Abstract
Objective:
In the largest cohort study of neuropsychological outcomes among HIV-infected women to date, we examined the association between HIV status and cognition in relation to other determinants of cognitive function (aim 1) and the pattern and magnitude of impairment across cognitive outcomes (aim 2).
Methods:
From 2009 to 2011, 1,521 (1,019 HIV-infected) participants from the Women's Interagency HIV Study (WIHS) completed a comprehensive neuropsychological test battery. We used multivariable regression on raw test scores for the first aim and normative regression-based analyses (t scores) for the second aim. The design was cross-sectional.
Results:
The effect sizes for HIV status on cognition were very small, accounting for only 0.05 to 0.09 SD units. The effect of HIV status was smaller than that of years of education, age, race, income, and reading level. In adjusted analyses, HIV-infected women performed worse than uninfected women on verbal learning, delayed recall and recognition, and psychomotor speed and attention. The largest deficit was observed in delayed memory. The association of low reading level with cognition was greater in HIV-infected compared to HIV-uninfected women. HIV biomarkers (CD4 count, history of AIDS-defining illness, viral load) were associated with cognitive dysfunction.
Conclusions:
The effect of HIV on cognition in women is very small except among women with low reading level or HIV-related comorbidities. Direct comparisons of rates of impairment in well-matched groups of HIV-infected men and women are needed to evaluate possible sex differences in cognition.
Compared to HIV-infected men, HIV-infected women may be at greater risk for cognitive decline due to a higher prevalence of risk factors common in predominantly minority, urban-dwelling women, such as poverty, low literacy levels, low education, substance abuse, poor mental health, early life stressors and trauma, barriers to health care service utilization, and environmental exposures.1,2 These factors might contribute to low cognitive reserve and confer increased risks of cognitive dysfunction. Several studies have examined cognition in HIV-infected women,3–11 but the maximum sample size has been 237. Larger studies are needed to understand the determinants and patterns of cognitive function in HIV-infected women.
The Women's Interagency HIV Study (WIHS) is the largest longitudinal study of the natural and treated history of HIV infection and clinical outcomes in women residing in the United States.12,13 Here we present the first findings from the largest comprehensive cohort study of cognitive function in HIV-infected (n = 1,019) and demographically similar HIV-uninfected women (n = 502). Our first aim was to investigate the association between HIV status and cognition in relation to other determinants of cognition. We predicted that low socioeconomic status, low reading level, illicit substance use, and depressive symptoms would contribute to decrements across a range of cognitive domains, and would more strongly influence cognition in HIV-infected women. Our second aim was to investigate the pattern and magnitude of impairment across cognitive outcomes. Based on prior WIHS findings,11,14 we predicted that HIV-infected women would show deficits in psychomotor speed, attention, verbal learning, and memory.
METHODS
Standard protocol approvals, registrations, and patient consents.
Previous reports detail the WIHS recruitment, retention, and study procedures and demonstrate that the WIHS cohort reflects the demographic and exposure risk characteristics of HIV-infected women in the United States.12,13,15 The study received institutional review board approval at each WIHS site.
Participants.
The WIHS was established in August 1994 at 6 clinical consortia: Brooklyn, New York; Bronx/Manhattan, New York; Washington, DC; Los Angeles, California; San Francisco/Bay Area, California; and Chicago, Illinois. In brief, the participants eligible for this cognitive substudy were enrolled during 2 waves of WIHS recruitment: the first between October 1994 and 1996 (n = 2,623) and the second in 2001–2002 (n = 1,143) for a total of 3,766 (2,791 HIV-infected and 975 HIV-uninfected). Participants in this cross-sectional investigation completed a cognitive assessment during the first wave (April 2009–2011) of a comprehensive neuropsychological test battery administered every 2 years in conjunction with WIHS semiannual core study visits. At each WIHS core visit, participants undergo a physical examination, medical and psychosocial interviews, and a blood draw to assess HIV status/viral load and immune, kidney, and liver function.
For cognitive testing, we broadly targeted all active English-speaking WIHS participants (n = 1,908) who completed any of the 4 semiannual WIHS visits. Exclusionary criteria were established in advance but applied after cognitive testing of the targeted group because variables acquired at the core semiannual visit (e.g., recent drug abuse) were needed to determine eligibility. Of these 1,908 women, 1,595 (84%) completed cognitive assessments. Limited attendance at WIHS visits contributed to the 16% missing data; 45% of women who did not complete cognitive testing attended 2 or fewer semiannual visits during the 2-year wave compared to 3% of women who did complete cognitive testing (p < 0.001). Compared to noncompleters, completers were more likely to be black non-Hispanic; to be less educated; to have an annual income; to be hepatitis C virus (HCV) antibody positive; to have recently smoked; to have recently used crack, cocaine, heroin, and/or marijuana; and to be HIV-seropositive (table e-1 on the Neurology® Web site at Neurology.org). Completers were more likely to be from the Bronx and Brooklyn and less likely to be from Los Angeles and Chicago.
We included 1,521 (1,019 HIV-infected; 95% of the active cohort) participants in analyses after excluding 74 participants meeting one or more of the following exclusion criteria: (1) presence of conditions that limit test validity (e.g., hearing loss, impaired vision, immediate influence of illicit substances; n = 11); (2) history of stroke/cerebrovascular accident (n = 13); and (3) self-reported use of antipsychotic medication in the past 6 months (n = 50). Sixty-four women had self-reported dementia or dementia by medical record and completed cognitive assessments. They were included to ensure representation across the range of cognitive performance.
