Abstract
Objectives:
To observe the natural time course of noncognitive symptoms before the onset of symptomatic Alzheimer disease dementia.
Methods:
Using the National Alzheimer's Coordinating Center Uniform Data Set from September 2005 to March 2013, data from cognitively normal individuals who were aged 50 years or older at first visit and had subsequent follow-up were analyzed. Survival analyses were used to examine the development of particular symptoms relative to each other on the Neuropsychiatric Inventory Questionnaire (NPI-Q), Functional Activities Questionnaire, and Geriatric Depression Scale, and to compare the development of individual symptoms for persons who did and did not receive a Clinical Dementia Rating (CDR) >0 (indicating abnormal cognition) during the follow-up period.
Results:
The order of symptom occurrence on the NPI-Q was similar for participants who remained at CDR 0 and for those who received a CDR >0 over the follow-up period, although the time to most NPI-Q symptoms was faster for participants who received a CDR >0 (p < 0.001). With the exception of memory, Geriatric Depression Scale symptoms reported by both CDR groups were similar.
Conclusions:
We found a significantly earlier presence of positive symptoms on the NPI-Q in cognitively normal patients who subsequently developed CDR >0. Among participants with no depression symptoms at baseline, results suggest that depressive symptoms may increase with aging regardless of incipient dementia. Such findings begin to delineate the noncognitive course of Alzheimer disease dementia in the preclinical stages. Future research must further elucidate the correlation between noncognitive changes and distinct dementia subtypes.
The natural course of Alzheimer disease (AD) dementia includes hallmark functional and behavioral deficits in addition to cognitive decline. Alternately termed behavioral and psychological symptoms in dementia or neuropsychiatric symptoms (NPS), this heterogeneous array of noncognitive impairment affects an estimated 90% of patients with AD.1 Earlier studies have shown that with advancing cognitive decline in AD dementia, noncognitive symptoms include apathy, depression, agitation, and aggression, to psychosis. With progressive cognitive and functional decline in AD dementia, the presence of NPS is postulated to represent increased neurodegradation across neural systems.2–5 Such NPS have been associated with worse prognosis, accelerated illness progression, increased use of services, and earlier institutionalization.2 However, variable neuropsychiatric measures and clinical definitions have clouded the true incidence and progression of NPS in patients with AD.
While noncognitive outcomes of AD after incident dementia are increasingly characterized, the relative time course of behavioral and functional deficits before onset of cognitive impairment in AD dementia has been rarely studied. Here, we examined the time course of noncognitive symptoms reported on the Functional Activities Questionnaire (FAQ),6 Geriatric Depression Scale (GDS),7 and Neuropsychiatric Inventory Questionnaire (NPI-Q),8 both in relation to one another and to diagnosis of cognitive impairment. Analyzing data from the National Alzheimer's Coordinating Center (NACC), we followed the course of noncognitive decline for 1,218 participants who were cognitively normal with no positive symptoms on the FAQ, GDS, or NPI-Q at baseline who subsequently progressed to Clinical Dementia Rating (CDR) >0 as well as 1,198 participants who remained CDR 0 throughout follow-up.
METHODS
Archival data from the NACC Uniform Data Set (UDS)9 were used. The UDS is a repository of prospectively collected data from 34 Alzheimer's Disease Centers across the United States supported by the National Institute on Aging. Participants at Alzheimer's Disease Centers take part in a standardized assessment battery to assess for the presence and severity of dementia. Each participant is accompanied by an informant who knows the participant well, usually a spouse, child, or friend. More detailed information regarding the UDS9,10 and NACC (http://www.alz.washington.edu/) has been published.
