Abstract
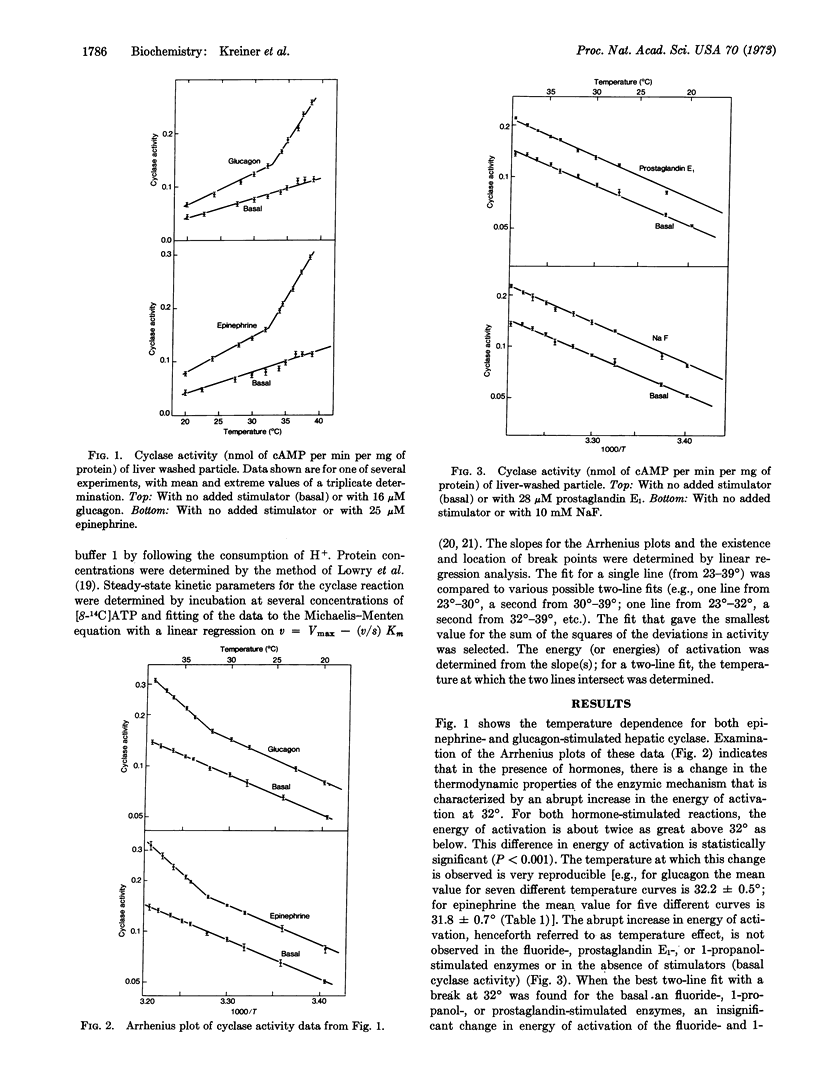
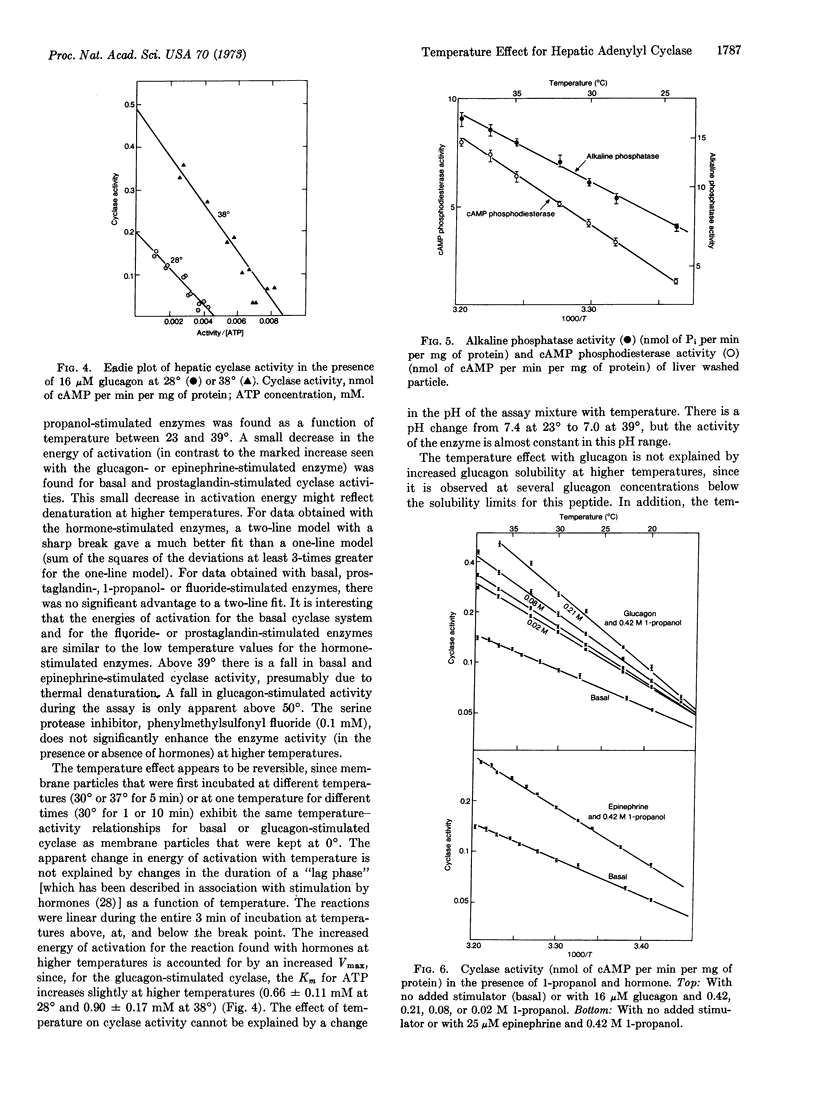
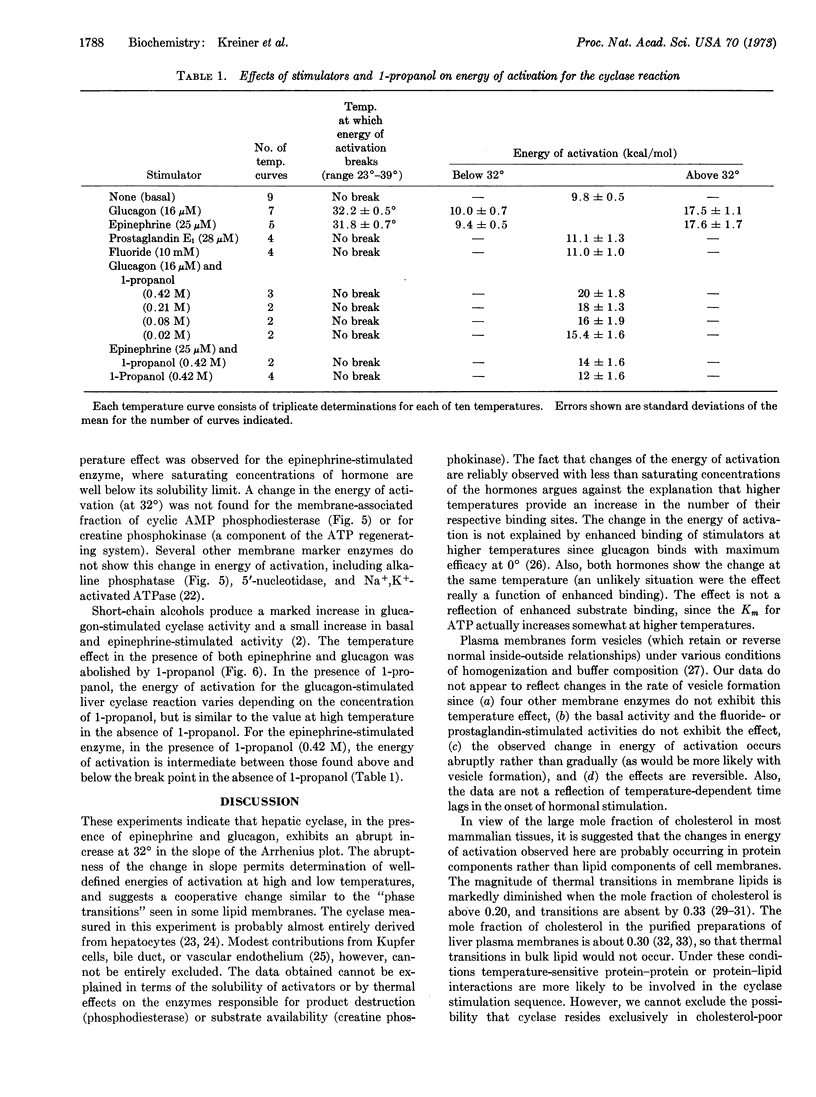
We describe an abrupt increase (at 32°) in the energy of activation for the reaction of hepatic adenylyl cyclase in the presence of glucagon or epinephrine. This increase is not seen in the presence of fluoride, prostaglandin E1, or 1-propanol, or in the absence of cyclase stimulators. The change in energy of activation found with hormones is abolished by 1-propanol. This change does not represent differences in hormone or substrate binding at different temperatures, but seems to reflect interactions among elements of the cyclase stimulation sequence. Similar changes in energy of activation were not observed for alkaline phosphatase, cyclic AMP-phosphodiesterase, 5′-nucleotidase, or ouabain-sensitive ATPase. Since the mole fraction of cholesterol in liver membranes is sufficiently high to preclude a phase change in bulk membrane lipids, our observation suggests either that cyclase is restricted to cholesterol-poor membrane regions or that the change in its energy of activation is largely restricted to protein components of the cyclase apparatus. The data are compatible with fundamental differences in the stimulation process(es) for the hormones (glucagon and epinephrine) as compared with those for fluoride and prostaglandin E1.
