Abstract
Objective:
To assess the involvement of small nerve fibers in Charcot-Marie-Tooth type 1A (CMT1A).
Methods:
We used indirect immunofluorescence and confocal microscopy on punch biopsies from glabrous (fingertip) and hairy (thigh and leg) skin of 20 unrelated patients with CMT1A to quantify somatic and autonomic nerve fibers. In particular, we quantified epidermal nerve fibers (ENF), Meissner corpuscles (MC), intrapapillary myelinated endings (IME), and sudomotor nerves. We correlated morphologic data with findings from quantitative sensory testing, sudomotor output, sympathetic skin response, and cardiovascular reflexes. A control population of healthy age- and sex-matched controls was included with a matching ratio of 1:2.
Results:
We found a length-dependent loss of ENFs that worsened with aging. We also observed a loss of MCs, IMEs, and sudomotor nerves. The loss of ENF at distal leg correlated with the increase in heat-pain thresholds (p < 0.05) and with tactile thresholds (p < 0.05). Sudomotor nerve fiber loss correlated with ENF density (p < 0.05) and sweating output (p < 0.001).
Conclusions:
We demonstrated through morphologic, physical, and psychophysical testing that small somatic and autonomic fibers are abnormal and cause symptoms in patients with CMT1A. Awareness of such symptoms by the clinician could lead to better treatment.
Charcot-Marie-Tooth (CMT) diseases are a heterogeneous group of inherited motor and sensory neuropathies with a general prevalence of 1:2,500.1,2 CMT type 1A (CMT1A) is the most common subtype and is caused by a duplication of the gene encoding peripheral myelin protein 22 kD (PMP22) within a 1.4-Mb duplicated region on chromosome 17p11.2. Clinically, CMT1A is characterized by progressive distal muscular atrophy and weakness, distal sensory loss, and decreased or absent deep tendon reflexes.3 Sensory symptoms in CMT1A are generally attributed to length-dependent large-fiber dysfunction (loss of touch, vibration, and position sense in the lower limbs). However, it has become increasingly evident that small-diameter sensory nerves must also be affected, as was previously hypothesized4 and reported anecdotally.3 Specifically, the presence of pain has been shown to be present and to negatively affect the quality of life in patients with CMT1A as a whole5,6 and even in children.7
Small fiber sensory abnormalities have been evaluated by a number of methods, including quantitative sensory testing (QST), laser-evoked potentials (LEP), and corneal confocal microscopy. Analysis of small sensory nerve fibers morphologically, through the use of skin biopsies, has been especially informative over the past decade.8 This has been among the most reliable and objective methods in the diagnosis of small fiber neuropathies, allowing the assessment of unmyelinated somatic and autonomic nerve fibers through the quantification of epidermal nerve fibers (ENF) and of sudomotor and pilomotor nerves.
We evaluated small nerve fibers in patients with CMT1A and compared our morphologic findings with findings from QST, dynamic sweat test (DST), sympathetic skin response (SSR), and cardiovascular reflexes (CVR).
METHODS
Patients.
Twenty unrelated patients (male/female 5/15; mean age 42.9 ± 8.4 years) from 20 kinships with a clinical and genetic diagnosis of CMT1A were included in the study. Patients were also screened to identify and exclude participants with possible alternative causes of involvement of small fibers, such as abnormalities of glucose metabolism, endocrine function, vitamin E, B12 and folic acid deficiency, hepatic or renal failure, HIV, or connective tissue disorders. All patients were older than 18 years at the time of enrollment. Clinical impairment was evaluated using the validated CMT neuropathy score (CMTNS).9 Patients were enrolled from the CMT center of the University of Naples, “Federico II,” where they were referred for diagnosis and follow-up.
Controls.
A control population of healthy age- and sex-matched controls was included for the morphologic studies. The patient:control matching ratio was 1:2.
Functional sensory and autonomic findings were compared to age- and sex-matched controls (patient:control ratio = 1:2, mean age 44.2 ± 11.5), extracted from the “Salvatore Maugeri Foundation, Institute of Telese Terme” database that included 300 healthy volunteers.
Standard protocol approvals, registrations, and patient consents.