Available data of interest spanned from September 2005 (the earliest time of UDS data availability) to the March 2013 NACC “data freeze.” Data from persons with a CDR11 of 0 (indicating normal cognition) at the first visit during this period, who were aged 50 years or older at that visit, and who had at least one additional visit after the initial assessment were included. Exclusion criteria were missing data on any of the demographic variables of interest (age, sex, race, education, and the presence of at least one APO ε4 allele). We first identified participants who received a CDR >0 over the follow-up period. CDRs of 0.5, 1, 2, and 3 represent very mild, mild, moderate, and severe dementia, respectively.11 Frequency matching was used to select a second sample of participants who did not receive a CDR above 0 during the follow-up period, and who were similar to the first sample regarding APO ε4 status, age (50–69 years, 70–80 years, >80 years), education (<14 years, 15–17 years, >17 years), and length of follow-up (1–3 years, 4–5 years, 6–7 years).
Outcome measures.
The NPI-Q consists of 12 items, each assessing 12 NPS domains. The informant indicates whether each symptom is present (yes or no). For each “yes” response, the informant is then asked to rate the severity of the symptom (mild, moderate, or severe).8 The GDS (short form) is a screening measure for depression in older adults made up of 15 items. Participants answer “yes” or “no” for each item.7 The 10-item FAQ assesses whether the participant had any difficulties in instrumental activities of daily living (IADL) (paying bills, shopping, etc.) as reported by the informant.6
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants. Research using the NACC database was approved by the University of Washington Institutional Review Board.
Statistical analyses.
Survival analyses were used to examine the development of particular symptoms relative to each other within an assessment instrument, and to compare the development of individual symptoms for persons who did and did not receive a CDR >0 during the follow-up period. Because we were interested in studying the relative time of onset of individual symptoms within an assessment instrument, only data from participants who had no positive symptoms at baseline on that instrument were included in analyses for that instrument. In these analyses, for a particular symptom, the dependent variable was the time from baseline assessment to the first clinical assessment when that symptom was endorsed. Data were censored at the time of last assessment for persons who did not endorse that symptom over the follow-up period. Endorsement of a symptom was taken as a “yes” response on NPI-Q and GDS items, and as any response indicating at least some difficulty (i.e., a response other than “normal”) on the FAQ items.
Kaplan-Meier survival curves were used to graph the time course of the development of individual NPI-Q symptoms relative to each other, among persons who had no NPI-Q symptoms at baseline. The curves were drawn separately for those who did and did not remain CDR 0 over the follow-up period. Cox proportional hazards models (adjusting for age, sex, education, race, and APO ε4 status) tested, within the same sample, whether the time from baseline to the appearance of each NPI-Q symptom differed for participants who did and did not receive a CDR >0 over the follow-up period. Similar analyses were conducted using the GDS and FAQ data.
RESULTS
Data from 2,416 participants meeting inclusion criteria were used (table 1). Almost all (1,184, 97.2%) of the 1,218 participants who developed incident cognitive impairment received a first CDR >0 of 0.5, a rating that indicates very mild dementia.11 Others were rated CDR 1 (25, 2.1%), CDR 2 (8, 0.7%), or CDR 3 (1, 0.1%). Sixty percent (n = 736) of participants who received at least one CDR >0 over the follow-up period had at least one additional clinical assessment after receiving the first CDR >0. Among these, and compared with the first CDR >0 received, 447 (60.7%) had no change, 101 (13.7%) progressed to a higher CDR, and 289 (23.7%) regressed to a lower CDR at their last assessment during the follow-up period. Two hundred eighty-seven (99.3%) of those who “regressed” went from a CDR 0.5 back to CDR 0. In our experience, it is common for longitudinally followed participants who will eventually consistently show clear symptoms of dementia (i.e., CDR 1 or greater) to vacillate between CDR 0 and 0.5 over the first few years of the course of the disease.
Table 1.
Demographics
The order of symptom occurrence on the NPI-Q was similar for participants who remained CDR 0 (figure 1A) and for those who received a CDR >0 (figure 2A) over the follow-up period, although the time to each NPI-Q symptom, other than elation or euphoria, was faster for participants who received a CDR >0 compared with those who did not (table 2). Few participants, regardless of whether they developed cognitive impairment or not, experienced elation or euphoria, or hallucinations, over the follow-up period.