After completing a standardized training process, certified testers administered the test battery to a subset (about 25%) of WIHS participants at each of 4 semiannual visits, thus including 100% of the target sample within a 2-year cycle. Test administrators were required to complete a 3-step certification process involving (1) face-to-face training at respective sites; (2) central review, scoring, and feedback for 2 audiotaped administrations of the test battery; and (3) central review of test administrations 6 and 12 months after initial certification and annually thereafter to control for “drift” in testing. These training procedures were shown to be effective, efficient, and low cost in previous work.16 The test battery was developed by WIHS investigators, reviewed by external neuroAIDS experts, and designed to enable future applications of existing HIV-associated neurocognitive disorders (HAND) research criteria17 and comparisons to the Multicenter AIDS Cohort Study cohort. A pilot study at the Chicago WIHS Consortium ensured that the battery was suitable for use in the WIHS cohort.11 Neuropsychological tests were the Hopkins Verbal Learning Test–Revised (HVLT-R)18; Stroop Test19; Trail Making Test Parts A and B; Symbol Digit Modalities Test (SDMT); Letter-Number Sequencing Test (LNS); Letter Fluency (F, A, S); Semantic Fluency (animals); and Grooved Pegboard.
Statistical analysis.
We examined differences in demographic characteristics of included participants by HIV status with independent t tests for continuous variables and χ2 tests for categorical variables. To assess predictors of neuropsychological outcomes, we focused on raw test scores. We conducted multivariable regression analyses to examine the association between HIV status and cognition, first after controlling for primary covariates of age, race/ethnicity, education, and reading level as measured by the Wide Range Abilities Test (WRAT-3 Reading Recognition subtest) score, and then also after controlling for study site (of 6), cohort (1994/1995 vs 2001/2002), annual household income (≤$12,000), Center for Epidemiologic Studies Depression Scale (cutoff score 16), recent self-reported use of antidepressant medication, HCV antibody status, smoking status (recent, former, never), marijuana/hashish use (recent, former, never), recent heavy alcohol use (heavy use >7 drinks/week or more than 4 drinks in one sitting, non-heavy alcohol use, abstainer), and crack, cocaine, and/or heroin use via any means of administration (recent, former, never). We also adjusted for number of prior exposures to the Stroop (range 1–4), HVLT (range 1–2), SDMT (range 1–5), and Trail Making (range 1–5) tests. We then examined the interactive effects of HIV status and primary covariates on raw scores.
