Catheter obstruction is a serious complication of peritoneal dialysis (PD). This problem can be due to non-mechanical obstruction (e.g. fibrin clots) or mechanical obstruction (e.g. omentum or mesentery wrapping, strangulation of uterine tube fimbriae). Urokinase is effective for dissolving fibrin clots but occasionally fails. Our method is useful for diagnosing whether or not there are fibrin clots left in the catheter. In the case of mechanical obstruction, surgery, which is very invasive, is usually required to release the wrapping or strangulating organ from the catheter. Our method allows for early diagnosis of the cause of obstruction and successful settlement. We suggest the new and non-invasive approach to detect and treat obstruction.
Case Report
A 77-year-old Japanese male with end-stage renal disease due to diabetes mellitus was introduced to automated PD in April 2011. He presented with outflow failure in May 2012. X-rays showed catheter tip migration, and he was operated to repair the malposition. Two weeks later, the dialysate contained a lot of fibrin and the outflow slowed again so urokinase was injected. Although the inflow and outflow speed resumed, there was soon a complete absence of outflow. To investigate and resolve the cause of the catheter obstruction, we used a bronchoscope for newborn infants (BF-N20, Olympus, Japan, Figure 1A) with a 2-mm diameter. After filling the catheter with glycerin, the bronchoscope could easily be inserted into a Tenckhoff catheter (inner diameter of 2.6 mm). This examination revealed that the tip of the catheter was touching the bladder wall, which operated like a check valve. Fat tissue of the visceral organ and fibrin clots obstructed the side holes of the catheter (Figure 2). The fat tissue and fibrin clots were released into the peritoneal cavity mechanically by delicately inserting a bronchoscope without further use of urokinase. This caused the position of the catheter to change, improving outflow. The peritoneum was pink and normal. The catheter subsequently functioned properly and the patient was able to continue with PD.
Figure 1A —
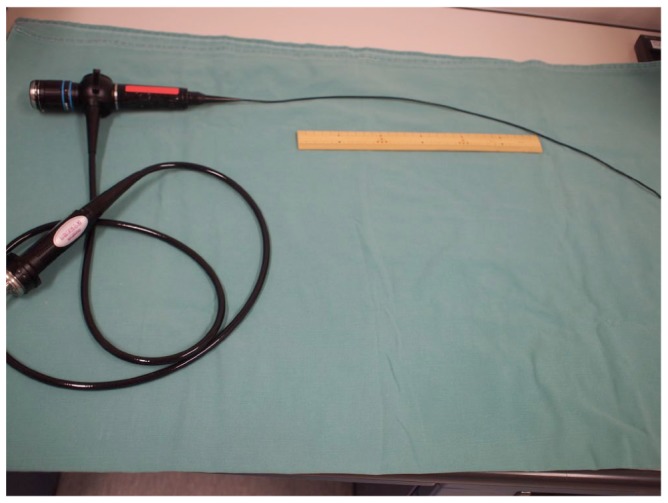
A bronchoscope for newborn infants (BF-N20, Olympus, Japan). Inner diameter is 2.2 mm. Working length is 55 cm, so it can reach the tip of the Tenckhoff catheter (MD-4, Medi-Tech, length 48.5 cm).
Figure 2 —
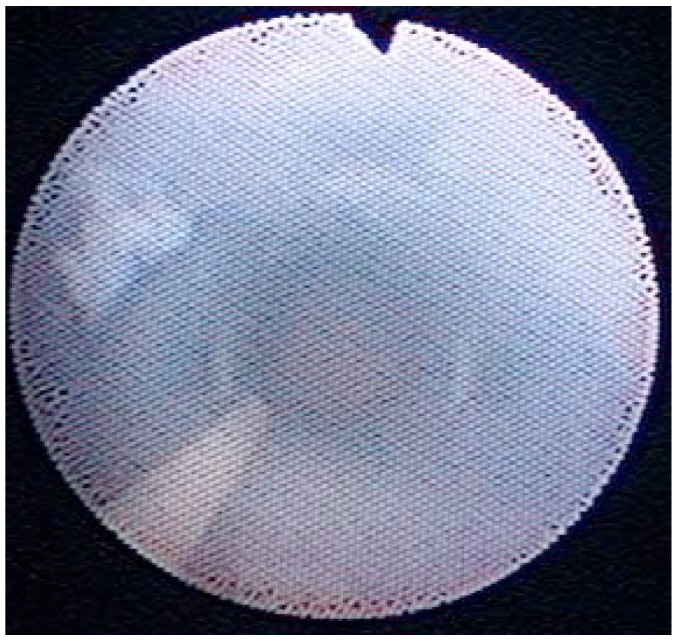
Inside luminal view of PD catheter. Most of the fibrin clots which obstructed the side holes of the catheter were dissolved by urokinase.
Discussion
It is difficult and unsuccessful to salvage a malfunctioning catheter using stiff wire manipulation under fluoroscopic guidance. This manipulation has shown a high incidence of recurrent malfunction and the need for repeat manipulation (1,2). Laparoscopic salvage offers 62 to 100% success rate and 0 to 39% recurrence (3,4,5) but is invasive. Our bronchoscopic method is non-invasive and provides direct visibility into the PD catheter. This enables early diagnosis of the cause of catheter obstruction. In cases of obstruction due to fibrin clots, urokinase should be used. In cases of mechanical obstruction, the issue can be resolved by delicately inserting a bronchoscope with glycerin, thereby avoiding the salvage surgery.