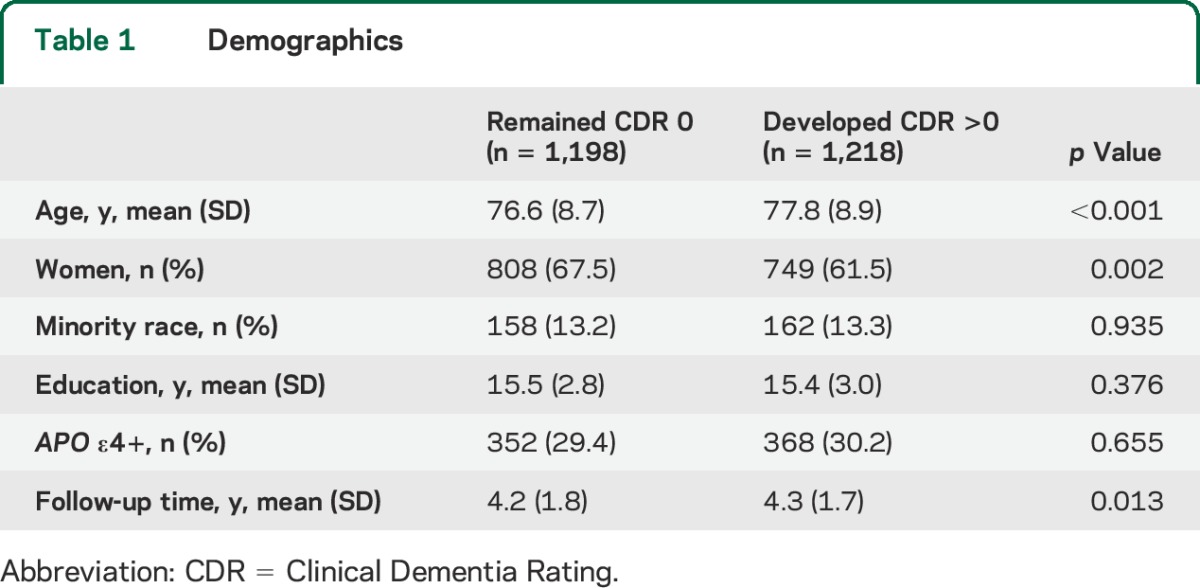
Figure 1. Time to symptoms for participants who maintained a Clinical Dementia Rating of 0.
Kaplan-Meier curves showing time to symptoms on the (A) Neuropsychiatric Inventory Questionnaire, (B) Geriatric Depression Scale, and (C) Functional Activities Questionnaire for participants who maintained a Clinical Dementia Rating of 0 over the follow-up period. Curves labeled with multiple symptoms are representative of clusters of symptoms that tend to emerge at around the same time. atten. = attention; beh. = behaviors; dist. = disturbance; rem. appts. = remembering appointments.
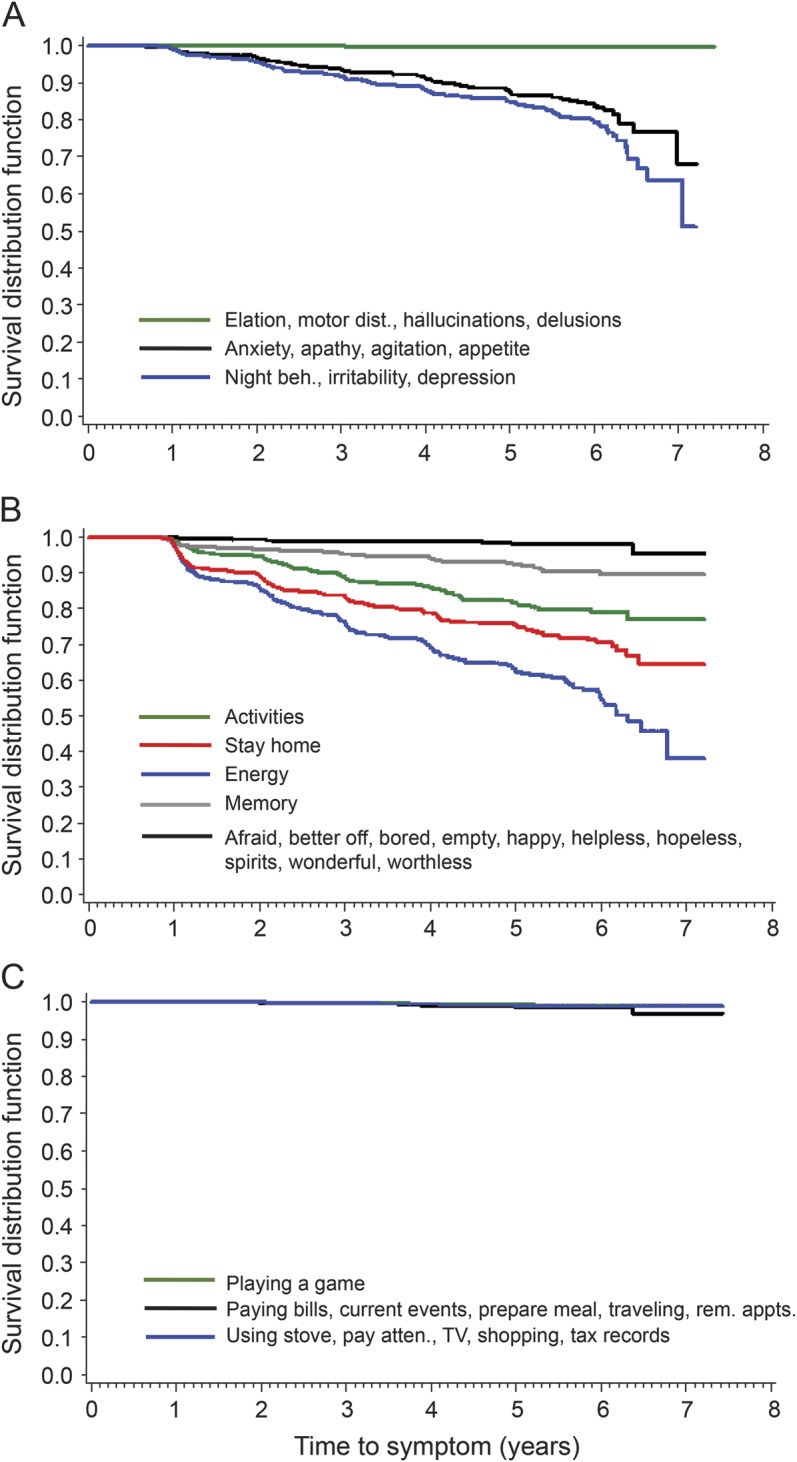
Figure 2. Time to symptoms for participants who received at least one Clinical Dementia Rating greater than 0.
Kaplan-Meier curves showing time to each symptom on the (A) Neuropsychiatric Inventory Questionnaire, (B) Geriatric Depression Scale, and (C) Functional Activities Questionnaire for participants who received at least one Clinical Dementia Rating greater than 0 over the follow-up period. Curves labeled with multiple symptoms are representative of clusters of symptoms that tend to emerge at around the same time. atten. = attention; beh. = behaviors; dist. = disturbance; rem. appts. = remembering appointments.
