Abstract
Objective:
To delineate the specific speech deficits in individuals with epilepsy-aphasia syndromes associated with mutations in the glutamate receptor subunit gene GRIN2A.
Methods:
We analyzed the speech phenotype associated with GRIN2A mutations in 11 individuals, aged 16 to 64 years, from 3 families. Standardized clinical speech assessments and perceptual analyses of conversational samples were conducted.
Results:
Individuals showed a characteristic phenotype of dysarthria and dyspraxia with lifelong impact on speech intelligibility in some. Speech was typified by imprecise articulation (11/11, 100%), impaired pitch (monopitch 10/11, 91%) and prosody (stress errors 7/11, 64%), and hypernasality (7/11, 64%). Oral motor impairments and poor performance on maximum vowel duration (8/11, 73%) and repetition of monosyllables (10/11, 91%) and trisyllables (7/11, 64%) supported conversational speech findings. The speech phenotype was present in one individual who did not have seizures.
Conclusions:
Distinctive features of dysarthria and dyspraxia are found in individuals with GRIN2A mutations, often in the setting of epilepsy-aphasia syndromes; dysarthria has not been previously recognized in these disorders. Of note, the speech phenotype may occur in the absence of a seizure disorder, reinforcing an important role for GRIN2A in motor speech function. Our findings highlight the need for precise clinical speech assessment and intervention in this group. By understanding the mechanisms involved in GRIN2A disorders, targeted therapy may be designed to improve chronic lifelong deficits in intelligibility.
Language and speech impairment are integral to the epilepsy-aphasia syndromes (EAS). At the severe end of the epilepsy-aphasia spectrum lie two disorders associated with regression and continuous spike and wave during sleep, defined by bilaterally synchronous discharges occupying >85% of slow-wave sleep. Language regression, typically with verbal auditory agnosia, is characteristic of Landau-Kleffner syndrome, often associated with treatable focal seizures. Global regression is usual in epileptic encephalopathy with continuous spike and wave during sleep (ECSWS) associated with multiple seizure types. Next in the continuum is intermediate epilepsy-aphasia disorder (IEAD) with abnormal cognitive development or regression, with or without seizures, with epileptiform activity occupying <85% sleep.1 At the mild end, impaired language and literacy skills are described in benign childhood epilepsy with centrotemporal spikes.2 Ictal oromotor and speech impairment as well as interictal speech sound disorder have also been reported.3,4 Speech dyspraxia occurs in rare families with rolandic epilepsy and cognitive impairment.5–7 Impairment in language (understanding and use of words) is central to the EAS, yet speech (how speech sounds are produced or articulated) has not been carefully investigated.
Inherited and de novo mutations in GRIN2A, encoding the NR2A subunit of the glutamate NMDA receptor, are found in 9% to 20% of probands with EAS.8–10 We identified mutations in GRIN2A in 4 of 519 patients with epileptic encephalopathies of unknown cause and found all 4 patients had EAS disorders.8 This finding was replicated in French and German studies.9,10 Here, we studied the speech phenotype of 3 families with EAS associated with GRIN2A mutations.
METHODS
We studied 11 individuals from 3 families with EAS and GRIN2A mutations.1,5,8 A range of tasks was performed to assess speech, oral motor skills, cognition, and language (table 1). Audiovisual recordings of assessments were made using a Marantz PMD671 digital recorder, Countryman Isomax headset microphone, and a Sony DCR-SR85 digital camera. Two speech pathologists (S.J.T., A.T.M.) independently rated the perceptual speech characteristics of conversational samples using a dysarthria rating scale,11 then reached consensus on discrepant ratings. Word and nonword repetition tasks (Nonword Memory Test, multisyllabic word repetition task) and subtests of the Apraxia Battery for Adults, Second Edition (ABA-2), were used to assess motor speech planning and programming. Word and nonword repetition raw scores were calculated as the number of words correctly produced and compared with adult normative data.12,13 Raw scores on the ABA-2 were compared with normative data.14 Maximum performance tasks (maximum vowel prolongation, maximum repetition rate of monosyllables and trisyllables) were used to independently assess subsystems required for accurate speech production: respiration, phonation, and articulation.15 Three trials of each task were performed, and the best performance was compared with adult normative data.16 The Frenchay Dysarthria Assessment, Second Edition, was used to examine nonspeech oral motor skills.17 Performance was measured on a 9-point scale, with scores 7 and below indicative of impairment.