To test our second hypothesis regarding the pattern of performance across neuropsychological tests, we used a regression-based approach to create demographically corrected normative standards (i.e., t scores) for individual neuropsychological tests.14,20,21 Based on prior research,14 each outcome was first regressed on age, years of education, WRAT-3 score, and race/ethnicity in the HIV-uninfected women (table e-2). We then used the unstandardized β weights of each predictor, the constant, and the standard error to calculate predicted scores for each test. Finally, to create t scores with a mean of 50 and SD of 10 in the controls, we estimated the difference between the predicted scores and observed (residual) scores (t score = [(observed score − predicted score)/standard error of the estimate of the regression] × 10 + 50). These t scores served as outcomes in a series of multivariable regression analyses for the same covariates (e.g., site, income) described above. For analyses restricted to HIV-infected women, we examined current and nadir CD4 T-lymphocyte count <200 cells/mm3, plasma HIV RNA (viral load) (>10,000 cp/mL, ≤10,000, undetectable), history of AIDS-defining illness, antiretroviral combination antiretroviral therapy (cART) medication use/adherence (no cART therapy, cART therapy + <95% adherent, cART therapy + ≥95% adherent), and duration of antiretroviral therapy use. The statistical significance level was set at p < 0.05. Analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC).
RESULTS
Study population.
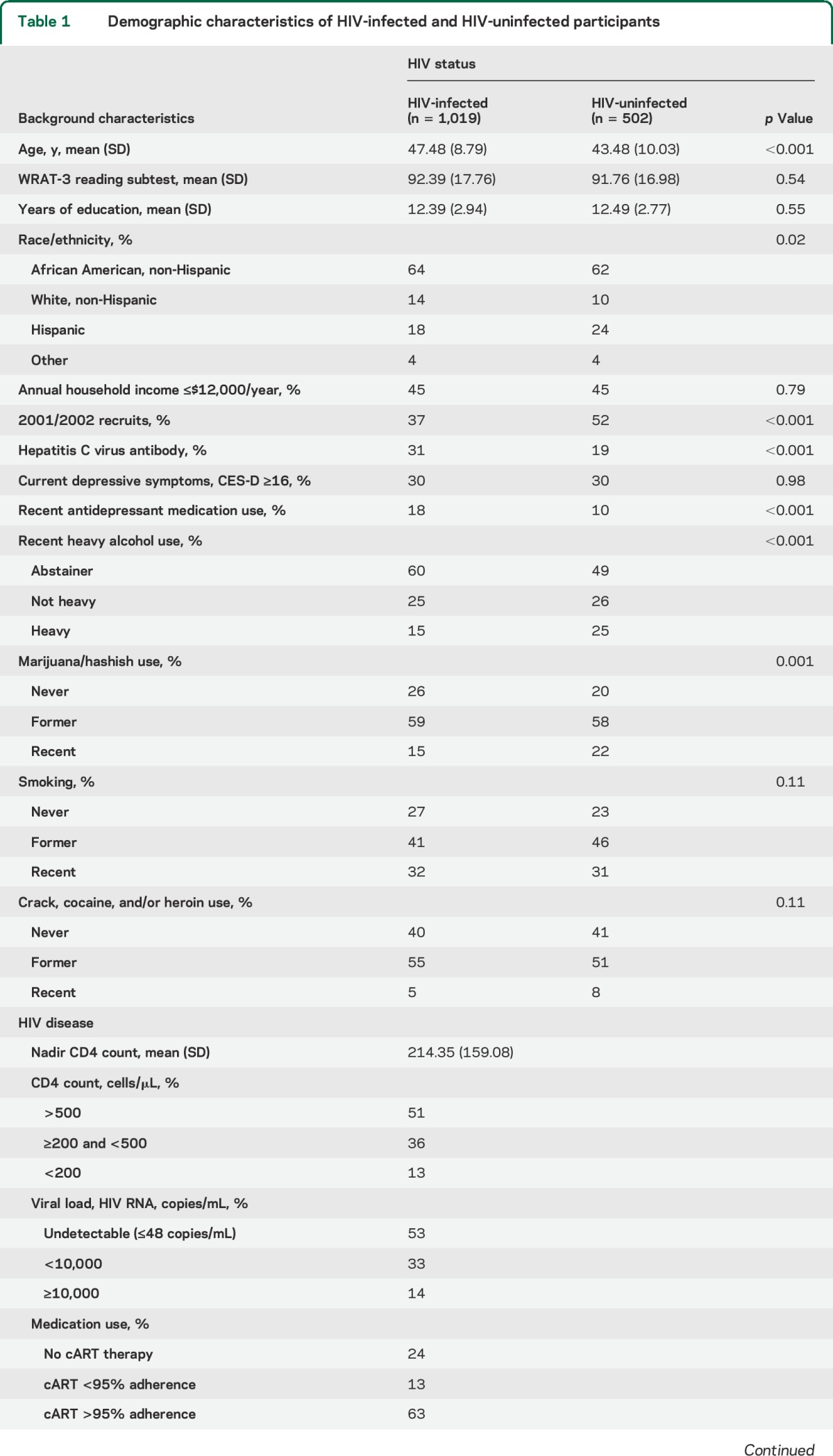
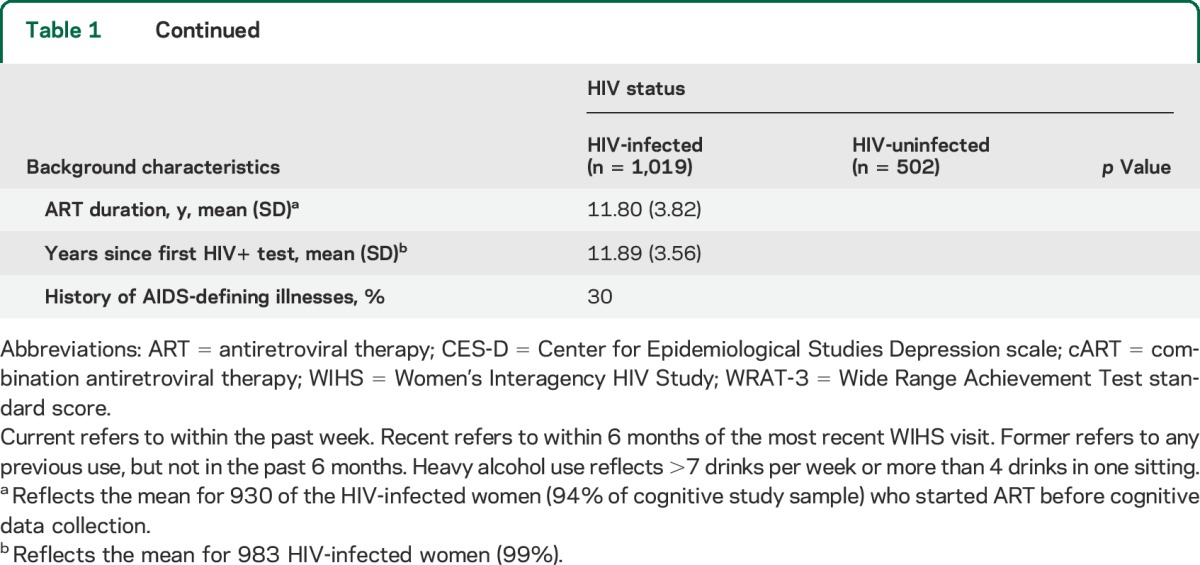
Participants included 1,019 HIV-infected and 502 HIV-uninfected women (table 1). Age ranged from 25 to 87 years (mean = 46.15, SD = 9.39; range 25.9–87.4 in HIV-infected and 25.5–77.5 in HIV-uninfected), with 64% non-Hispanic African American and 20% Latina and 42% from the 2001/2002 cohort.12,13 Lifetime use of illicit substances was common: 59% formerly used marijuana and 54% formerly used crack, cocaine, and/or heroin. Only 21% reported recent use of illicit substances (6% crack, cocaine, and/or heroin; 17% marijuana), where recent was defined as use since the previous visit, typically 6 months earlier. HIV-infected women were less likely than HIV-uninfected women to report recent use of marijuana and recent hazardous alcohol use (p < 0.001) and were more likely to be hepatitis C infected and to report use of antidepressant medication. Among HIV-infected women, 13% had a current CD4 T-lymphocyte count <200 cells/μL, 53% had undetectable plasma HIV RNA, and 63% were adherent by self-report to ≥95% of prescribed cART doses. A total of 97% of women completed the neuropsychological assessment at the core visit, and 3% at a separate visit.