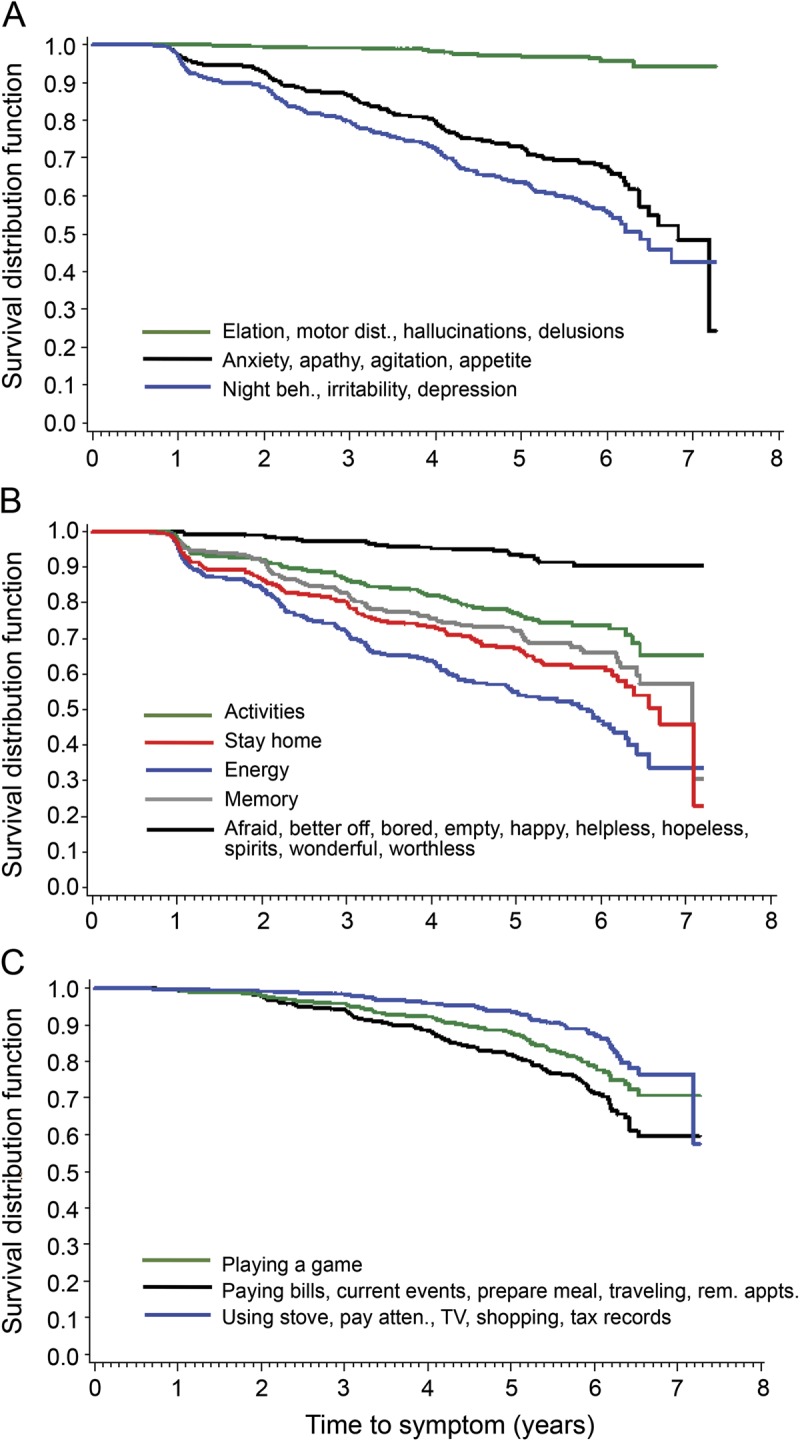
Table 2.
Adjusted hazard ratios of time to each symptom for individuals who received a Clinical Dementia Rating above zero compared with those who did not
Given large numbers of participants, even small absolute differences may be statistically significant, although the results may have little clinical significance. This seems to be the case with our GDS results. Although most differences between the CDR groups were significant at the α < 0.05 level, most group differences on symptoms assessed by the NPI-Q and FAQ were significant at p < 0.001. Likewise, hazard ratio estimates were smaller for GDS symptoms compared with those on the other scales. With one exception, GDS symptoms reported by both CDR groups were similar (table 2, figures 1B and 2B). Both participants who did and did not develop CDR >0 were faster to deny that they were full of energy, to report that they had dropped activities and interests, and to report that they preferred to stay home, compared with the other GDS symptoms. The expected exception was that participants who developed CDR >0 reported more memory problems with time (figure 2B). The remaining GDS symptoms occurred relatively infrequently across the follow-up period.
Few persons who maintained CDR 0 status across the follow-up period were viewed by their informant as having any difficulty with IADL, as indicated by responses to the FAQ (figure 1C). By contrast, difficulties with IADL were much more likely to occur for those who received at least one CDR >0 (table 2, figure 1C).