The Institutional Ethics Committee approved the study and all participants gave written informed consent.
Study methods.
All patients completed the Small Fiber Neuropathy Symptoms Inventory Questionnaire (SFN-SIQ)10 and underwent skin biopsy. A subgroup of 10 patients (P1 to P10) underwent an additional extensive protocol to assess autonomic and sensory function. To avoid interferences from stress or fatigue with the results of psychophysical QST and the cardiovascular autonomic nervous system testing, the evaluations were performed on 3 separate days according to the following schedule: CVRs on day 1, DST and SSR on day 2, and QST (tactile and thermal thresholds, and mechanical pain perception) on day 3.
The 13-item SFN-SIQ questionnaire was used to provide data on clinical symptoms of small nerve fiber impairment. Patients were questioned on the occurrence of autonomic and sensory symptoms in their daily life. For each item, patients were assigned a score based on occurrence of the symptom: 0 = never; 1 = sometimes; 2 = often; 3 = always. The scoring ranges from 0 (no symptoms) to 39 (all symptoms are always present).
QST testing was performed on the dorsum of right hand and foot for 6 sensory modalities (tactile threshold, thresholds to warm and cold innocuous and noxious stimuli, mechanical pain perception) as previously described.11
Postganglionic sudomotor function was evaluated using the DST.12 Briefly, after stimulation with 1% pilocarpine by iontophoresis (5 minutes at 2 mA) on the lateral aspect of distal leg (union of middle third with the lower third), sweating output, visualized with colorimetric method, was recorded using a video camera. Activated sweat gland density per cm2 and sweat volume per gland and per cm2 were calculated analyzing sweat drop imprints on seriate frames from digital recording.
SSR was recorded at hands and feet after random single 1-ms stimuli at the wrist, using surface electrodes; a bandpass of 0.2–100 Hz was used. The skin temperature was maintained between 32 and 34°C.
To assess CVRs, the following tests were performed: heart rate variability at rest and during deep breathing, Valsalva ratio, 30/15 ratio, and blood pressure changes to standing and to handgrip.13
Skin biopsy.
Skin samples were obtained with a 3-mm punch from the thigh and distal leg (union of middle third with the lower third) and by 2-mm punch from fingertip. Specimens were fixed in cold Zamboni solution, cryoprotected in 20% sucrose phosphate-buffered saline, and cut in 50-µm-thick sections by means of a sliding microtome. Free-floating sections were incubated overnight with a panel of primary antibodies to mark vessels and both myelinated and unmyelinated somatic and autonomic nerve fibers. Secondary antibodies conjugated with cyanine 2 and 3 were used to visualize the structures of interest. ULEX Europaeus agglutinin 1 coupled with cyanine 5 was used to visualize blood vessels and epidermis. Primary antibody abbreviations, source, and dilution are listed in table e-1.
Quantification of cutaneous nerves.
Somatic nerves.
ENF density (number per linear mm) was calculated on 4 sections double-stained with protein gene product (PGP) 9.5 and collagen IV (Col IV) according to standard criteria.8 A qualitative evaluation of peptidergic nerve fiber populations was performed on 2 sections for each biopsy marked with substance P (SubP) and calcitonin gene-related peptide (CGRP).
Quantification of Meissner corpuscles (MC) and intrapapillary myelinated endings (IME) was performed in glabrous skin samples. Density values (numbers of structures per square millimeter) were obtained following previously described procedures.14
Autonomic nerves.
A qualitative evaluation of sudomotor, pilomotor, and vasomotor nerves was performed in all skin samples using the pan-neuronal marker PGP and selective cholinergic and noradrenergic markers vasoactive intestinal peptide (VIP) and dopamine β-hydroxylase.15,16 A quantitative assessment of sudomotor nerves in skin samples from the leg from 10 subjects (P1 to P10) was also performed. For this purpose, using a nonlaser confocal microscope (AxioImagerM2 with Apotome2, Zeiss, Jena, Germany), 3D digital images of sweat glands were acquired using a 20× objective (Zeiss EC Plan NeoFluar 20×/0.5). To avoid nonrepresentative sweat gland fragments, only sweat glands at least 25 mm thick were included in quantifications. The total length of sudomotor nerves immunoreactive for PGP and VIP was calculated using the nerve tracing module Autoneuron, a part of Neurolucida software (Microbrightfield Bioscience, Williston, VT). This enabled us to trace nerve fibers through 2-µm optical sections of confocal z-stacks. Sweat gland volumes were calculated by manually tracing the outside contour on alternate optical sections of composite confocal images showing PGP 9.5 or VIP with Col IV and Ulex. A value of nerve density per gland volume (nm/µm3) was obtained. This method of quantification was previously validated17 by comparison with an unbiased estimation of sudomotor nerve total length by stereologic measures.