Table 1.
Demographic characteristics of HIV-infected and HIV-uninfected participants
HIV effects on cognition.
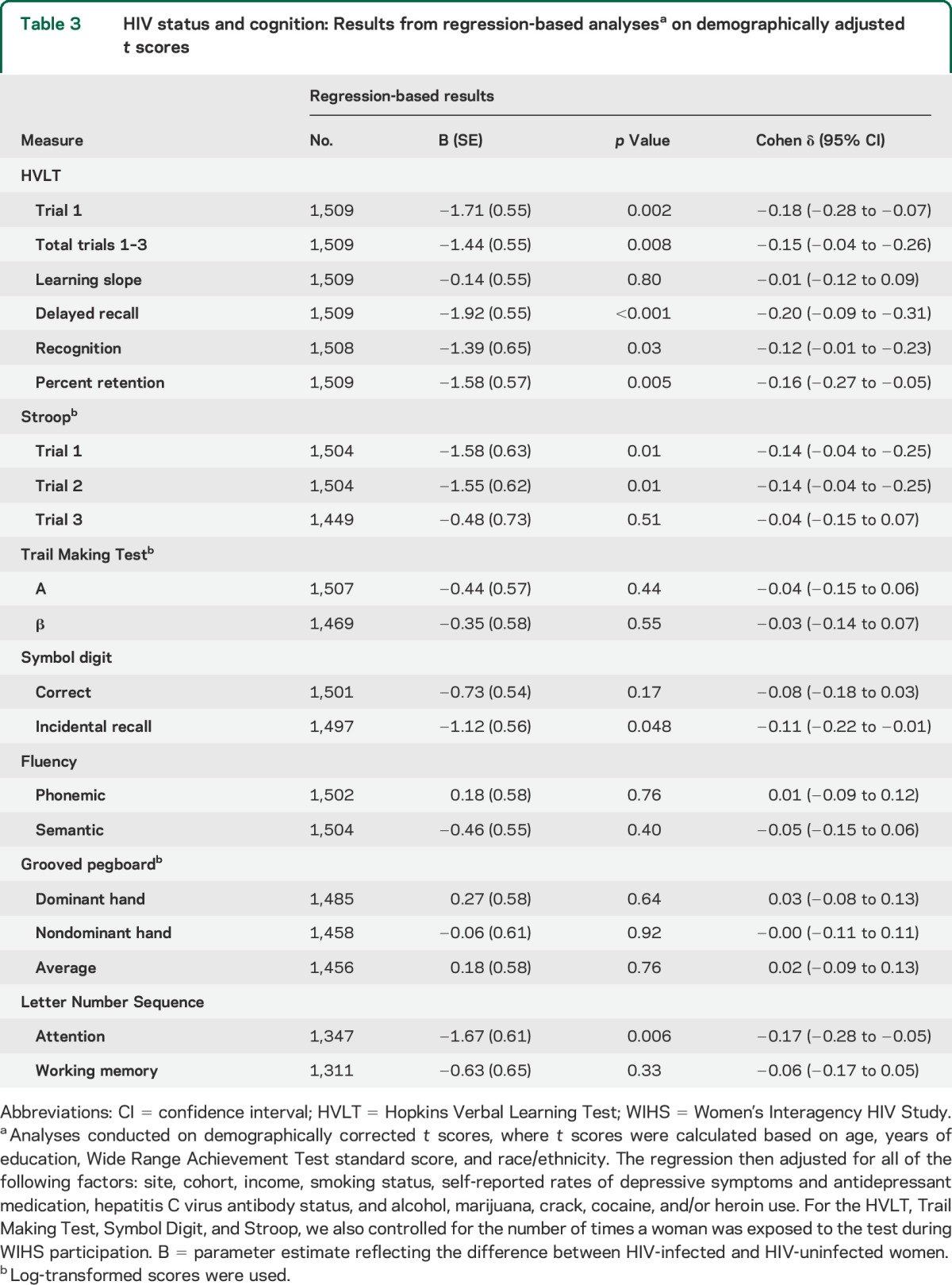
Table 2 shows the comparison of neuropsychological test scores between groups. HIV-uninfected women served as the reference group and negative β coefficients referred to worse performance among infected women. In unadjusted models, significant group differences were observed on the HVLT, Stroop, Trail Making Test, SDMT, Grooved Pegboard, Semantic Fluency, and LNS Attention. In adjusted analyses, HIV-infected women performed worse on all but one (learning slope) HVLT outcome measure (trial 1 learning, total learning across trials 1–3, delayed recall, recognition, percent recognition) (ps < 0.05) and on measures of attention (Stroop trial 1 and 2; LNS attention trial) (ps < 0.05). HIV-infected women also performed worse on incidental recall on the SDMT. These same patterns of HIV effects were mirrored in regression analyses using demographically adjusted t scores (table 3). In both analyses, the largest HIV effect was observed for HVLT delayed recall (Cohen δ = −0.20). This effect remained after controlling for the total words learned (p = 0.02; Cohen δ = −0.13).
Table 2.