DISCUSSION
Our sample demonstrates significant noncognitive declines associated with eventual progression to CDR >0. That participants who progressed to CDR >0 exhibited a greater decline on FAQ items compared with those who remained CDR 0 is consistent with the clinical definition of dementia. Decreased ability to perform functional activities is an essential diagnostic criterion for AD dementia.12 Therefore, that participants who developed CDR >0 showed significant impairment in IADL such as paying bills, shopping, and preparing meals further supports the clinical dementia diagnosis.
In addition to greater impairment on the FAQ, those participants who developed CDR >0 also exhibited an accelerated decline on the NPI-Q. Both participants who progressed beyond as well as those who remained CDR 0 exhibited a similar sequence of positive symptoms on the NPI-Q. These noncognitive symptoms trend into 3 “phases”: first, irritability, depression, and nighttime behavior changes; next, anxiety, appetite changes, agitation, and apathy; and last, elation, motor disturbances, hallucinations, delusions, and disinhibition. Although these trends are sequentially similar, the development of symptoms on the NPI-Q happens sooner among the patients who develop CDR >0 in follow-up.
Moreover, this progression of noncognitive symptoms is similar to that described in patients with diagnosed AD dementia. Previously, the presence of more severe noncognitive symptoms, such as elation, motor disturbances, hallucinations, delusions, and disinhibition, is described in conjunction with more advanced AD dementia.2 Our sample suggests that this arc of noncognitive changes begins in preclinical AD dementia. Such a finding corroborates our previous study, which showed that depression scores and other behavioral symptoms monitored by the NPI-Q worsen faster among individuals with preclinical AD dementia at baseline as defined by CSF biomarkers.13
While depression measured by the NPI-Q is associated with preclinical AD dementia in this study, with the exception of memory, the occurrence of GDS items with time did not differ dramatically between participants who remained at and those who progressed beyond CDR 0. Both groups showed significant decreases in energy and interest in activities alongside an increased preference for staying home on the GDS. This discordance between the 2 surveys may reflect that the NPI-Q relies on informant report while the GDS relies on self-report. Anosognosia is common in early dementia.14 Likewise, it is possible that a portion of participants with preclinical dementia may have anosognosia, confounding their GDS responses. Together, these NPI-Q and GDS results suggest that, among those with no depression symptoms at baseline, these symptoms may increase with the aging process regardless of incipient dementia, but may appear earlier in participants who eventually progress beyond CDR 0.
Such findings add to the conflicting studies regarding depression and dementia. It remains unclear whether depression is a psychological response to the process of AD or a manifestation of the same underlying pathology. Greater numbers of amyloid plaques and neurofibrillary tangles have been described in depressed participants at autopsy.15–17 However, varying cross-sectional studies show that the biomarker CSF β-amyloid 1–42 is increased,18 decreased,19 and unrelated20 to depression. Similarly, studies examining the association between psychological and cognitive symptoms have borne variable results. Overall, they suggest that depression may be a modest risk factor for patients with preclinical dementia.4,5
Although we used a large sample and frequency matching to minimize confounding variables and adequately detect significant differences between the 2 groups, there remain limitations to the study. The vast majority of participants who developed CDR >0 will exhibit pathology consistent with AD. However, invariably, some of these participants when studied at autopsy will be found to have non-AD dementing illnesses. It remains unclear whether the noncognitive changes observed on the FAQ, NPI-Q, and GDS are an epiphenomenon of dementia or whether specific symptoms are associated with distinct phenotypes. As such, signals for the latter could be lost in such a large, generalized analysis.
Because we were interested in the time of appearance of symptoms relative to each other, we restricted the analyses for each test to persons who had none of those symptoms at baseline. However, this means that the analyses may reflect persons who are more psychologically and functionally “healthy” than others. For example, depressive symptoms occur frequently in the older adult population.21
We found a significantly earlier presence of positive symptoms on the NPI-Q in cognitively normal individuals who subsequently developed CDR >0. Such findings begin to delineate the noncognitive course of AD dementia in the preclinical stages. Future research must further elucidate the association between noncognitive changes and distinct subtypes of dementia. Furthermore, as the NACC database continues to grow, longer follow-up of these participants will provide additional insight into the noncognitive course of both healthy aging and dementia.
