Encapsulating peritoneal sclerosis (EPS) is the most serious and life-threatening complication of peritoneal dialysis (PD) therapy, as mortality is extremely high once it occurs. According to recent reports (1–5), mortality rate of EPS is 14 – 84%. Extensive fibrosing of the peritoneum in patients with EPS leads to symptoms of malnutrition and small bowel obstruction (ileus). Encapsulation can progress so that the bowel eventually becomes completely cocooned. The outcomes of several medical approaches such as tamoxifen, steroids, and newer immunosuppressive agents to retard EPS progression (6) remain poor. Moreover, in some alive cases, surgical approach (enterolysis, peritonectomy) is inevitable to cure symptoms of ileus (6).
Dissolved hydrogen (H2) has a unique biological antioxidative and anti-inflammatory capacity (7). Accumulating evidence suggests that H2 ameliorates organ damage in various models of ischemia and inflammation (8). Clinical applications of H2 to pro-inflammatory disorders have been investigated, particularly for use during hemodialysis (HD) therapy (9–15). In addition, we recently showed that peritoneal dialysate containing high H2 concentrations could reduce both peritoneal and systemic oxidative stress in the clinical setting (16).
In the present case study, dialysate enriched with H2 for both hemodialysis (HD) therapy and peritoneal lavage caused symptoms and laboratory signs of EPS to decrease.
Case History
A 63-year-old Japanese man with a 6-year history of continuous ambulatory peritoneal dialysis (CAPD) presented to our emergency room with constipation and nausea of 7 days’ duration.
The patient had been diagnosed with diabetes mellitus at the age of 40, and had begun PD at the age of 57. Peritoneal permeability in the fast peritoneal equilibrium test remained at a high average. He had been prescribed with 2.27% glucose dialysate (2 L/bag, 2 bags/day) and 7.5% icodextrin dialysate (2 L/bag, 1 bag/day). He had no past history of infectious peritonitis nor hemoperitoneum.