Statistical analysis.
We used Student t test for unpaired data and Mann-Whitney test (when analyzing nonparametric data) to compare morphologic and functional findings from patients and controls, and linear correlation (Pearson) to correlate patient data with age and disease severity and to correlate morphologic parameters with functional findings. A p value of <0.05 was considered significant.
RESULTS
Phenotypic characterization.
Disease severity, as determined by the CMTNS, ranged between mild and moderate impairment (scores between 4 and 20, mean 10.7 ± 3.8). Individual scores along with additional clinical information about the patients are provided in table e-2.
Skin biopsy findings.
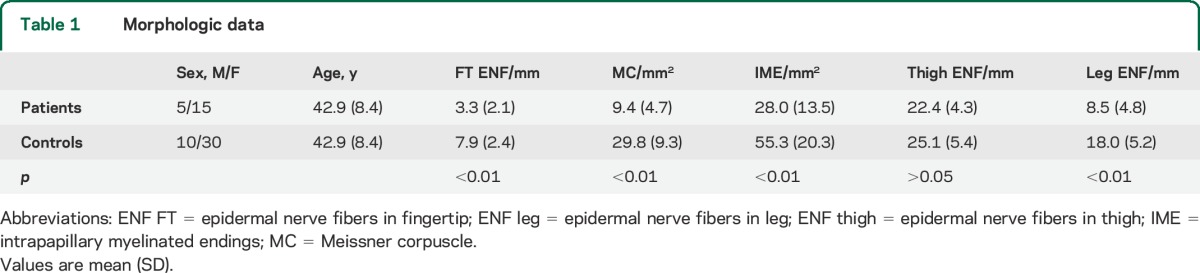
Our findings demonstrated a loss of somatic and autonomic nerve fibers in the distal leg and fingertip while cutaneous innervation from the thigh was relatively spared (figure 1). Quantitative assessment of ENF, MC, and IME are summarized in table 1. ENF density in fingertip (figure 1, A and B) and leg (figure 1, E and F) was lower (p < 0.001) in patients compared to controls, supporting a length-dependent small fiber neuropathy. A loss of MCs and IMEs (figure 1, A and B) was also found (p < 0.001). In our patients the loss of ENF at distal leg correlated with the increase in heat-pain thresholds (p < 0.05) and with tactile thresholds (p < 0.05). No ENF density differences were found between patients with and without burning pain. Peptidergic (SubP and CGRP immunoreactive) fibers appeared poorly represented in skin samples of patients compared to controls.
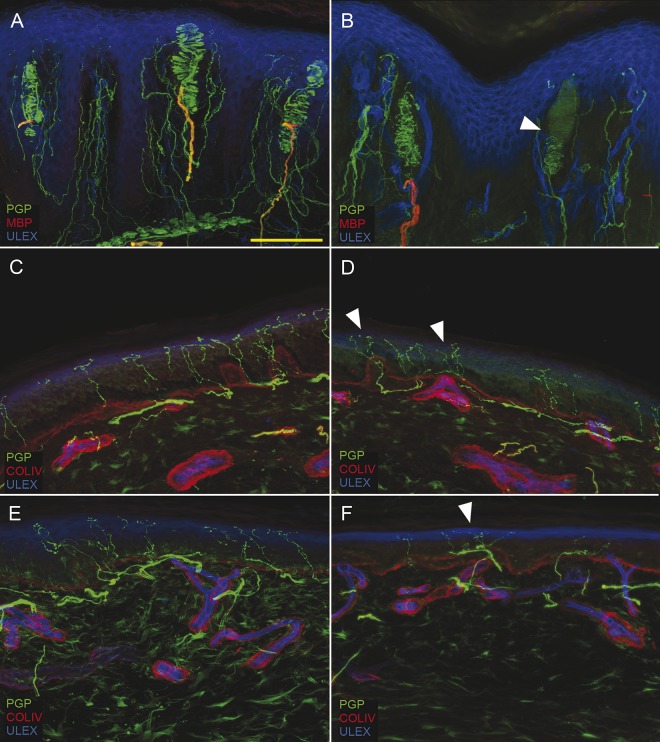
Figure 1. Somatic denervation in glabrous and hairy skin.