GLOSSARY
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- FAQ
Functional Activities Questionnaire
- GDS
Geriatric Depression Scale
- IADL
instrumental activities of daily living
- NACC
National Alzheimer's Coordinating Center
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- NPS
neuropsychiatric symptom
- UDS
Uniform Data Set
AUTHOR CONTRIBUTIONS
Ms. Masters: study concept and design, data analysis and interpretation, drafting and critical revision of manuscript. Dr. Morris: data analysis and interpretation, critical revision of manuscript, study supervision. Dr. Roe: study concept and design, data analysis and interpretation, drafting and critical revision of manuscript.
STUDY FUNDING
Funding for this study was provided by the Longer Life Foundation, the National Institute on Aging (NIA) (P50 AG005681, P01 AG003991, and P01 AG026276), Fred Simmons and Olga Mohan, the Farrell Family Research Fund, and the Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Knight Alzheimer's Disease Research Center (ADRC). The NACC database is funded by NIA grant U01 AG016976.
DISCLOSURE
M. Masters reports no disclosures relevant to the manuscript. J. Morris reports disclosures: Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris has participated or is currently participating in clinical trials of antidementia drugs sponsored by Janssen Immunotherapy and Pfizer. Dr. Morris has served as a consultant for the following companies: Eisai, Esteve, Janssen Alzheimer Immunotherapy Program, GlaxoSmithKline, Novartis, and Pfizer. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants P50AG005681, P01AG003991, P01AG026276, and U19AG032438. C. Roe reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Savva GM, Zaccai J, Matthews FE, Davidson JE, McKeith I, Brayne C. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry 2009;194:212–219. [DOI] [PubMed] [Google Scholar]
- 2.Eustace A, Coen R, Walsh C, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer's disease. Int J Geriatr Psychiatry 2002;17:968–973. [DOI] [PubMed] [Google Scholar]
- 3.Mok WY, Chu LW, Chung CP, Chan NY, Hui SL. The relationship between non-cognitive symptoms and functional impairment in Alzheimer's disease. Int J Geriatr Psychiatry 2004;19:1040–1046. [DOI] [PubMed] [Google Scholar]
- 4.Raudino F. Non-cognitive symptoms and related conditions in the Alzheimer's disease: a literature review. Neurol Sci 2013;34:1275–1282. [DOI] [PubMed] [Google Scholar]
- 5.Li XL, Hu N, Tan MS, Yu JT, Tan L. Behavioral and psychological symptoms in Alzheimer's disease. Biomed Res Int 2014;2014:927804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986;5:165–173. [Google Scholar]
- 8.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 12.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's Disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roe CM, Fagan AM, Grant EA, Holtzman DM, Morris JC. CSF biomarkers of Alzheimer disease: “noncognitive” outcomes. Neurology 2013;81:2028–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris RG, Mograbi DC. Anosognosia, autobiographical memory and self knowledge in Alzheimer's disease. Cortex 2013;49:1553–1565. [DOI] [PubMed] [Google Scholar]
- 15.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry 2006;63:161–167. [DOI] [PubMed] [Google Scholar]
- 16.Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry 2008;16:168–174. [DOI] [PubMed] [Google Scholar]
- 17.Meynen G, Van Stralen H, Smit J, Kamphorst W, Swaab DF, Hoogendijk WJ. Relation between neuritic plaques and depressive state in Alzheimer's disease. Acta Neuropsychiatr 2010;22:14–20. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsson P, Skoog I, Waern M, et al. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry 2007;15:832–838. [DOI] [PubMed] [Google Scholar]
- 19.Pomara N, Bruno D, Sarreal AS, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry 2012;169:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelborghs S, Maertens K, Vloeberghs E, et al. Neuropsychological and behavioural correlates of CSF biomarkers in dementia. Neurochem Int 2006;48:286–295. [DOI] [PubMed] [Google Scholar]
- 21.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113:372–387. [DOI] [PubMed] [Google Scholar]