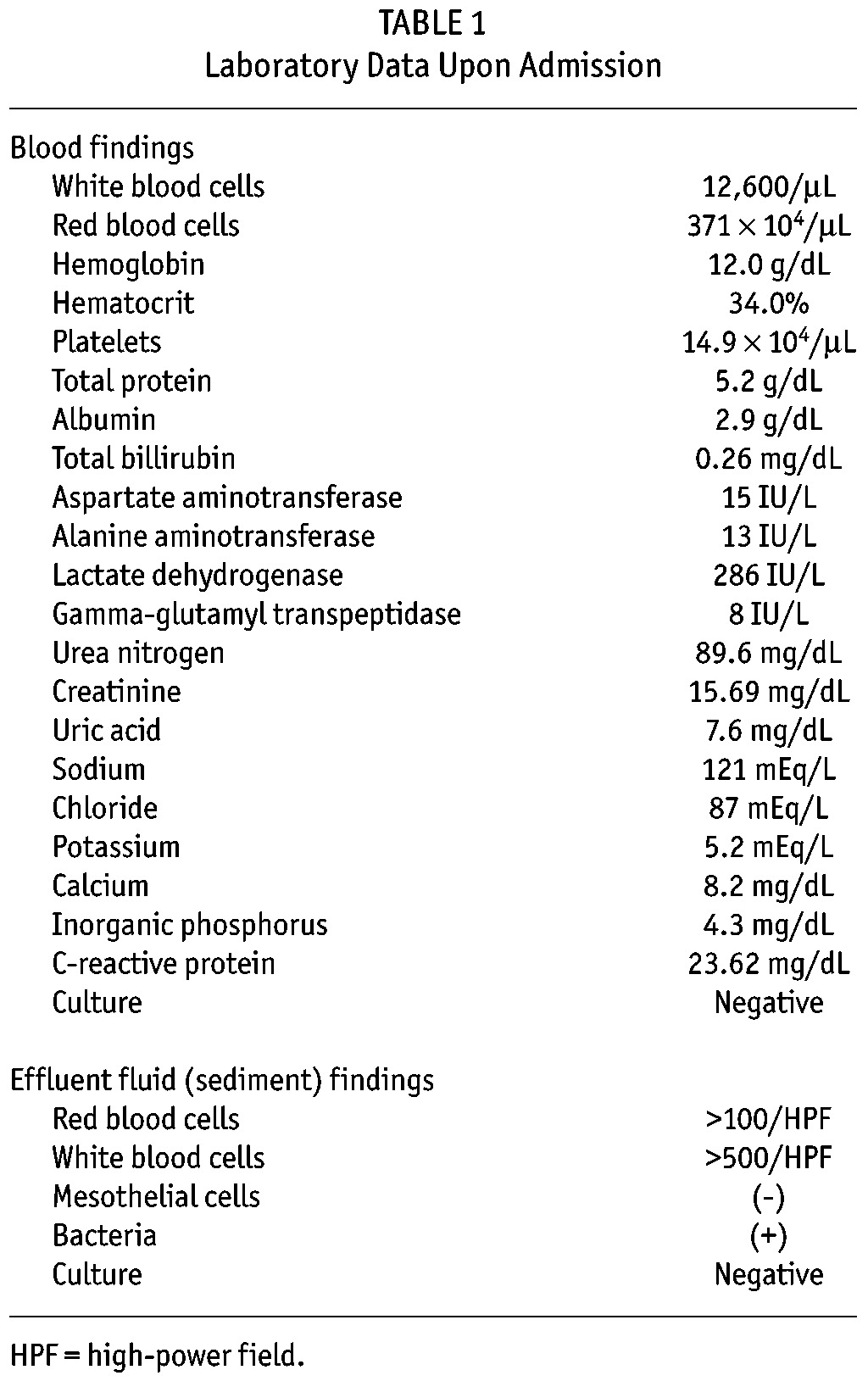
Defecation ability ceased 7 days before presenting at our emergency room with constipation and nausea. His blood pressure was 155/80 mmHg, pulse 70 beats/min and temperature 37.8°C. His abdomen was distended with slight diffuse tenderness. A glycerin enema failed to elicit bowel movement and he was admitted with suspected EPS. Table 1 shows the laboratory findings. Inflammatory markers (white blood cells and C-reactive protein [CRP]) were elevated (12,600 /μL and 23.62 mg/dL, respectively). Effluent fluid was slightly cloudy with elevated numbers of white blood cells in the sediment. Infectious peritonitis was tentatively diagnosed based on these findings.
TABLE 1.
Laboratory Data Upon Admission
The patient’s oral intake was stopped and total parenteral nutrition was started. Peritoneal therapy was discontinued and hemodialysis (HD) was started on hospital day 3. The patient had nausea that was not improved by intravenous ceftazidime and the effluent remained cloudy. Repetitive effluent fluid culture remained negative.
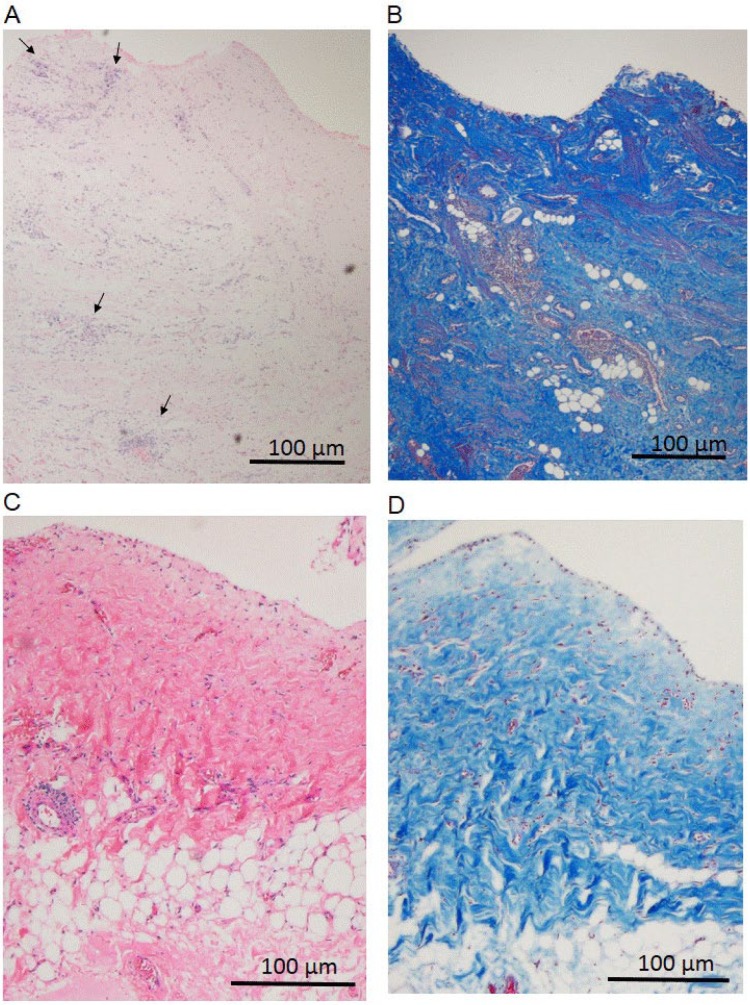
The peritoneal catheter was laparoscopically removed and re-implanted on hospital day 10. A laparoscopic examination revealed that the intestine was encapsulated by a thin white membrane (Figure 1). Figure 2A shows the histological findings of a biopsy specimen from the parietal peritoneum (hemotoxilin-eosin stain). Visualization using Masson trichrome revealed a peritoneal surface that was up to 2,000 μm thick and mainly comprised red or blue homogeneous or lamellar fibrinous material containing scatted fibroblasts. Figure 2B shows fibrin or organized fibrin components. Mesothelium was not identified. These findings and the clinical course indicated a final diagnosis of EPS. Prednisolone (30 mg/day) combined with peritoneal lavage using peritoneal dialysate somewhat improved the nausea and decreased the serum CRP level. However, the nausea worsened and CRP became elevated when the prednisolone dose was reduced to 20 mg/day on hospital day 21.