Keywords: epinephrine, glucagon, prostaglandin E1, fluoride
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Miller W. H. Digitonin effects on photoreceptor adenylate cyclase. Science. 1972 Mar 24;175(4028):1363–1364. doi: 10.1126/science.175.4028.1363. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Neufeld A. H., King R. A specific, reversible, macromolecular inhibitor of hepatic glucagon responsive adenyl cyclase. Endocrinology. 1971 Nov;89(5):1242–1249. doi: 10.1210/endo-89-5-1242. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Russell V., Blanco M. Independent variation of glucagon and epinephrine responsive components of hepatic adenyl cyclase as a function of age, sex and steroid hormones. Endocrinology. 1970 Jan;86(1):154–159. doi: 10.1210/endo-86-1-154. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Russell V., Robertson W. Evidence for separate epinephrine and glucagon responsive adenyl cyclase systems in rat liver. Biochem Biophys Res Commun. 1968 Jun 10;31(5):706–712. doi: 10.1016/0006-291x(68)90619-0. [DOI] [PubMed] [Google Scholar]
- Dod B. J., Gray G. M. The lipid composition of rat-liver plasma membranes. Biochim Biophys Acta. 1968 Apr 29;150(3):397–404. doi: 10.1016/0005-2736(68)90138-7. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L., Duncan L. Adenyl cyclase. Kinetic properties and nature of fluoride and hormone stimulation. J Biol Chem. 1971 Jul 10;246(13):4166–4173. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. VI. Differences in the effect of temperature on the ATPase and (Na+-K+)-ATPase activities of plasma membranes isolated from rat liver and hepatoma. Biochim Biophys Acta. 1968 Apr 29;150(3):354–363. doi: 10.1016/0005-2736(68)90134-x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Esfahani M., Crowfoot P. D., Wakil S. J. Molecular organization of lipids in Escherichia coli membranes. II. Effect of phospholipids on succinic-ubiquinone reductase activity. J Biol Chem. 1972 Nov 25;247(22):7251–7256. [PubMed] [Google Scholar]
- Gorman R. E., Bitensky M. W. Selective activation by short chain alcohols of glucagon responsive adenyl cyclase in liver. Endocrinology. 1970 Nov;87(5):1075–1081. doi: 10.1210/endo-87-5-1075. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Krishna G., Harwood J. P. Requirement for guanosine triphosphate in the prostaglandin activation of adenylate cyclase of platelet membranes. J Biol Chem. 1972 Apr 10;247(7):2253–2254. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lande S., Gorman R., Bitensky M. Selectively blocked and des-histidine-glucagons: preparation and effects on hepatic adenylate cyclase activity. Endocrinology. 1972 Mar;90(3):597–604. doi: 10.1210/endo-90-3-597. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pastan I. Effects of calcium on ACTH stimulation of the adrenal: separation of hormone binding from adenyl cyclase activation. Nature. 1970 Nov 28;228(5274):864–866. doi: 10.1038/228864a0. [DOI] [PubMed] [Google Scholar]
- Leray F., Chambaut A. M., Hanoune J. Role of GTP in epinephrine and glucagon activation of adenyl cyclase of liver plasma membrane. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1385–1391. doi: 10.1016/0006-291x(72)90866-2. [DOI] [PubMed] [Google Scholar]
- Levey G. S. Solubilization of myocardial adenyl cyclase: less of hormone responsiveness and activation by phospholipids. Ann N Y Acad Sci. 1971 Dec 30;185:449–457. doi: 10.1111/j.1749-6632.1971.tb45272.x. [DOI] [PubMed] [Google Scholar]
- Marinetti G. V., Ray T. K., Tomasi V. Glucagon and epinephrine stimulation of adenyl cyclase in isolated rat liver plasma membranes. Biochem Biophys Res Commun. 1969 Jul 23;36(2):185–193. doi: 10.1016/0006-291x(69)90313-1. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Menon K. M., Smith M. Characterization of adenyl cyclase from the testis of Chinook salmon. Biochemistry. 1971 Mar 30;10(7):1186–1190. doi: 10.1021/bi00783a015. [DOI] [PubMed] [Google Scholar]
- Perkins J. P., Moore M. M. Adenyl cyclase of rat cerebral cortex. Activation of sodium fluoride and detergents. J Biol Chem. 1971 Jan 10;246(1):62–68. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Jones A. B. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966 Jan 10;241(1):140–142. [PubMed] [Google Scholar]
- Rothman J. E., Engelman D. M. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. doi: 10.1038/newbio237042a0. [DOI] [PubMed] [Google Scholar]
- SMITH R. E., HOCK R. J. Brown fat: thermogenic effector of arousal in hibernators. Science. 1963 Apr 12;140(3563):199–200. doi: 10.1126/science.140.3563.199. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Sweat F. W., Hupka A. Adenyl cyclase in hepatic parenchymal and reticuloendothelial cells. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1436–1442. doi: 10.1016/s0006-291x(71)80246-2. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Kreiner P., Barrnett R. J., Bitensky M. W. Biochemical characterization and cytochemical localization of a catecholamine-sensitive adenylate cyclase in isolated capillary endothelium. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3175–3179. doi: 10.1073/pnas.69.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]