Digital images from fingertip (A, B), thigh (C, D), and leg (E, F) show epidermal and dermal denervation in patients with Charcot-Marie-Tooth type 1A (CMT1A). A moderate length-dependent loss of epidermal nerve fibers is present in patients with CMT1A compared to controls (B, D, F compared to A, C, E). Morphologic abnormalities (arrowhead) and loss of Meissner corpuscles and intrapapillary myelinated endings are also evident in B. In addition to degenerative signs, frequent aspects of nerve remodeling (clusters) are observed (arrowheads in D and F). Scale bar: 100 µm. COLIV = collagen IV; MBP = myelin basic protein; PGP = protein gene product.
Table 1.
Morphologic data
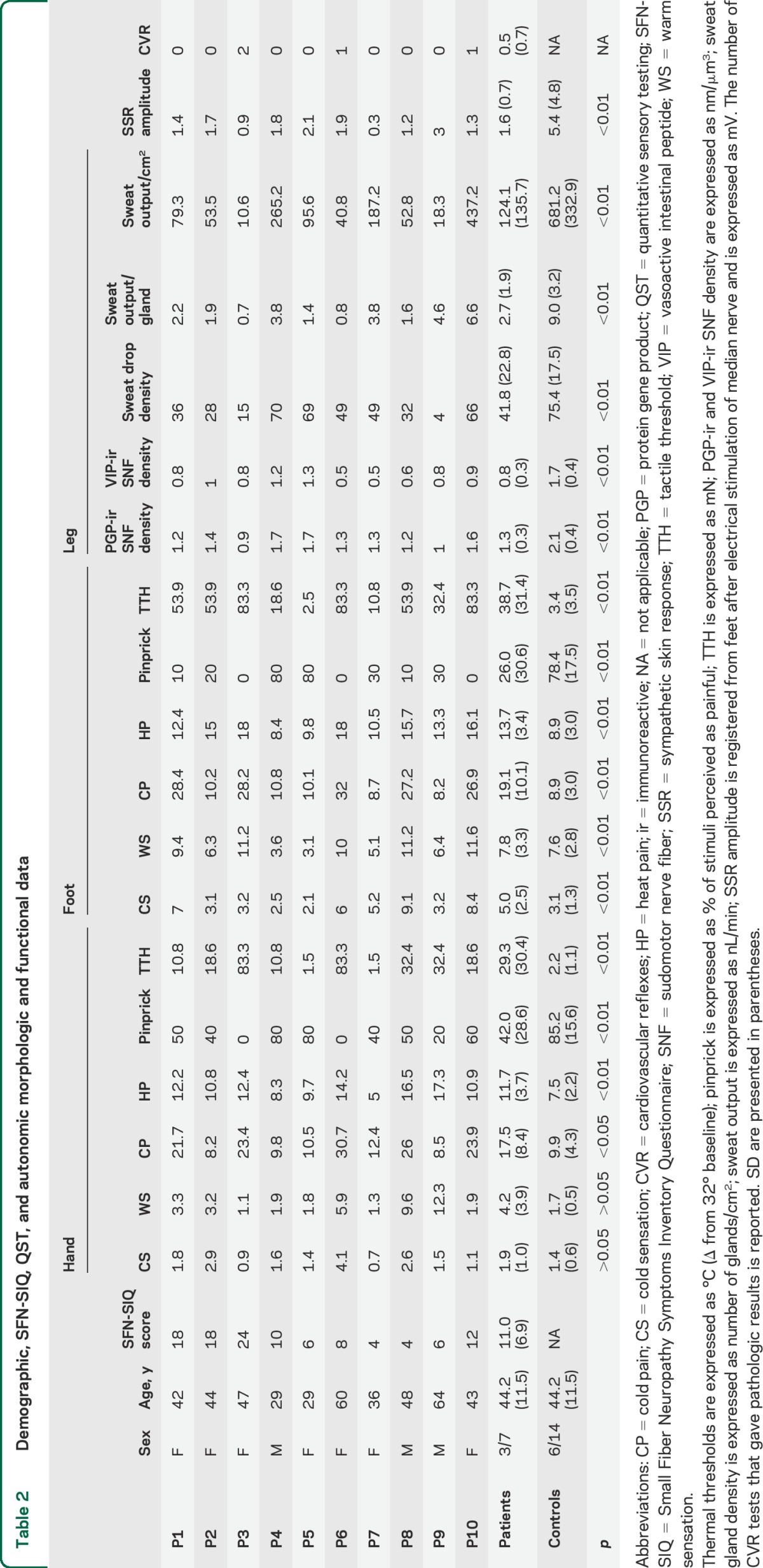
All dermal adnexa showed aspects of derangement of nerve supply with involvement of sudomotor (figure 2, A and B), vasomotor (figure 2, C and D), and pilomotor (figure 2, E and F) fibers. The most frequently observed morphologic abnormalities were nerve thickening, a chaotic distribution of nerve fibers around dermal annexes, and a loss of the regular coiling around tubules or vessels. Quantitative evaluation of