Figure 1 —
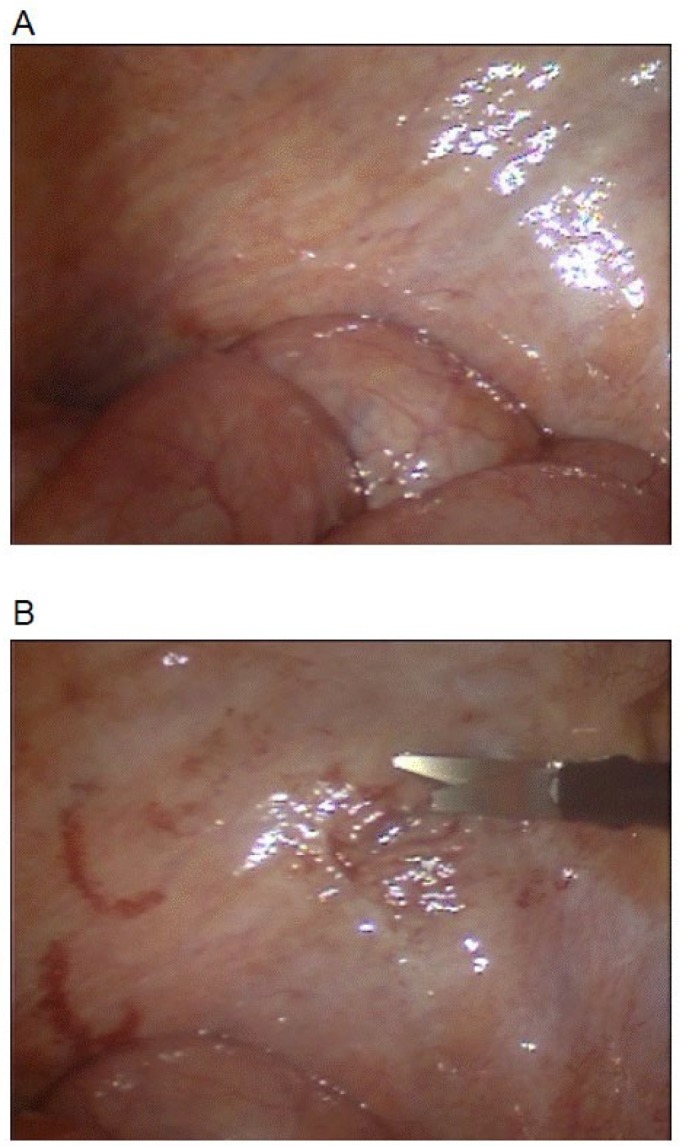
Macroscopic findings at laparoscopic examination. (A) Both visceral and parietal peritonea are whitish and thickened. Intestine is encapsulated by thin membrane. (B) Specimen of parietal peritoneum obtained under laparoscopic monitoring. 254×366mm (72 × 72 DPI)
Figure 2 —
Histological findings of parietal peritoneum before (A, B) and after (C, D) treatment with hydrogen-enriched dialysate. (A) Thin membrane covering peritoneum consists mainly of homogeneous or lamellar fibrinous material containing fibroblasts stained with hematoxylin and eosin (HE). Mononuclear cell infiltration is visible in some areas (arrowheads). (B) Fibrinous materials (red or blue with Masson trichrome stain) suggest fibrin or organized fibrin components. (C) Sub-mesothelial connective tissue (compact zone) has become obviously thinner after treatment with H2 (mean: 190 μm; HE stain). (D) Surface is covered by mesothelial monolayer and fibrinous materials are absent (Masson trichrome stain). 254×366mm (72 × 72 DPI)
Considering the possible contribution of oxidative stress on the pathogenesis of EPS, HD using H2-enriched dialysate (12) was started on hospital day 31. This decreased the CRP value (Figure 3) and improved the nausea. Oral intake was started on hospital day 38 without the recurrence of nausea. Peritoneal lavage using H2-enriched peritoneal solution was started on hospital day 40 to minimize peritoneal damage as described (16). This strategy obviously diminished the cloudiness of the effluent fluid due to blood (Figure 3). The prednisolone dose was gradually tapered to 0 by hospital day 77 and the patient was discharged on hospital day 94.
