Manns B, Klarenbach S, Thompson S, et al. Impact of remote location on quality care delivery and relationships to adverse health outcomes in patients with diabetes and chronic kidney disease. Nephrol Dial Transplant 2012; July 2 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Gong Z, Peng X, Tang S, Bi M, Huang W. Clinical characteristics and outcomes of rural patients with ESRD in Guangxi, China: one dialysis center experience. Int Urol Nephrol 2010; 42:195–204. [DOI] [PubMed] [Google Scholar]
- 19. Maripuri S, Arbogast P, Ikizler TA, Cavanaugh KL. Rural and micropolitan residence and mortality in patients on dialysis. Clin J Am Soc Nephrol 2012; 7:1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehrotra R, Story K, Guest S, Fedunyszyn M. Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit Dial Int 2012; 32:322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 22. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171:110–8. [DOI] [PubMed] [Google Scholar]
- 23. Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59:e1–420. [DOI] [PubMed] [Google Scholar]
- 24. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van de Luijtgaarden MW, Noordzij M, Stel VS, Ravani P, Jarraya F, Collart F, et al. Effects of comorbid and demographic factors on dialysis modality choice and related patient survival in Europe. Nephrol Dial Transplant 2011; 26:2940–7. [DOI] [PubMed] [Google Scholar]
- 26. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol 2007; 18:2781–8. [DOI] [PubMed] [Google Scholar]