sudomotor innervation was performed on a total of 195 sweat glands (65 from patients and 130 from controls, 87 with PGP and 108 with VIP). The density of sudomotor nerves was lower (p < 0.001) compared to normal healthy controls using both the pan-neuronal marker PGP and the selective cholinergic marker VIP (table 2) and correlated (p < 0.001) with the density of activated sweat gland density (figure e-1A). Eighty percent of patients had a sudomotor innervation below 5% cutoff evaluating PGP-immunoreactive fibers and 90% evaluating VIP-immunoreactive fibers. The loss of MC correlated with the increase in tactile thresholds (see below) and with the loss of ENFs in the same site. The loss of ENF from distal leg correlated with the loss of sudomotor nerve fibers from the same site (p < 0.05), suggesting a parallel involvement of unmyelinated autonomic and somatic nerve fiber populations. Activated sweat gland density after pilocarpine stimulation correlated with sudomotor innervation as well as with ENF (p < 0.01) and MC loss (p < 0.05).
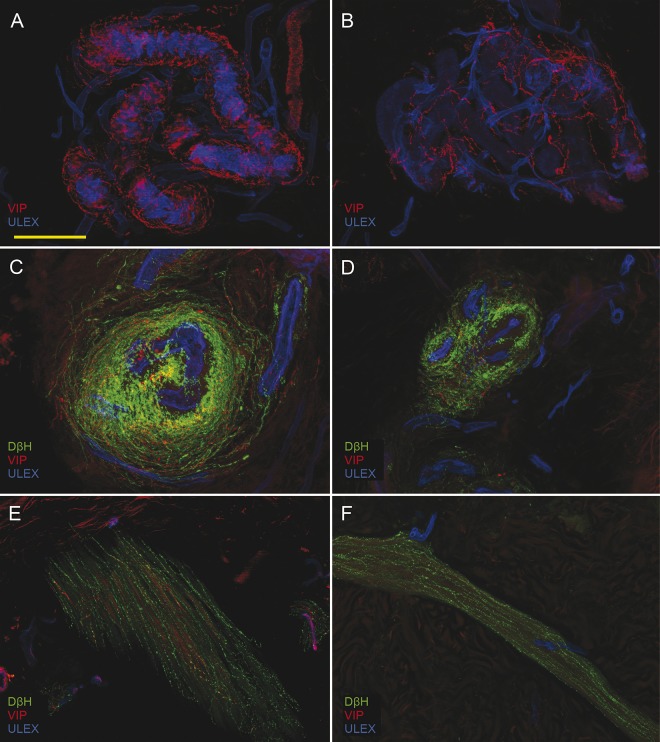
Figure 2. Cutaneous autonomic denervation.