Results from unadjusted and adjusted analyses of HIV status on raw cognitive test scores
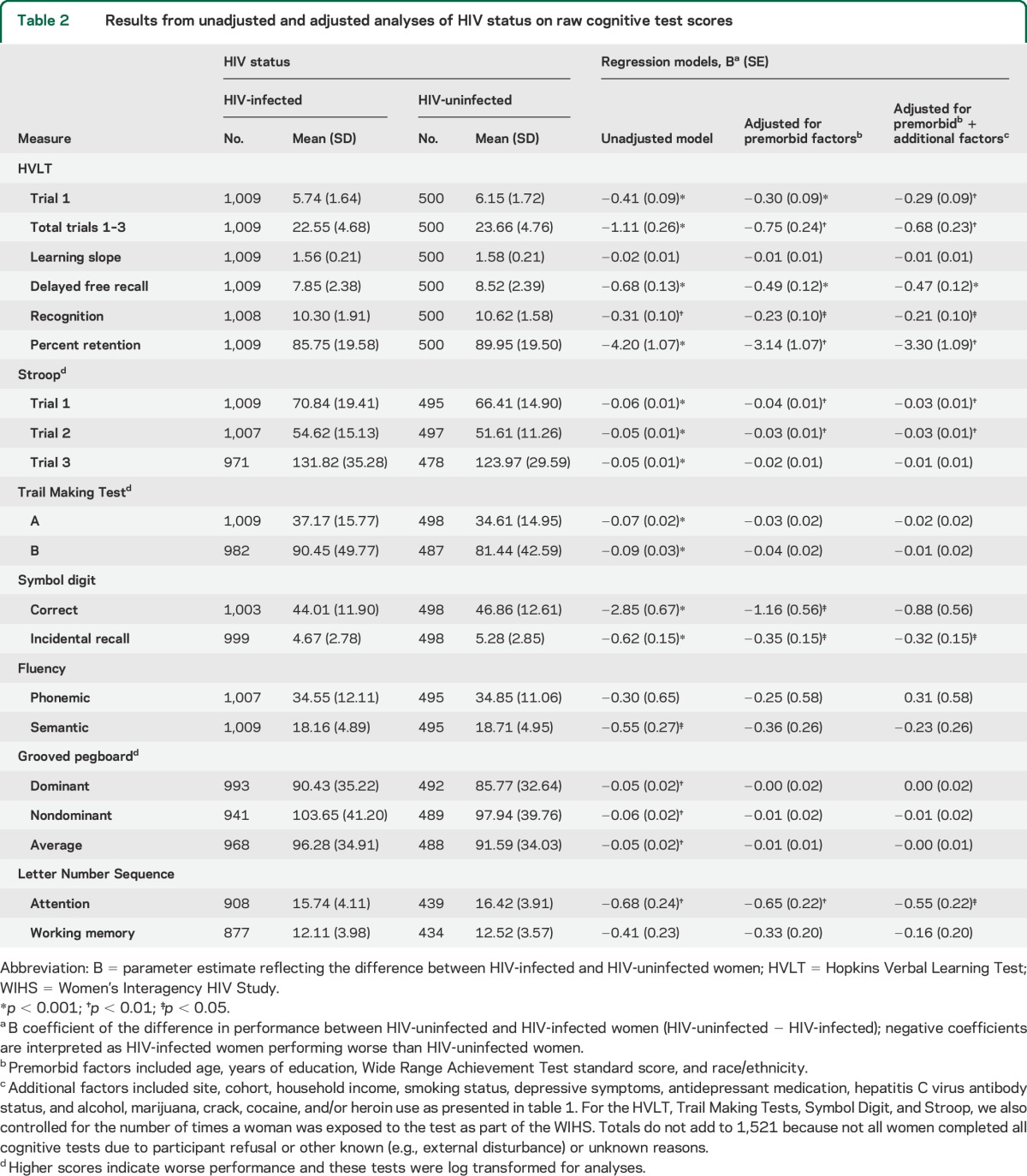
Table 3.
HIV status and cognition: Results from regression-based analysesa on demographically adjusted t scores
Effect of HIV status in relation to other determinants of cognitive function.