Table 1.
Tasks used to assess speech, oral motor skills, cognition, and language

Perceptual speech characteristics and performance on word and nonword repetition and maximum performance tasks were used to distinguish the different motor speech disorders. Dysarthria was diagnosed based on the presence of speech deficits at any level of the speech subsystem (respiration, phonation, articulation, resonance, prosody) due to abnormalities in the strength, speed, range, steadiness, tone, or accuracy of movements, specified in the Mayo dysarthria classification system.16 Diagnosis of speech dyspraxia was based on features identified in the American Speech and Hearing Association Childhood Apraxia of Speech Technical Report,18 including inconsistent errors, disrupted coarticulatory transitions, and inappropriate prosody.
Receptive and expressive language skills were measured using the Clinical Evaluation of Language Fundamentals, Fourth Edition, with normative data available up to 21 years.19 For adults older than 21 years, receptive vocabulary skills were examined using the Peabody Picture Vocabulary Test, Fourth Edition, expressive vocabulary using the Expressive Vocabulary Test, Second Edition, and comprehension of grammatical contrasts using the Test for Reception of Grammar, Second Edition.20–22 Standard scores were computed using normative data provided for each test. Information regarding early language skills, as well as electroclinical and imaging data, was obtained from the families and confirmed from their medical records. Cognitive function was measured using the 4-subtest form of the Wechsler Abbreviated Scale of Intelligence, Second Edition,23 or the 2-subtest form when there were time constraints during testing.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Human Research Ethics Committees of The Royal Children's Hospital and Austin Health (RCH HREC 27053, Austin HREC H2011/04390). Written informed consent was obtained from all participants, including from parents in the case of minors or those with intellectual disability. Consent covered use of video footage for publication.
RESULTS
The cohort comprised 11 individuals from 3 families named according to the codes (A, C, D) used in the report identifying GRIN2A as the causative gene for ease of reference.1,5,8 Family A included 5 members with autosomal dominant rolandic epilepsy with speech dyspraxia (ADRESD).5 Family C was a father–son pair with ECSWS who had the same GRIN2A mutation and shared an identical haplotype with family A, but the families were not known to be related. Recent genealogic work has determined the relationship between the 2 families (figure, family A-C). Family D comprised 2 brothers with IEAD, their sister with ECSWS, and their mother who did not have a history of seizures and had not received antiepileptic medication (figure).
Figure. Pedigrees of families A–C and D.
Family A was originally reported in Scheffer et al.,5 1995; family D is family I in Tsai et al.,1 2013.
The median age of the affected individuals studied was 48 years (mean 38 years, range 16–64 years). At the time of this study, none of the 10 individuals with a seizure disorder had ongoing seizures. Only 3 individuals were on antiepileptic medication (AC-V-1, AC-IV-5, D-II-1); in one individual, the antiepileptic medication was for behavioral management rather than seizures (D-II-1). No epileptiform abnormalities were present on the last EEG in 7 individuals (table 3). Three (AC-IV-2, D-II-2, D-II-6) had epileptiform abnormalities on their studies at ages 8 to 12 years but have not had subsequent studies. Six individuals underwent brain MRI at the time of the study, which was normal in 4 (AC-III-2, AC-III-5, AC-IV-2, AC-V-1). A Chiari I malformation was found in AC-IV-5, and left hippocampal sclerosis in AC-IV-7. Brain MRI was reported as normal in 3 (AC-III-4, D-II-1, and D-II-6). D-I-2 did not undergo EEG or MRI studies and her son D-II-2 did not have an MRI study.
Table 3.