Confocal images show, in patients compared to controls, loss of cholinergic (vasoactive intestinal peptide [VIP]) sudomotor nerves (B compared to A) and loss of cholinergic and noradrenergic fibers in arteriovenous anastomoses (D compared to C) and arrector pili muscle (F compared to E). Scale bar: 100 µm.
Table 2.
Demographic, SFN-SIQ, QST, and autonomic morphologic and functional data
Other measures of small fiber dysfunction.
SFN-SIQ.
All patients reported at least 2 symptoms related to dysfunction of small nerve fibers (table 2). Sweating disorders (hypohidrosis/hyperhidrosis) (60%), palpitations (50%), and gastrointestinal complaints (50%) were most frequently reported. Burning feet occurred in 6 out of 20 patients.
QST findings.
Mean values of sensory thresholds for all modalities were higher (p < 0.01) than those in controls (table 2). All patients but one had increased thresholds in sensory modalities involving both C and A-δ fibers, as well as an increase in A-β-fiber–related tactile thresholds. Abnormalities in the feet were greater than those in the hands. None of the patients showed thermal hyperalgesia.
DST.
Patients, compared to controls, had a marked reduction of both density of activated sweat glands and mean sweat output for each gland (p < 0.001) (table 2). Hypohidrosis (sweat output/cm2 less than 5° percentile cutoff) was present in 7 of the 10 patients analyzed.
SSR was obtained in all patients in both hands and feet. The amplitude of SSR recorded at feet was lower in patients than in age- and sex-matched controls (p < 0.05) (table 2).
CVRs.
Only 1 patient showed a cardiac dysautonomia, with abnormalities in 2 tests (30/15 ratio and blood pressure changes to standing), while 2 patients showed borderline results with abnormalities in only 1 test (30/15 ratio) (table 2).
Correlations with age.
The loss of ENF in all the 3 sites, as well as the sensory thresholds, the loss of sudomotor nerves, and the deficit in sweating output increased with age. The age effect appeared particularly evident on the loss of leg ENF; the age–disease interaction was significant (p = 0.04) for this parameter in a model of multiple linear regression (figure e-1B).
DISCUSSION
Our results demonstrated length-dependent small fiber abnormalities in all patients tested with CMT1A. Specifically, morphologic studies revealed a length-dependent decrease in ENF density that correlated with other physical and psychophysical measures of small fiber somatic and autonomic functions such as QST, reduced numbers of activated sweat glands, and decreased numbers of sudomotor nerve fibers. Thus, small nerve fiber abnormalities are clearly present in patients with CMT1A.
These small nerve fiber abnormalities have clinical consequences for patients. C unmyelinated and A-δ small myelinated nerve fibers carry pain and temperature modalities that terminate in the skin as ENFs.18 The reduction in ENF density would therefore explain the length-dependent reduction of pain and temperature sensation observed in most patients with CMT1A.19,20 Abnormalities in these nerves may also explain the pain that occurs in some patients with CMT1A. Pain is a common problem in CMT1A that may begin in childhood7 and impairs quality of life in both children21 and adults.5,6 While much of the pain may be related to structural or joint abnormalities that are present from childhood,7 neuropathic pain has been estimated to occur in approximately 20% of patients with CMT1A.22 A study using LEPs to explore the nociceptive pathway in 16 patients with CMT1A reported a length-dependent impairment of A-δ fibers.23 Our study also supports the involvement of A-δ fibers in CMT1A since these are myelinated, and PMP22 has a fundamental role in myelination.24 However, some of our findings (warm threshold abnormalities and ENF loss) also suggest an involvement of C fibers in CMT1A, although we cannot have morphologic evidence, since it is not possible to distinguish between C and A-δ fibers, whose cutaneous terminal branches are both unmyelinated.25,26 The involvement of small fibers in conditions affecting mainly large fibers has been previously reported,27–29 implying that congenital or acquired mechanisms underlying the degeneration of large fibers may also affect small fibers. In CMT1A, abnormalities of the interaction between axon and Schwann cells may play a causative role in the distal degeneration of unmyelinated fibers, since PMP22 is also present in the plasma membrane of nonmyelinating Schwann cells.30 In addition, although the exact role of PMP22 is still under investigation, it has been observed that PMP22 overexpression may affect Schwann cell proliferation, differentiation, and death.31 The correlation of ENF loss with both MC density and tactile thresholds suggests that small fiber and large fiber degeneration in CMT1A follow a parallel course.
