Abstract
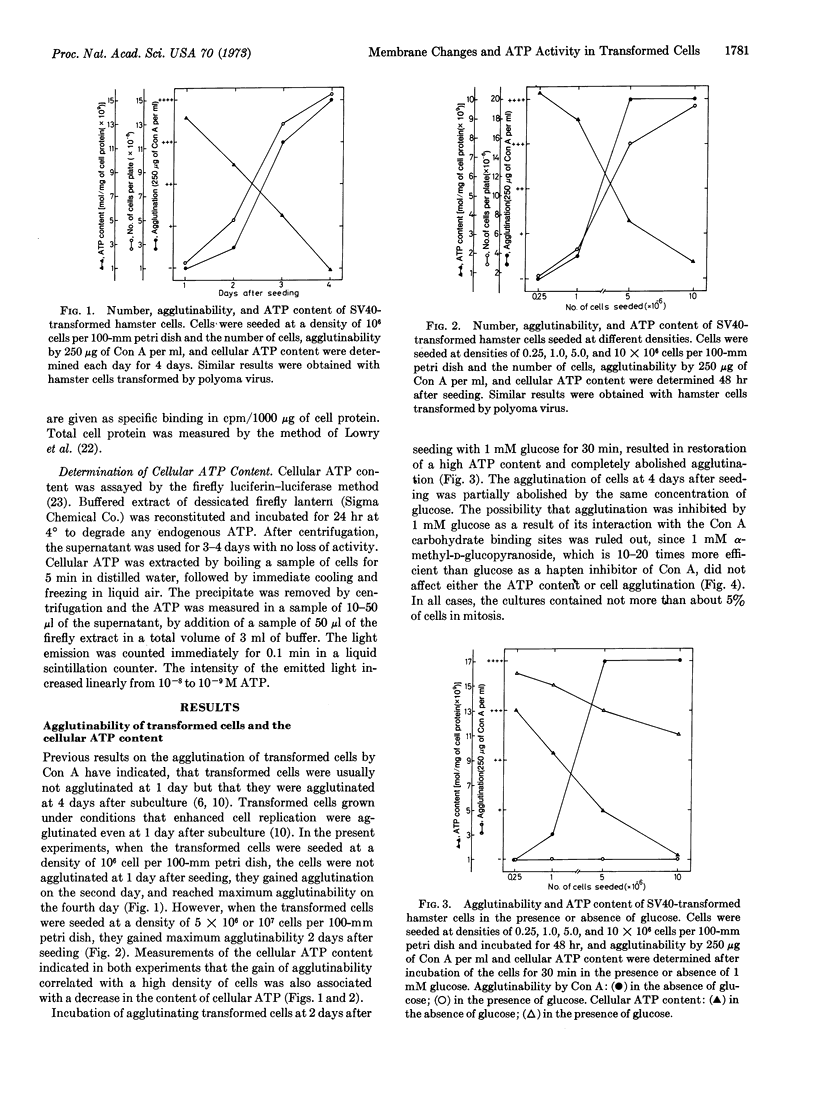
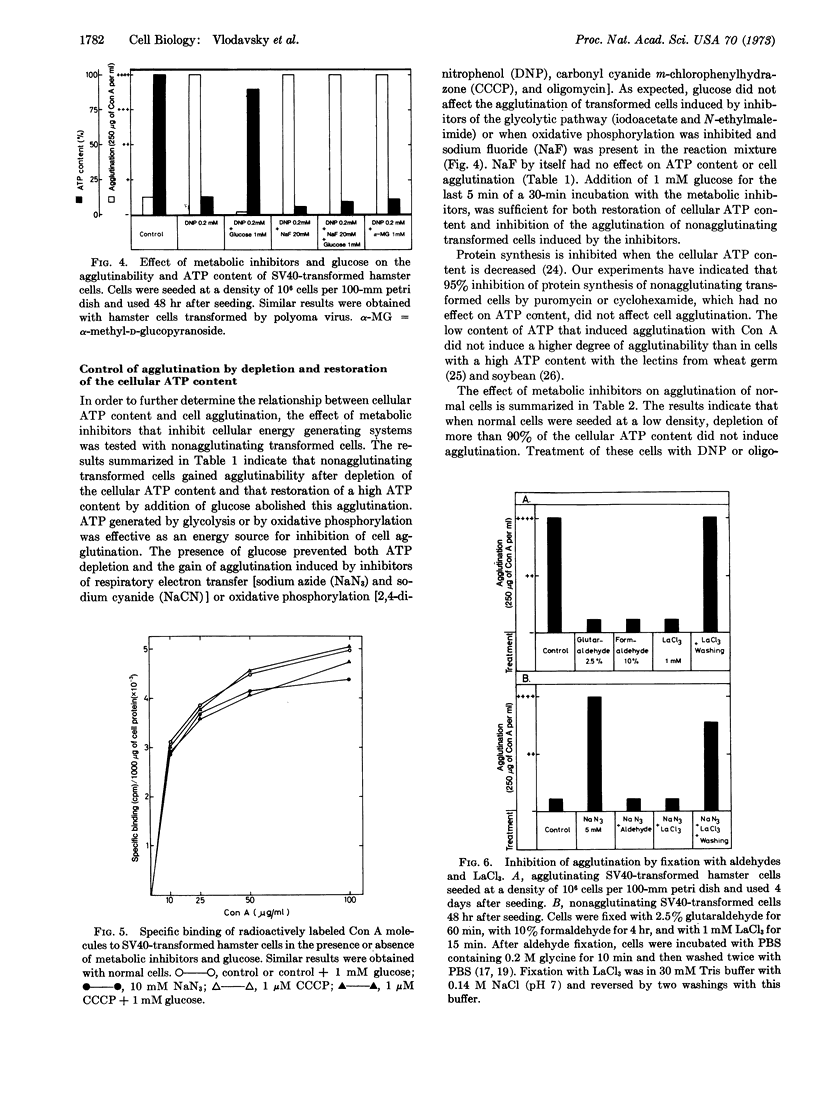
Transformed fibroblasts had a low content of ATP when grown at a high cell density and a high content of ATP when grown at a low cell density. Concanavalin A agglutinated transformed cells with a low, but not those with a high, ATP content. Transformed cells with a high ATP content gained agglutinability after ATP depletion by inhibitors of the energy-generating systems, and those with a low ATP content lost their agglutinability after restoration of a high ATP content by glucose. Fixation of the surface membrane by formaldehyde, glutaraldehyde, or LaCl3, inhibited agglutination of cells with an ATP content that allows agglutination. Normal fibroblasts grown at a high or a low cell density were not agglutinated by concanavalin A. Depletion of the cellular ATP content of normal cells induced agglutination only in cells grown at a high, but not at a low, cell density. A similar number of concanavalin A molecules was bound to the surface membrane of agglutinating and nonagglutinating fibroblasts. It is suggested that a high content of ATP inhibits the movement of concanavalin A binding sites, and that a low content of ATP allows, in transformed cells, a new distribution of binding sites to form the clusters required for cell agglutination. Agglutinability of transformed cells is determined by ATP content, and in normal cells changes in the content of ATP are by themselves not sufficient to induce agglutination. Transformed cells, therefore, do not have a control, presumably for membrane stability, that exists in normal cells.
Keywords: concanavalin A, simian virus 40, golden hamster embryo