Results of speech and language tasks
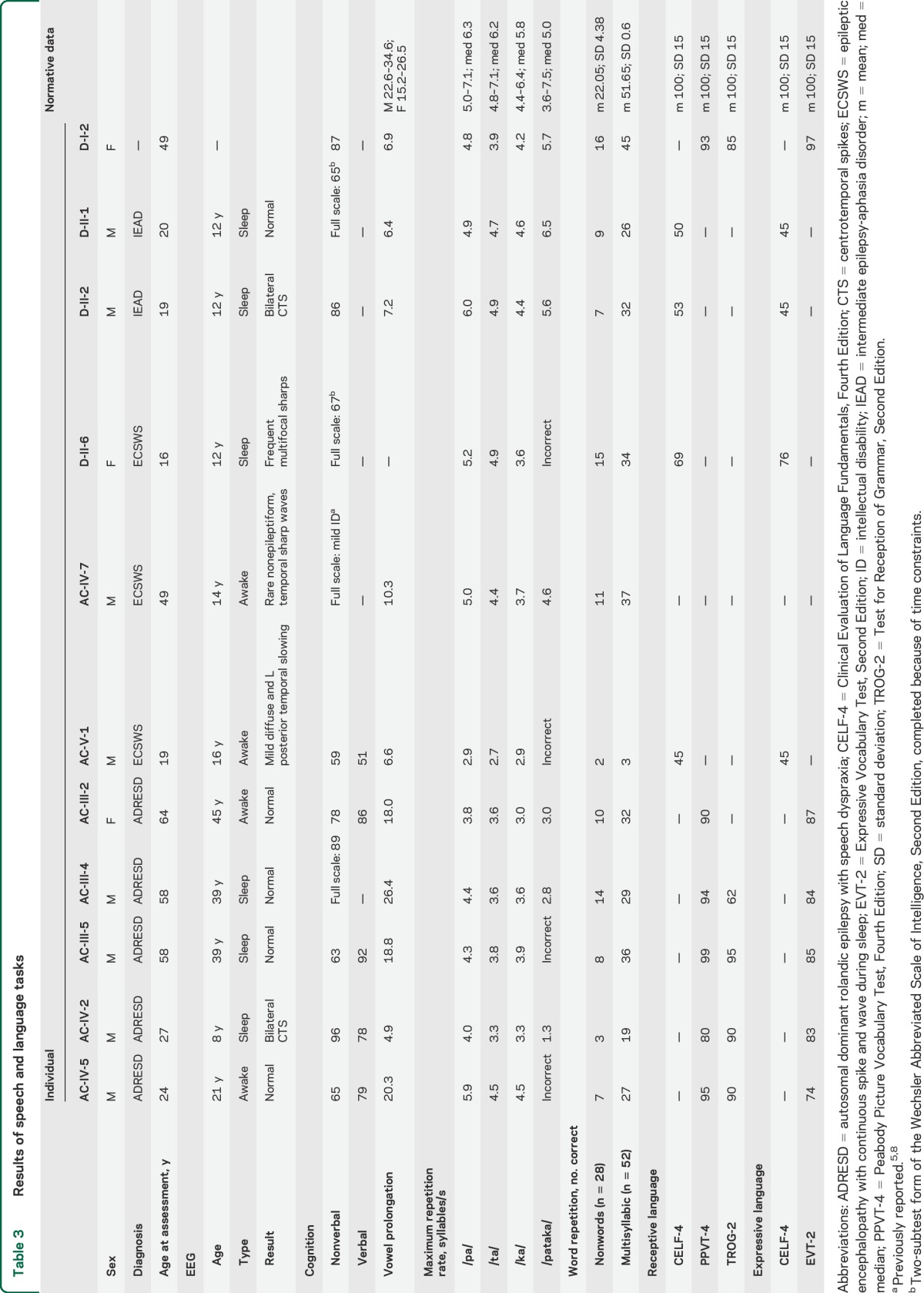
The more severe epilepsy phenotypes may be associated with more severe speech phenotypes, but larger numbers of cases are required to show whether this is a true correlation.