The use of a gastrofiberscope for percutaneous endoscopic gastrostomy whose diameter was 2.4 mm has also been reported (6). In this article, we discussed the use of the bronchoscope, whose inner diameter is 2.2 mm. We would like to add the information of the smaller scope, however, whose diameter was 1.8 mm (BP2-1865, Machida, Japan, Figure 1B). We used this smaller scope in several cases after this patient’s treatment.
Figure 1B —
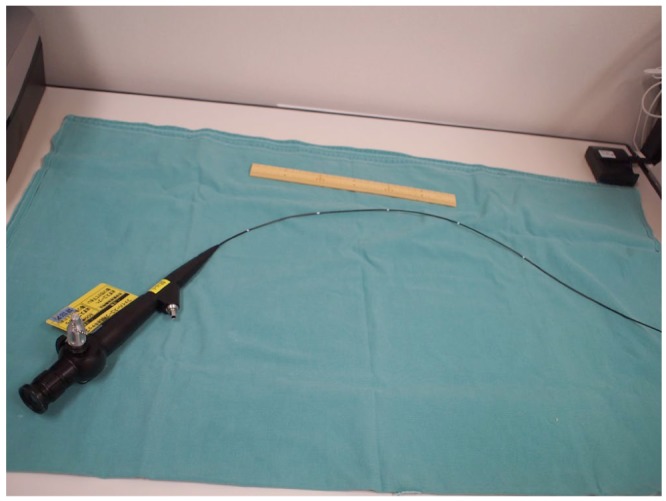
A bronchoscope for newborn infants (BP2-1865, Machida, Japan). Inner diameter is 1.8 mm and working length is 65 cm.
Moreover, the pouch of Douglas, where the peritoneum might be most damaged, can be directly observed (Figure 3). If the mesothelial surface turns brownish, this may indicated the accumulation in the peritoneum of advanced glycosylation end-products (AGEs) and degeneration (7). In the future, peritoneal damage could be diagnosed without peritoneal biopsy.
Figure 3 —
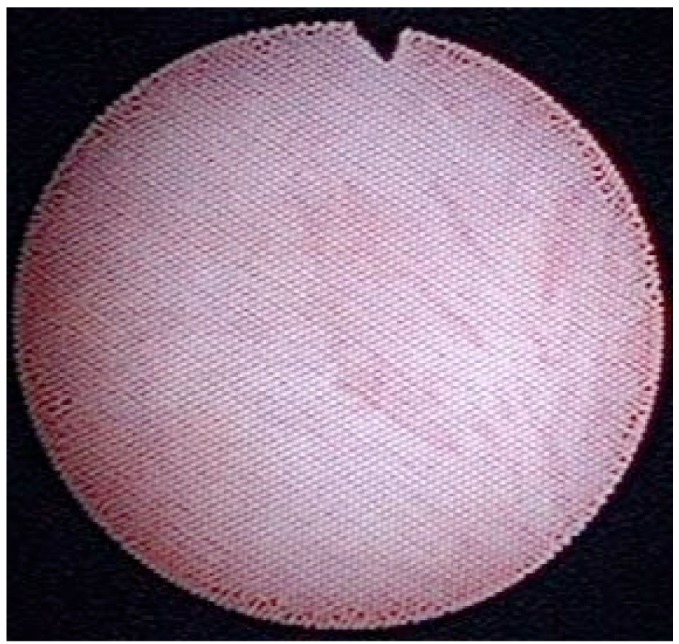
Inside luminal view of pouch of Douglas. The color of the peritoneum was normal, not brown, which could indicate accumulation in the peritoneum of advanced glycosylation end-products.
In conclusion, this approach makes possible not only early diagnosis of catheter obstruction and treatment but also noninvasive diagnosis of peritoneal degeneration in the future.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Moss JS, Minda SA, Newman GE, Dunnick NR, Vernon WB, Schwab SJ. Malpositioned peritoneal dialysis catheters: a critical reappraisal of correction by stiff-wire manipulation. Am J Kidney Dis 1990; 15:305–8. [DOI] [PubMed] [Google Scholar]
- 2. Diaz-Buxo JA, Turner MW, Nelms M. Fluoroscopic manipulation of Tenckhoff catheters: outcome analysis. Clin Nephrol 1997; 47:384–8. [PubMed] [Google Scholar]
- 3. Amerling R, Vande Maele D, Spicak H, Lo AY, White P, Beaton H, et al. Laparoscopic salvage of malfunctioning peritoneal catheters. Surg Endosc 1997; 11:249–52. [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Donovan JF. Laparoscopic omentectomy for salvage of peritoneal dialysis catheters. J Endourol 2002; 16:241–4. [DOI] [PubMed] [Google Scholar]
- 5. Barone GW, Johnson DD, Webb JW. A practical approach to laparoscopic surgery for malfunctioning peritoneal dialysis catheters. J Laparoendosc Adv Surg Tech A 1998; 8:19–23. [DOI] [PubMed] [Google Scholar]
- 6. Takara Y, Ishibashi Y, Fujishiro M, Fujita T. Endoscopic treatment of obstructed peritoneal catheter. Kidney Int 2011; 80:679. [DOI] [PubMed] [Google Scholar]
- 7. Di Paolo N, Sacchi G. Atlas of peritoneal histology. Perit Dial Int 2000; 20:S5–96. [PubMed] [Google Scholar]