ENF loss substantially increased with age, suggesting an additive effect on the modest decline in density observed in normal cohorts of controls.32 This decline could not be appreciated in our age- and sex-matched control population representing a small cohort with a nonhomogeneous distribution of subjects among the different age decades.
The autonomic nervous system (ANS) is divided into sympathetic and parasympathetic components. Along the sympathetic pathway, only preganglionic sympathetic fibers are myelinated.33 Preganglionic sympathetic fibers originate from the spinal cord in the Clarke column and project to peripheral sympathetic ganglia. Preganglionic parasympathetic fibers originate from neurons located in the brainstem or sacral spinal cord and have a long course before the synapsis in the parasympathetic ganglia sited close to their target organs. In general, it is widely thought that signs and symptoms of ANS involvement such as urinary dysfunction, gastrointestinal disturbances, or orthostatic complaints are unusual in CMT1A patients.19 For example, prior reports have found that cardiac dysautonomia is relatively uncommon in CMT1A.34 Our findings were in agreement as features of cardiac dysautonomia were found in only 1 out of 10 patients we analyzed. Despite the low occurrence of CVR abnormalities, we observed a moderate loss of vasomotor nerves in all our patients. This discrepancy was in some way expected. In fact, we have learned from other longstanding autonomic conditions that cutaneous vasomotor denervation may not be associated with CVR abnormalities.35 Alternatively, we found abnormalities of sweating, sympathetically mediated, to be frequent. In our patient population, through the SFN-oriented questionnaire, hypohidrosis emerged as the most frequently reported autonomic symptom (60%). We evaluated the entire polysynaptic sudomotor pathway using SSR and also used DST to explore the function of postganglionic sudomotor nerves. SSR was present in all our patients but the amplitude of the potentials in the distal site was lower compared to healthy age- and sex-matched controls. For diagnostic purposes only, the absence of the response is considered abnormal; however, SSR amplitude has been recently shown to correlate with sweat output.36 DST revealed hypohidrosis in 70% of our patients. DST is an objective and reliable test that is independent of patient cooperation.12 Both SSR and DST indicated a sudomotor functional impairment that goes parallel to the loss of sudomotor nerves. Taken together, our data demonstrate that some autonomic abnormalities are present in patients with CMT1A. Why some modalities are more affected than others and whether abnormalities result from dysfunction of presynaptic sympathetic fibers is beyond the scope of the present manuscript but will be important in understanding the pathogenesis of clinical abnormalities in patients with CMT1A.
We demonstrated through morphologic, physical, and psychophysical testing that small somatic nerve fibers and some sympathetic autonomic fibers are abnormal and cause symptoms in patients with CMT1A. Our findings build on a small but growing body of research that aims to demonstrate that CMT1A not only affects large myelinated fibers but also involves small fibers. Since SFN-related disturbances can negatively affect the clinical picture and the quality of life of patients with CMT1A, awareness of such symptoms by the clinician could lead to better treatment and an improvement of disease course.