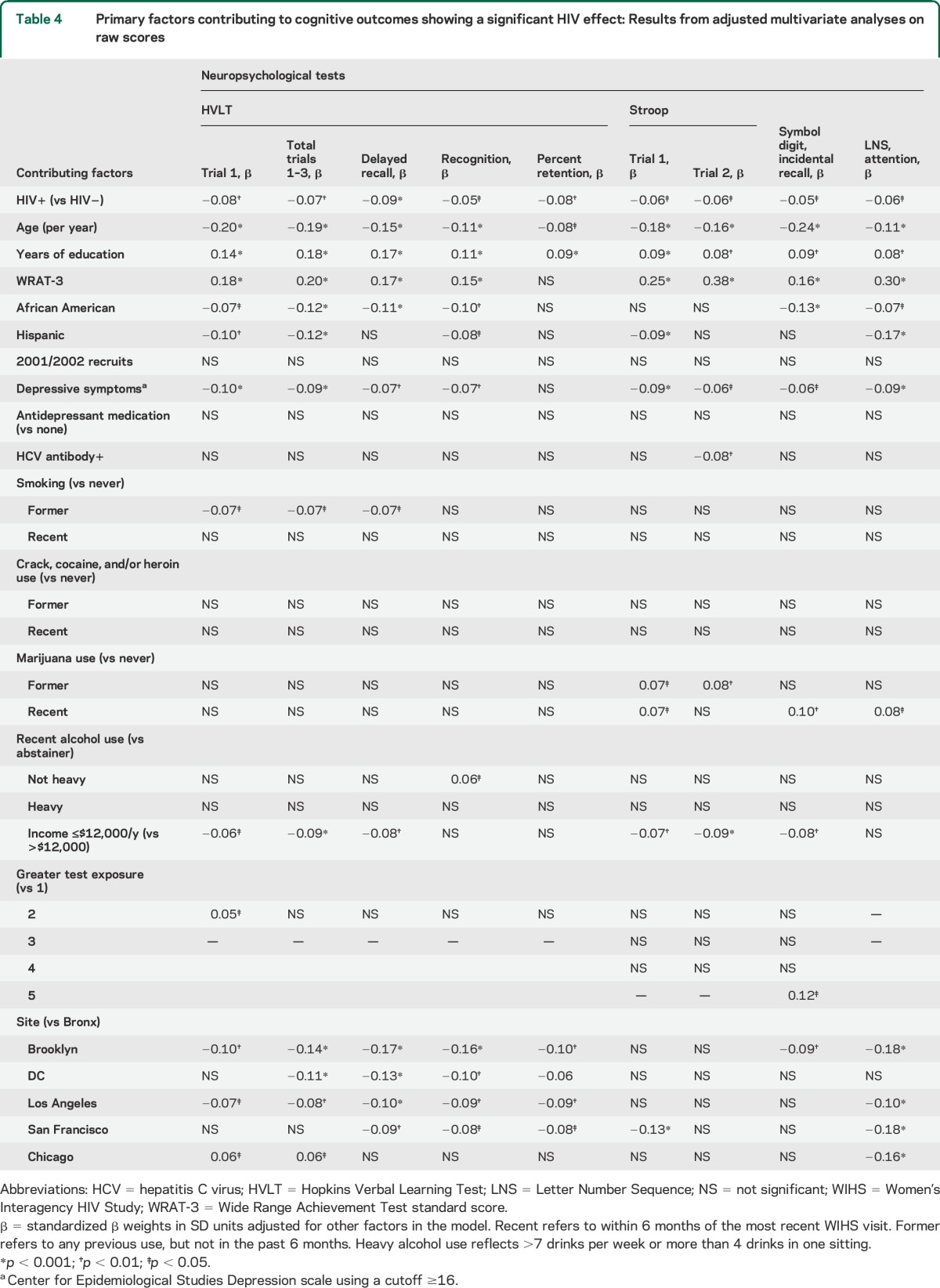
Table 4 shows the standardized β coefficients from the multivariable regression on raw test scores, where the coefficients are adjusted for all factors in the model. Generally, the effect sizes for HIV status on cognition were very small, accounting for 0.05 to 0.09 SD units. The HIV effect was smaller than effects of years of education, age, race/ethnicity, annual household income, and reading level as indexed by the WRAT-3, which is used as a marker of education quality.22 Reading level was the strongest predictor of cognition, predicting all but one outcome and yielding effect sizes equivalent to 0.15 to 0.38 SD units. By comparison, although significant, the effect size for years of education ranged from 0.08 to 0.18 SD units.
Table 4.
Primary factors contributing to cognitive outcomes showing a significant HIV effect: Results from adjusted multivariate analyses on raw scores
Interaction between HIV status and cohort.
To evaluate whether the effect of HIV serostatus on cognition varied by cohort (1994/1995 vs 2001/2002), we conducted a separate model that also included a serostatus by cohort interaction term. No interactions were significant on any outcome.
Interaction between HIV status and education/age.
HIV status significantly interacted with WRAT-3 on Stroop trials 1 and 2 (ps < 0.01), Trails B (p = 0.03), and Grooved Pegboard (dominant hand, p = 0.02). For Stroop trial 1, a negative effect of HIV serostatus was evident when WRAT scores were 0.5 SD below the mean (i.e., scaled score of <95), and for Stroop trial 2, Trails B, and Grooved Pegboard when WRAT scores were at least 2 SD below the mean (i.e., <68). For semantic fluency, HIV-infected women performed worse than HIV-uninfected women when years of education were 9 years or less (ps < 0.05). Age did not interact with HIV status to influence cognitive function.
Clinical determinants of cognitive dysfunction.
Among HIV-infected women, current CD4 T-lymphocyte count <200 cells/µL was associated with lower t scores on Stroop trial 1 (p = 0.03) and LNS working memory (p = 0.02). Having a prior AIDS-defining illness was associated with worse performance on HVLT delayed recall (p = 0.04) and the LNS attention trial (p = 0.007). Detectable HIV plasma viral load was associated with slower average performance on the Grooved Pegboard (<10,000 vs undetectable, p = 0.02; >10,000 vs undetectable, p = 0.005).
DISCUSSION
We report findings from the largest comprehensive cognitive study to date in HIV-infected women with a demographically similar uninfected comparison group (n = 1,521). In analyses adjusting for age, years of education, reading level (WRAT-3),22 race/ethnicity, and depressive symptoms, HIV-infected women showed lower performance only on measures of verbal learning and memory (HVLT), speed of information processing (Stroop trials 1 and 2), and attention (LNS control test). These effects were very small (0.05–0.09 SD units) but were significant given the large sample size. Reading level, age, years of education, and race were more strongly associated with cognitive performance than HIV status. Certain HIV-infected women were vulnerable to greater cognitive deficits, including those with low education and those with low CD4 counts, high viral load, or an AIDS-defining illness. Generally, these findings confirm previous findings suggesting that low cognitive reserve might exacerbate cognitive deficits in HIV-infected individuals.1,23,24
In male-dominant HIV cohorts,25 including the HIV Neurobehavioral Research Center (HNRC) cohort, deficits in learning and executive function are the most frequent cognitive impairments post cART.26 The HNRC examined the categorical outcome of cognitively impaired vs unimpaired, whereas this analysis of WIHS focused on cognition as a continuous outcome. In the WIHS, the largest effect was found on verbal memory, but that effect was very small (0.08 SD units). To examine possible sex differences in clinically relevant levels of cognitive impairment in HIV, it is necessary to directly compare the rates of cognitive impairment in women and men who are matched on demographic variables and comorbidities.