Individuals with GRIN2A mutations showed abnormalities in both motor speech planning/programming (i.e., speech dyspraxia) and execution (i.e., dysarthria).
Speech features.
Conversational speech intelligibility was moderately impaired in 3 individuals (AC-V-1, D-II-1, D-II-2) and mildly reduced in 7 (AC-III-2, AC-III-4, AC-III-5, AC-IV-2, AC-IV-5, AC-IV-7, D-I-2) (table 2; see video on the Neurology® Web site at Neurology.org). Impairments occurred across the domains of articulation, phonation, resonance, and prosody. All individuals demonstrated impaired articulation, characterized by imprecise production of consonants (11/11, 100%) and vowels (8/11, 73%). Phonological-level speech production errors included substitution of consonants and vowels, reduction of consonant clusters, and omission of sounds and/or syllables. A number of phonological errors were heard, for example, /f/ for /θ/ (e.g., “fing” for “thing”) and /d/ or /v/ for /ð/ (e.g., “dat” for “that”). Prosodic impairments were common, and speech was typically slow, with stress errors (7/11, 64%), shortening of phrases (6/11, 55%), and prolonged intervals between syllables and words (5/11, 45%). Breath support for speech was generally adequate. Laryngeal impairments manifested as difficulty modulating pitch (monopitch 10/11, 91%; pitch fluctuations 5/11, 45%) as well as hoarse, harsh, or breathy vocal quality (11/11, 100%; harsh and breathy voice in AC-IV-5). Altered resonance was characterized by hypernasality (7/11, 64%; mixed nasality in AC-IV-7), with nasal flare and nasal air escape at times. No speech features were rated as severely impaired.
Table 2.
Conversational speech abnormalities: Frequency (n = number of individuals/11) and severity ratings

Two individuals (AC-IV-7, AC-V-1) underwent nasendoscopy by an otolaryngeal surgeon. This revealed normal structures and excluded obvious velopharyngeal weakness. Difficulty with motor control of velopharyngeal closure was noted. AC-V-1 had minimal air escape on non-nasal sustained sounds, which increased in connected speech. AC-IV-7 had a small granuloma on the left vocal fold process of the arytenoid, but laryngeal function was normal.
Speech tasks.
Individuals had dysarthria with motor execution difficulties on maximum performance tasks (table 3). Maximum vowel duration was reduced in 8 of 11 individuals, with the task not completed by one (D-II-6). Maximum repetition rate of monosyllables was also slow in 10 of 11 individuals, with repetition of /ta/ impaired in 9 of 11, followed by /ka/ (8/11) and /pa/ (7/11). Two individuals (AC-IV-2, AC-V-1) ran out of breath during these tasks, and 2 (AC-IV-2, AC-IV-7) were unable to maintain loudness during vowel prolongation.
Speech dyspraxia with difficulties in motor planning and programing was observed (table 3). Most individuals (7/11) had difficulty repeating a trisyllabic sequence (pataka). Four (AC-IV-5, AC-III-5, AC-V-1, D-II-6) were unable to repeat the sequence correctly, with sequencing errors noted for most individuals (8/11) across multiple repetitions. All individuals also had difficulty repeating nonwords and multisyllabic words compared with control data, with more errors as word length increased (2 syllables 14%–71% correct compared with 5 syllables 0%–43% correct on Nonword Memory Test; mild-severe impairment on ABA-2 Increasing Word Length subtest).
Oral motor assessment.
AC-III-5 and AC-IV-2 had involuntary movements of the tongue at rest. Dystonic posturing of the tongue at rest and deviation to the left was noted in D-II-1. Slow or poorly coordinated tongue movement was evident in all individuals but one (D-II-2). Tongue movement was generally poorer on nonspeech tasks (protrusion, elevation) compared with speech.