Figure 3 —
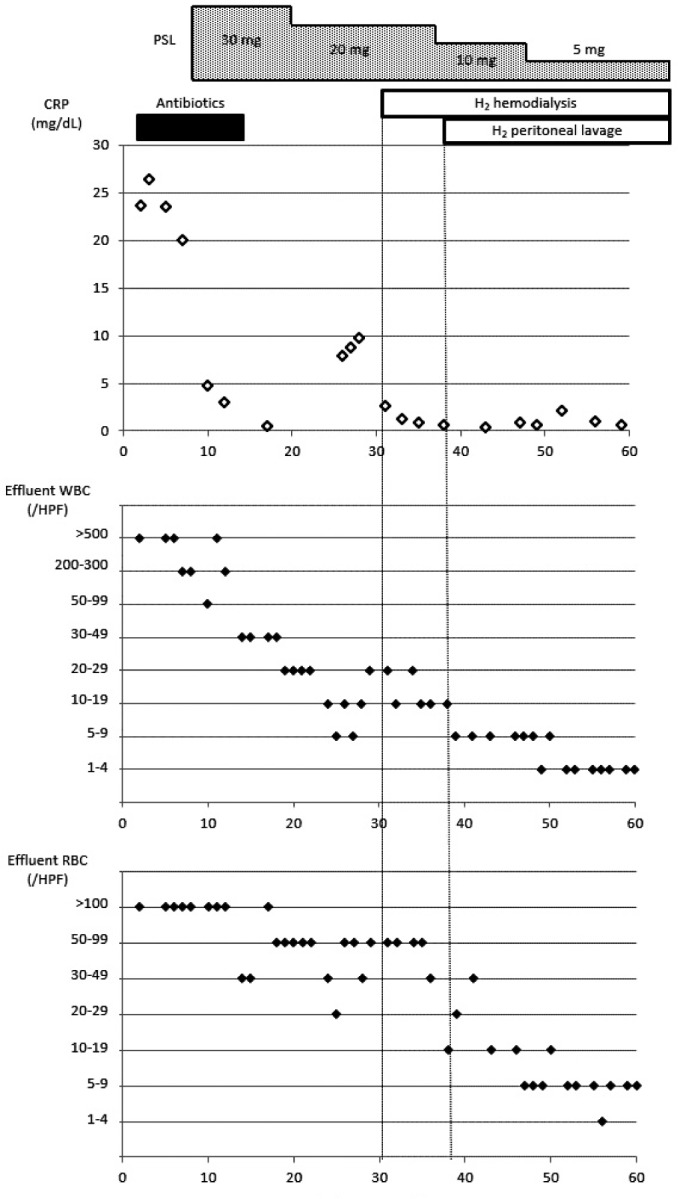
Course of serum C-reactive protein and effluent white and red blood cells. 254x366mm (72 × 72 DPI) CRP = C-reactive protein; WBC = white blood cells; RBC = red blood cells; HPF = high-power field.