The prominent deficit in verbal memory observed in the WIHS might reflect the influence of sex steroid hormones, stress/trauma, or other factors. Women show a lifelong advantage in verbal memory compared to men,27 due at least in part to their higher estradiol levels,28 which promote hippocampal and prefrontal function.29–31 Verbal memory worsens during the menopausal transition,32,33 and the average age of women in our study was 47 years. Thus, reproductive age might be associated with changes in verbal memory. Additionally, compared to men, women are more vulnerable to the negative effects of stress hormones on hippocampal-dependent tests.34 Childhood trauma (31%) and domestic violence (66%) are common in WIHS.35
A previous WIHS functional MRI study suggests that the deficits in verbal learning and memory observed in this study might reflect hippocampal dysfunction.11 Compared to HIV-uninfected controls, HIV-infected women showed hippocampal hyperactivation during verbal encoding and hippocampal hypoactivation during recall.11 Alterations in hippocampal function during verbal encoding and recall were associated with lower HVLT performance. Alterations in prefrontal cortex also likely contribute to the observed deficit in delayed verbal memory, because HIV-associated deficits in verbal memory are characterized by deficits in executive control of encoding and retrieval mechanisms,25 a pattern consistent with frontal-subcortical involvement.
Although verbal memory was the cognitive domain showing the largest association with HIV serostatus, reading level, years of education, and age showed stronger associations. For verbal learning, the effect size for HIV was about one-third of the effect sizes for reading level, age, and years of education, but similar to the effect sizes for depressive symptoms and poverty. Generally, low reading level was the strongest predictor of performance, predicting all but one outcome and yielding effect sizes equivalent to 0.15 to 0.38 SD units. By comparison, although significant, the effect size for years of education ranged from 0.08 to 0.18 SD units. That finding is consistent with findings from studies in predominantly male cohorts36,37 and extends previous demonstrations that reading level is a stronger predictor of cognitive function than years of school in cohorts with large African American representation,22 such as the WIHS.14
Our large sample size and well-matched control group provided sufficient statistical power to investigate the hypothesis that low education and education quality (as indexed by reading level) might interact with HIV status, resulting in a more negative effect in HIV-infected women. Our analyses supported that hypothesis for processing speed, executive function, and fine motor skills, but not verbal learning and memory. Broadly, those findings indicate that a lack of cognitive reserve may predispose women with HIV to cognitive impairments.
This study had several limitations. First, the sample underrepresented women who miss semiannual visits and excluded participants with advanced dementia who do not attend WIHS study visits. These sampling issues likely counterbalance each other because women with dementia perform poorly and women who missed visits had higher educational achievement and other cognitive advantages. Second, 11% of participants missed LNS outcomes, leading to a probable underestimation of the association between HIV and working memory. Third, our ability to detect any interaction between age and HIV on cognition is limited by the small number of women over age 60 (n = 93) and survival bias. Fourth, we measured HIV effects on cognitive scores measured as continuous outcomes, so the clinical significance of these effects is unclear. Assessments of HAND are underway in the WIHS and will address questions about the clinical significance of cognitive deficits and whether the patterns of cognitive deficits are similar when cognitive impairment is the outcome. Fifth, it is surprising that the effect of HIV on cognition was not influenced by cohort (1995/1996 vs 2001/2002), even though the earlier recruits were less likely to be exposed to cART early in the course of the disease. Survivor bias might lead to an underrepresentation of women with cognitive impairment in the earlier cohort and might minimize any cognitive differences between the cohorts. Nevertheless, the WIHS women are generally representative of the HIV epidemic in the United States.12,13,15 Finally, the design is cross-sectional and a longitudinal study is underway.
Overall, this investigation addresses the need for large-scale investigations to improve scientific knowledge about cognitive function in HIV-infected women compared to well-matched controls. Clinicians can advise female patients that the magnitude of the HIV effect is very small, and most evident among women with low reading levels and those with HIV-associated comorbidities, low CD4, or high viral load.
Supplementary Material
GLOSSARY
- cART
combination antiretroviral therapy
- HAND
HIV-associated neurocognitive disorders
- HCV
hepatitis C virus
- HNRC
HIV Neurobehavioral Research Center
- HVLT-R
Hopkins Verbal Learning Test–Revised
- LNS
Letter-Number Sequencing Test
- SDMT
Symbol Digit Modalities Test
- WIHS
Women's Interagency HIV Study
- WRAT
Wide Range Abilities Test
Footnotes
Editorial, page 220
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
P.M. Maki: designed the study, conceptualized the study, analyzed data in the study, interpreted data in the study, drafted the manuscript, and revised the manuscript. L.H. Rubin: analyzed data in the study, interpreted data in the study, drafted data analysis and results section of the manuscript, revised the manuscript. V. Valcour: interpreted data in the study, revised the manuscript. E. Martin: conceptualized the study, revised the manuscript. H. Crystal: conceptualized the study, interpreted data in the study, revised the manuscript. M. Young: conceptualized the study, interpreted data in the study, revised the manuscript. K.M. Weber: conceptualized the study, interpreted data in the study, revised the manuscript. J. Manly: designed the study, conceptualized the study, interpreted data in the study. J. Richardson: interpreted data in the study, revised the manuscript. K. Anastos: interpreted data in the study, revised the manuscript.