In speech, lip movements were reduced and/or poorly coordinated, with the posture of the top lip suggestive of increased tone (AC-IV-5, AC-III-5, AC-IV-7, D-II-6). Subtle asymmetry in lip retraction was noted in 2 individuals (AC-III-5, AC-V-1), and as the lips came to rest in one (AC-IV-7). Lip seal was adequate. Mild oral dysphagia was reported (AC-IV-5, AC-III-5, AC-V-1) including difficulty chewing or food “getting stuck,” together with instances of expectoration (AC-V-1) or aspiration (e.g., peanut inhalation in AC-III-5). Early saliva control difficulties were noted in AC-IV-5 and AC-V-1, and required medication (AC-V-1).
Language.
Moderate to severe language impairment was present in individuals younger than 21 years with ECSWS and IEAD, with receptive and expressive language skills below the first percentile in AC-V-1, D-II-1, and D-II-2 (table 3). No specific pattern of impairment was evident across the domains of language (e.g., semantics, syntax, morphology; see table 4). The degree of impairment in the brothers with IEAD was greater than anticipated given their cognitive skills. Language was congruent with cognitive skills in the individuals with ECSWS (AC-V-1, D-II-6). Adults with ADRESD performed comparatively better, and apart from a few (AC-IV-5, AC-IV-2, AC-III-4), scores fell within 1 SD of the mean on receptive and expressive language tasks. Delayed language development or impaired language skills were documented before onset of seizures in 7 individuals. Early language skills were also delayed in AC-III-5 and normal in AC-III-2 and AC-III-4 based on parental report.
Table 4.
CELF-4 subtest scores

D-I-2, who had no history of seizures or regression, had average cognitive and language skills; however, it is impossible to exclude that she had difficulties as a child. The language assessment was not completed in AC-III-4 and AC-IV-7.
DISCUSSION
GRIN2A has recently been identified as the first gene associated with EAS and therefore is likely to play a critical role in speech and cognitive-linguistic function. This present cohort is the largest studied to date with comprehensive speech and language data. The GRIN2A speech phenotype consists of a combination of speech dyspraxia with impaired motor planning and programing, and dysarthria with impairments in speech execution. Although variations among affected individuals were noted, their speech was typified by imprecise articulation of consonants and vowels and hypernasality, with prosodic disturbance. Poorly coordinated lip and tongue movements were seen, with abnormal tone and reduced and asymmetrical lip movement. These abnormalities, distinguished by listening to their speech, are supported by findings on quantifiable assessments of dysarthria. Performance on maximum vowel prolongation and diadochokinesis (alternating rapid movements) tasks examining maximum repetition rate of monosyllabic and trisyllabic sequences was poor. Impaired trisyllabic repetition is characteristic of speech dyspraxia.15 Reduced vowel prolongation and slow monosyllabic repetition are also observed in dysarthria associated with spastic quadriplegia; our patients did not have cerebral palsy.15 Although speech dyspraxia has been previously recognized in EAS disorders,5 dysarthria has not been a key feature.
Significant language impairment was also seen in adolescents and young adults with ECSWS and IEAD. The language of adults with ADRESD seemed comparatively better. This may have been due to continual improvement in language performance through life. Alternatively, different language assessments were used with individuals older than 21 years, so we cannot rule out that the contrast in language phenotype from adolescence to late adulthood was attributable to methodologic differences. Cognitive impairment was present in most cases. Earlier assessment in the original family with ADRESD revealed impaired comprehension of linguistic-semantic concepts and deficits in expressive vocabulary.5
Deficits in language skills were evident before seizure onset in 8 of 10 individuals with seizures, and 7 of 10 had a nonepileptiform EEG prior to speech and language assessment. This suggests that epileptiform abnormalities were not the cause of the speech and language impairments. Of note, GRIN2A mutations have been identified in individuals with speech disorder in the absence of seizures, in 2 members of the families studied here (AC-II-1 deceased,5 D-I-2) and 3 unrelated families with atypical rolandic epilepsy and speech dyspraxia.9 As we have studied a relatively small sample of 11 affected individuals, it is possible that the deficits observed are attributable to other familial genetic or environmental determinants. It is, however, noteworthy that we found similar impairments across the families with different mutations of GRIN2A. Larger numbers of cases with GRIN2A mutations will further refine the phenotypic spectrum of this disease.