Two months later, the peritoneal catheter was laparoscopically removed and a peritoneal biopsy was obtained. Intestinal encapsulation was not evident. The mean thickness of the sub-mesothelial connective tissue (compact zone) was 190 μm, and it was covered by a monolayer of mesothelial cells (Figure 2C and D). The patient has remained free of signs of EPS including ileus until now (for 18 months after the catheter removal).
Discussion
This case study showed that delivering H2 via HD and peritoneal lavage, in addition to oral prednisolone therapy, ameliorated EPS. Thus, H2 might have potential as a novel medical treatment for EPS. We cannot point out the definitive trigger of EPS occurrence in this case. However, the possible contribution of bacterial translocation (profound bacterial peritonitis) might be considered.
Several case reports have described medical treatment of EPS using mainly tamoxifen and/or corticosteroid (17–20). One case-control study found that prophylactic tamoxifen suppressed EPS occurrence (21), and a nationwide cohort study in Japan showed that corticosteroid therapy caused 38.5% of EPS to go into remission (22). However, harmful effects such as ischemic stroke and pulmonary embolism are associated with tamoxifen and opportunistic infection is associated with corticosteroid. In contrast, H2 has been tested particularly in the field of deepwater diving, and no toxicity was identified even at high concentrations (23). Thus, H2 has therapeutic potential for pathological states that are related to oxidative stress and inflammation (24).
Dissolved hydrogen had been regarded as chemically inactive until the discovery of its antioxidant effects (5). Since then, accumulating reports have indicated that H2 could be a useful new therapeutic modality in many biomedical fields. Consuming H2-enriched water exhibits anti-oxidative capabilities without detrimental effects in experimental (25–29) and clinical settings, including type II diabetes mellitus (30), metabolic syndrome (31), myopathies (progressive muscular dystrophy and polymyositis/dermatomyositis) (32), and rheumatoid arthritis (33). In addition, we also reported the clinical feasibility of using H2-enriched water as the dialysate for HD (13–15) and PD (16). The findings of these previous reports and the present case study indicate that H2 delivered via HD and peritoneal lavage could be useful as a novel medical treatment for EPS. In addition, regular use of H2-enriched peritoneal dialysate might be an interesting focus of clinical trials from the viewpoint of peritoneal preservation.
Conclusion
The present case report shows the efficacy of delivering H2 via HD and peritoneal lavage in EPS treatment. This experience suggests that hydrogen has potential as a novel medical treatment for EPS, and thus further investigation regarding this treatment is warranted.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Hong KD, Bae JH, Jang YJ, Jung HY, Cho JH, Choi JY, et al. Encapsulating peritoneal sclerosis: case series from a university center. Korean J Intern Med 2013; 28:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vidal E, Edefonti A, Puteo F, Chimenz R, Gianoglio B, Lavoratti G, et al. Encapsulating peritoneal sclerosis in paediatric peritoneal dialysis patients: the experience of the Italian Registry of Pediatric Chronic Dialysis. Nephrol Dial Transplant 2013; 28:1603–9. [DOI] [PubMed] [Google Scholar]
- 3. Habib SM, Korte MR, Betjes MG. Lower mortality and inflammation from post-transplantation encapsulating peritoneal sclerosis compared to the classical form. Am J Nephrol 2013; 37:223–30. [DOI] [PubMed] [Google Scholar]
- 4. Shroff R, Stefanidis CJ, Askiti V, Edefonti A, Testa S, Ekim M, et al. Encapsulating peritoneal sclerosis in children on chronic PD: a survey from the European Paediatric Dialysis Working Group. Nephrol Dial Transplant 2013; 28:1908–14. [DOI] [PubMed] [Google Scholar]
- 5. Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, Lang T, et al. Encapsulating peritoneal sclerosis: a rare, serious but potentially curable complication of peritoneal dialysis-experience of a referral centre in Germany. Nephrol Dial Transplant 2013; 28:1021–30. [DOI] [PubMed] [Google Scholar]
- 6. Lo WK, Kawanishi H. Encapsulating peritoneal sclerosis - medical and surgical treatment. Perit Dial Int 2009; 29:S211–4. [PubMed] [Google Scholar]
- 7. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13:688–94. [DOI] [PubMed] [Google Scholar]
- 8. Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des 2011; 17:2241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang KC, Yang CC, Lee KT, Chien CT. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int 2003; 64:704–14. [DOI] [PubMed] [Google Scholar]