STUDY FUNDING
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff and Deborah Gustafson); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien); Los Angeles County/Southern California Consortium (Alexandra Levine and Marek Nowicki); Chicago Consortium (Mardge Cohen); and the Data Coordinating Center (Stephen Gange and Elizabeth Golub). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-AI-34993, and U01-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI grant number UL1 RR024131). Dr. Valcour's work is supported by K24-MH098759. Dr. Rubin's effort was supported by grant 1K01MH098798-01 from the National Institute of Mental Health. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. J Clin Exp Neuropsychol 2000;22:208–218. [DOI] [PubMed] [Google Scholar]
- 2.Farinpour R, Miller EN, Satz P, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol 2003;25:654–670. [DOI] [PubMed] [Google Scholar]
- 3.Fox-Tierney RA, Ickovics JR, Cerreta CL, Ethier KA. Potential sex differences remain understudied: a case study of the inclusion of women in HIV/AIDS-related neuropsychological research. Rev Gen Psychol 1999;3:44–54. [Google Scholar]
- 4.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol 2001;23:149–163. [DOI] [PubMed] [Google Scholar]
- 5.Wojna V, Skolasky RL, Hechavarria R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol 2006;12:356–364. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Boland R, Paul R, et al. Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS 2001;15:341–345. [DOI] [PubMed] [Google Scholar]
- 7.Mason AC, Muller NL. The role of computed tomography in the diagnosis and management of human immunodeficiency virus (HIV)-related pulmonary diseases. Semin Ultrasound CT MR 1998;19:154–166. [DOI] [PubMed] [Google Scholar]
- 8.Stern RA, Arruda JE, Somerville JA, et al. Neurobehavioral functioning in asymptomatic HIV-1 infected women. J Int Neuropsychol Soc 1998;4:172–178. [DOI] [PubMed] [Google Scholar]
- 9.Richardson JL, Martin EM, Jimenez N, et al. Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J Int Neuropsychol Soc 2002;8:781–793. [DOI] [PubMed] [Google Scholar]
- 10.Richardson JL, Nowicki M, Danley K, et al. Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. Aids 2005;19:1659–1667. [DOI] [PubMed] [Google Scholar]
- 11.Maki PM, Cohen MH, Weber K, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 2009;72:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study: WIHS collaborative study group. Epidemiology 1998;9:117–125. [PubMed] [Google Scholar]
- 14.Manly JJ, Smith C, Crystal HA, et al. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV- women: the Women's Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol 2011;33:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol 2001;154:563–573. [DOI] [PubMed] [Google Scholar]
- 16.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials 2004;1:440–450. [DOI] [PubMed] [Google Scholar]
- 17.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandt J, Benedict RHB. Hopkins Verbal Learning Test–revised. Odessa, FL: PAR; 2001. [Google Scholar]
- 19.Comalli PE, Jr, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol 1962;100:47–53. [DOI] [PubMed] [Google Scholar]
- 20.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 21.Testa SM, Winicki JM, Pearlson GD, Gordon B, Schretlen DJ. Accounting for estimated IQ in neuropsychological test performance with regression-based techniques. J Int Neuropsychol Soc 2009;15:1012–1022. [DOI] [PubMed] [Google Scholar]
- 22.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc 2002;8:341–348. [DOI] [PubMed] [Google Scholar]
- 23.Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS Behav 2012;16:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satz P, Morgenstern H, Miller EN, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: findings from the Multicenter AIDS Cohort Study (MACS). J Acquir Immune Defic Syndr 1993;6:503–511. [PubMed] [Google Scholar]
- 25.Woods SP, Scott JC, Dawson MS, et al. Construct validity of Hopkins Verbal Learning Test–Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol 2005;20:1061–1071. [DOI] [PubMed] [Google Scholar]
- 26.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer J, Delis D, Daniel M. Sex differences in verbal learning. J Clin Psychol 1988;44:907–915. [Google Scholar]
- 28.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab 1996;81:2545–2549. [DOI] [PubMed] [Google Scholar]
- 29.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 2006;13:411–422. [DOI] [PubMed] [Google Scholar]
- 30.Maki PM, Dennerstein L, Clark M, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res 2011;1379:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging 2000;21:373–383. [DOI] [PubMed] [Google Scholar]
- 32.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol 2014;142:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab 2013;98:3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab 1997;82:2458–2465. [DOI] [PubMed] [Google Scholar]
- 35.Cohen M, Deamant C, Barkan S, et al. Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. Am J Public Health 2000;90:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohit M, Levine A, Hinkin C, et al. Education correction using years in school or reading grade-level equivalent? Comparing the accuracy of two methods in diagnosing HIV-associated neurocognitive impairment. J Int Neuropsychol Soc 2007;13:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan EL, Baird R, Mindt MR, et al. Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: effects of education and reading level in participant characterization. J Int Neuropsychol Soc 2005;11:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