The speech deficits described suggest an important role for GRIN2A and NMDA receptors in normal speech production. The NR2A subunit of the glutamate NMDA receptor, encoded by GRIN2A, is expressed in regions involved in speech production24 including the anterior cingulate, thalamus, putamen, cerebellum, anterior and dorsolateral prefrontal cortex, and caudate.25,26 NR2 subunits are crucial to NMDA receptor functioning, controlling cell surface expression and localization,27 providing glutamate binding sites,28 and modifying channel properties.25 Patients with anti-NMDA-receptor encephalitis, with antibodies against NR1-NR2 subunits, have absent or unintelligible speech and echolalia.29 Mice expressing truncated NR2A show impaired motor coordination, as well as deficits in synaptic plasticity and reorganization.30 Speech motor planning and execution deficits were observed in our cohort. Discovery of GRIN2A mutations in cohorts with speech disorder without epilepsy will add further support to the importance of this gene in normal speech production.
Supplementary Material
ACKNOWLEDGMENT
The authors sincerely thank the families for their participation in this study. The authors thank Associate Professor Lynette Sadleir for referring a family and Professor Susan Gathercole for use of the Nonword Memory Test. The authors are grateful to Professor Eliane Roulet-Perez for helpful advice regarding the manuscript.
GLOSSARY
- ADRESD
autosomal dominant rolandic epilepsy with speech dyspraxia
- EAS
epilepsy-aphasia syndromes
- ECSWS
epileptic encephalopathy with continuous spike and wave during sleep
- GRIN2A
glutamate receptor, ionotropic, N-methyl d-aspartate 2A
- IEAD
intermediate epilepsy-aphasia disorder
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.J.T., A.T.M., and I.E.S. designed the study and wrote the manuscript. A.T.M. and I.E.S. supervised the study. S.A.M. analyzed the MRI brain scans. S.J.T., A.K.M., A.T.M., and A.V. performed phenotypic analysis.
STUDY FUNDING
S.J.T. is supported by a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship (101777) and Speech Pathology Australia Nadia Verrall Memorial Research Grant. A.T.M. is supported by an NHMRC Career Development Award (607315). I.E.S. is supported by an NHMRC Program Grant (628952) and Practitioner Fellowship (1006110). This project was also supported by an Australian Research Council (ARC) Discovery Project (DP120100285) to A.T.M. and I.E.S.
DISCLOSURE
S. Turner, A. Mayes, A. Verhoeven, S. Mandelstam, and A. Morgan report no disclosures relevant to the manuscript. I. Scheffer serves on the editorial boards of the Annals of Neurology, Neurology®, and Epileptic Disorders; may accrue future revenue on a pending patent re: therapeutic compound; has received speaker honoraria from Athena Diagnostics, UCB, GSK, and Transgenomic; has received funding for travel from Athena Diagnostics, UCB, and GSK; and receives/has received research support from the NHMRC, ARC, Health Research Council of New Zealand, The University of Melbourne, American Epilepsy Society, the Jack Brockhoff Foundation, the Weizmann Institute, CURE, US Department of Defense, and the Perpetual Charitable Trustees. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Tsai MH, Vears DF, Turner SJ, et al. Clinical genetic study of the epilepsy-aphasia spectrum. Epilepsia 2013;54:280–287. [DOI] [PubMed] [Google Scholar]
- 2.Overvliet GM, Besseling RM, Vles JS, et al. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: review of the literature. Epilepsy Behav 2010;19:550–558. [DOI] [PubMed] [Google Scholar]
- 3.Clarke T, Strug LJ, Murphy PL, et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia 2007;48:2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundberg S, Frylmark A, Eeg-Olofsson O. Children with rolandic epilepsy have abnormalities of oromotor and dichotic listening performance. Dev Med Child Neurol 2005;47:603–608. [PubMed] [Google Scholar]