- 10. Huang KC, Hsu SP, Yang CC, Ou-Yang P, Lee KT, Morisawa S, et al. Electrolysed-reduced water reduced hemodialysis-induced erythrocyte impairment in end-stage renal disease patients. Kidney Int 2006; 70:391–8. [DOI] [PubMed] [Google Scholar]
- 11. Huang KC, Hsu SP, Yang CC, Ou-Yang P, Lee KT, Morisawa S, et al. Electrolysed-reduced water dialysate improves T-cell damage in end-stage renal disease patients with chronic haemodialysis. Nephrol Dial Transplant 2010; 25:2730–7. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama M, Kabayama S, Terawaki H, Nakayama K, Kato K, Sato T, et al. Less-oxidative hemodialysis solution rendered by cathode-side application of electrolyzed water. Hemodial Int 2007; 11:322–7. [DOI] [PubMed] [Google Scholar]
- 13. Nakayama M, Kabayama S, Nakano H, Zhu WJ, Terawaki H, Nakayama K, et al. Biological effects of electrolyzed water in hemodialysis. Nephron Clin Pract 2009; 112:c9–15. [DOI] [PubMed] [Google Scholar]
- 14. Nakayama M, Nakano H, Hamada H, Itami N, Nakazawa R, Ito S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: a clinical trial. Nephrol Dial Transplant 2010; 25:3026–33. [DOI] [PubMed] [Google Scholar]
- 15. Terawaki H, Zhu WJ, Matsuyama Y, Terada T, Takahashi Y, Sakurai K, et al. Effect of a hydrogen (H2)-enriched solution on the albumin redox of hemodialysis patients. Hemodial Int 2014;18:459–66. [DOI] [PubMed] [Google Scholar]
- 16. Terawaki H, Hayashi Y, Zhu WJ, Matsuyama Y, Terada T, Kabayama S, et al. Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients. A novel approach to suppress oxidative stress in the peritoneal cavity. Med Gas Res 2013; 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allaria PM, Giangrande A, Gandini E, Pisoni IB. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol 1999;12:395–7. [PubMed] [Google Scholar]
- 18. Evrenkaya TR, Atasoyu EM, Unver S, Basekim C, Baloglu H, Tulbek MY. Corticosteroid and tamoxifen therapy in sclerosing encapsulating peritonitis in a patient on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2004;19:2423–4. [DOI] [PubMed] [Google Scholar]
- 19. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8. [DOI] [PubMed] [Google Scholar]
- 20. Bhandari S, Wilkinsos A, Sellars L. Sclerosing peritonitis: value of immunosuppression prior to surgery. Nephrol Dial Transplant 1994; 9:436–7. [PubMed] [Google Scholar]
- 21. del Peso G, Bajo MA, Gil F, Aguilera A, Ros S, Costero O, et al. Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Perit Dial 2003; 19:32–5. [PubMed] [Google Scholar]
- 22. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37. [PubMed] [Google Scholar]
- 23. Fontanari P, Badier M, Guillot C, Tomei C, Burnet H, Gardette B, et al. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur J Appl Physiol 2000; 81:325–8. [DOI] [PubMed] [Google Scholar]
- 24. Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des 2011; 17:2241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohsawa I, Nishimaki K, Yamagata K, Ishikawa M, Ohta S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys Res Commun 2008; 377:1195–8. [DOI] [PubMed] [Google Scholar]
- 26. Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, et al. Hydrogenrich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun 2008; 375:346–50. [DOI] [PubMed] [Google Scholar]
- 27. Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol 2009; 64:753–61. [DOI] [PubMed] [Google Scholar]
- 28. Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, et al. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int 2010; 77:101–9. [DOI] [PubMed] [Google Scholar]
- 29. Zhu WJ, Nakayama M, Mori T, Nakayama K, Katoh J, Murata Y, et al. Intake of water with high levels of dissolved hydrogen (H2) suppresses ischemia-induced cardio-renal injury in Dahl salt-sensitive rats. Nephrol Dial Transplant 2011; 26:2112–8. [DOI] [PubMed] [Google Scholar]
- 30. Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 2008; 28:137–43. [DOI] [PubMed] [Google Scholar]
- 31. Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome - an open label pilot study. J Clin Biochem Nutr 2010; 46:140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito M, Ibi T, Sahashi K, Ichihara M, Ito M, Ohno K. Open-label trial and randomized, double-blind, placebo-controlled crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med Gas Res 2011; 1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y, et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res 2012; 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]