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat H., Inbar M., Sachs L. Requirement for cell replication after SV40 infection for a structural change of the cell surface membrane. Virology. 1970 Apr;40(4):854–859. doi: 10.1016/0042-6822(70)90131-5. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg H., Mager J. Studies on the mechanism of the inhibition of protein synthesis induced by intracellular ATP depletion. Biochim Biophys Acta. 1971 Mar 25;232(3):537–555. doi: 10.1016/0005-2787(71)90608-3. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. A specific metabolic activity on the surface membrane in malignant cell-transformation. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2748–2751. doi: 10.1073/pnas.68.11.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Inhibition of ascites tumor development by concanavalin A. Int J Cancer. 1972 Jan 15;9(1):143–149. doi: 10.1002/ijc.2910090117. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Temperature-sensitive activity on the surface membrane in the activation of lymphocytes by lectins. Exp Cell Res. 1973 Jan;76(1):143–151. doi: 10.1016/0014-4827(73)90429-1. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Miller I. R., Great H. Protein labeling by acetylation. Biopolymers. 1972;11(12):2533–2536. doi: 10.1002/bip.1972.360111212. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Shoham J., Sachs L. Differences in the binding of fluorescent concanavalin A to the surface membrane of normal and transformed cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2479–2482. doi: 10.1073/pnas.69.9.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Edelman G. M. Unusual fragments in the subunit structure of concanavalin A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1130–1134. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman Y., Sachs L. Mapping of sites on the surface membrane of mammalian cells. II. Relationship of sites for concanavalin A and an ornithine, leucine copolymer. J Membr Biol. 1972;10(1):1–10. doi: 10.1007/BF01867844. [DOI] [PubMed] [Google Scholar]
- Yanovsky A., Loyter A. The mechanism of cell fusion. I. Energy requirements for virus-induced fusion of Ehrlich ascites tumor cells. J Biol Chem. 1972 Jun 25;247(12):4021–4028. [PubMed] [Google Scholar]