- 5.Scheffer IE, Jones L, Pozzebon M, Howell RA, Saling MM, Berkovic SF. Autosomal dominant rolandic epilepsy and speech dyspraxia: a new syndrome with anticipation. Ann Neurol 1995;38:633–642. [DOI] [PubMed] [Google Scholar]
- 6.Roll P, Rudolf G, Pereira S, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet 2006;15:1195–1207. [DOI] [PubMed] [Google Scholar]
- 7.Kugler SL, Bali B, Lieberman P, et al. An autosomal dominant genetically heterogeneous variant of rolandic epilepsy and speech disorder. Epilepsia 2008;49:1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvill GL, Regan BM, Yendle SC, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet 2013;45:1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesca G, Rudolf G, Bruneau N, et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet 2013;45:1061–1066. [DOI] [PubMed] [Google Scholar]
- 10.Lemke JR, Lal D, Reinthaler EM, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet 2013;45:1067–1072. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch BE. Dysarthria: A Physiological Approach to Assessment and Treatment. Cheltenham, UK: Stanley Thornes; 1998. [Google Scholar]
- 12.Lewis BA, Freebairn L. Residual effects of preschool phonology disorders in grade school, adolescence, and adulthood. J Speech Hear Res 1992;35:819–831. [DOI] [PubMed] [Google Scholar]
- 13.Gathercole SE, Baddeley AD. Nonword Memory Test. Bristol, UK: University of Bristol; 1996. [Google Scholar]
- 14.Dabul BL. Apraxia Battery for Adults, 2nd ed Austin: PRO-ED; 2000. [Google Scholar]
- 15.Thoonen G, Maassen B, Wit J, Gabreels F, Schreuder R. The integrated use of maximum performance tasks in differential diagnostic evaluations among children with motor speech disorders. Clin Linguist Phon 1996;10:311–336. [Google Scholar]
- 16.Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis and Management, 3rd ed St. Louis: Mosby; 2013. [Google Scholar]
- 17.Enderby P, Palmer R. Frenchay Dysarthria Assessment, 2nd ed Austin: PRO-ED; 2008. [Google Scholar]
- 18.ASHA. Technical Report: Childhood Apraxia of Speech. Available at: www.asha.org/policy/tr2007-00278.htm#sec1.1. Accessed March 12, 2010. [Google Scholar]
- 19.Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals, Australian Standardised Edition, 4th ed Marrickville, Australia: Harcourt Assessment; 2006. [Google Scholar]
- 20.Dunn LM, Dunn DM. The Peabody Picture Vocabulary Test, 4th ed Minneapolis: NCS Pearson Inc.; 2007. [Google Scholar]
- 21.Williams KT. Expressive Vocabulary Test, 2nd ed London: Pearson Assessment; 2007. [Google Scholar]
- 22.Bishop DJ. Test for Reception of Grammar, 2nd ed London: Pearson Assessment; 2003. [Google Scholar]
- 23.Wechsler D. The Wechsler Abbreviated Scale of Intelligence, 2nd ed London: Pearson Assessment; 2011. [Google Scholar]
- 24.Liegeois FJ, Morgan AT. Neural bases of childhood speech disorders: lateralization and plasticity for speech functions during development. Neurosci Biobehav Rev 2012;36:439–458. [DOI] [PubMed] [Google Scholar]
- 25.Monyer H, Sprengel R, Schoepfer R, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 1992;256:1217–1221. [DOI] [PubMed] [Google Scholar]
- 26.Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb Cortex 1999;9:110–120. [DOI] [PubMed] [Google Scholar]
- 27.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 1995;269:1737–1740. [DOI] [PubMed] [Google Scholar]
- 28.Kendrick SJ, Lynch DR, Pritchett DB. Characterization of glutamate binding sites in receptors assembled from transfected NMDA receptor subunits. J Neurochem 1996;67:608–616. [DOI] [PubMed] [Google Scholar]
- 29.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprengel R, Suchanek B, Amico C, et al. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 1998;92:279–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