Supplementary Material
GLOSSARY
- ANS
autonomic nervous system
- CGRP
calcitonin gene-related peptide
- CMT
Charcot-Marie-Tooth
- CMT1A
Charcot-Marie-Tooth type 1A
- CMTNS
Charcot-Marie-Tooth neuropathy score
- Col IV
collagen IV
- CVR
cardiovascular reflexes
- DST
dynamic sweat test
- ENF
epidermal nerve fibers
- IME
intrapapillary myelinated endings
- LEP
laser-evoked potentials
- MC
Meissner corpuscles
- PGP
protein gene product
- PMP22
peripheral myelin protein 22 kD
- QST
quantitative sensory testing
- SFN-SIQ
Small Fiber Neuropathy Symptoms Inventory Questionnaire
- SSR
sympathetic skin response
- SubP
substance P
- VIP
vasoactive intestinal peptide
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M. Nolano: study concept and design, drafting and revising the manuscript, statistical analysis, patient recruitment. F. Manganelli: data acquisition, data analysis, revising the manuscript. V. Provitera: study concept and design, drafting and revising the manuscript, statistical analysis, patient recruitment. C. Pisciotta: data acquisition, data analysis. A. Stancanelli: data acquisition, data analysis. G. Caporaso: data analysis, revising the manuscript. R. Iodice: patient recruitment, data acquisition. M.E. Shy: interpretation of the data, revising the manuscript. L. Santoro: study concept and design, interpretation of the data, revising the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet 1974;6:98–118. [DOI] [PubMed] [Google Scholar]
- 2.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry 1997;62:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shy M, Lupski JR, Chance PF, Klein CJ, Dyck P. The hereditary motor and sensory neuropathies: an overview of the clinical, genetic, electrophysiologic and pathologic features. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy, 4th ed Philadelphia: WB Saunders; 2005:1623–1658. [Google Scholar]
- 4.Brooks AP. Abnormal vascular reflexes in Charcot-Marie-Tooth disease. J Neurol Neurosurg Psychiatry 1980;43:348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colomban C, Micallef J, Lefebvre MN, et al. Clinical spectrum and gender differences in a large cohort of Charcot-Marie-Tooth type 1A patients. J Neurol Sci 2014;336:155–160. [DOI] [PubMed] [Google Scholar]
- 6.Padua L, Shy ME, Aprile I, et al. Correlation between clinical/neurophysiological findings and quality of life in Charcot-Marie-Tooth type 1A. J Peripher Nerv Syst 2008;13:64–70. [DOI] [PubMed] [Google Scholar]
- 7.Ramchandren S, Jaiswal M, Feldman E, Shy M. Effect of pain in pediatric inherited neuropathies. Neurology 2014;82:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912. [DOI] [PubMed] [Google Scholar]
- 9.Shy ME, Blake J, Krajewski K, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005;64:1209–1214. [DOI] [PubMed] [Google Scholar]
- 10.Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve 2014;49:329–336. [DOI] [PubMed] [Google Scholar]
- 11.Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131:1903–1911. [DOI] [PubMed] [Google Scholar]
- 12.Provitera V, Nolano M, Caporaso G, Stancanelli A, Santoro L, Kennedy WR. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 2010;74:50–56. [DOI] [PubMed] [Google Scholar]
- 13.Vita G, Princi P, Calabro R, Toscano A, Manna L, Messina C. Cardiovascular reflex tests: assessment of age-adjusted normal range. J Neurol Sci 1986;75:263–274. [DOI] [PubMed] [Google Scholar]
- 14.Nolano M, Provitera V, Crisci C, et al. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol 2003;54:197–205. [DOI] [PubMed] [Google Scholar]
- 15.Donadio V, Nolano M, Provitera V, et al. Skin sympathetic adrenergic innervation: an immunofluorescence confocal study. Ann Neurol 2006;59:376–381. [DOI] [PubMed] [Google Scholar]
- 16.Nolano M, Provitera V, Caporaso G, Stancanelli A, Vitale DF, Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology 2010;75:1089–1097. [DOI] [PubMed] [Google Scholar]
- 17.Provitera V, Nolano M, Caporaso G, et al. Postganglionic sudomotor denervation in patients with multiple system atrophy. Neurology 2014;82:2223–2229. [DOI] [PubMed] [Google Scholar]
- 18.Shy ME. Peripheral neuropathies. In: Shafer G, ed. Goldman's Cecil Medicine. Philadelphia: 2012:2396–2409. [Google Scholar]
- 19.Thomas PK, Marques W, Davis MB, et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain 1997;120:465–478. [DOI] [PubMed] [Google Scholar]
- 20.Krajewski KM, Lewis RA, Fuerst DR, et al. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain 2000;123:1516–1527. [DOI] [PubMed] [Google Scholar]
- 21.Burns J, Ramchandren S, Ryan MM, Shy M, Ouvrier RA. Determinants of reduced health-related quality of life in pediatric inherited neuropathies. Neurology 2010;75:726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laura M, Hutton EJ, Blake J, et al. Pain and small fiber function in Charcot-Marie-Tooth disease type 1A. Muscle Nerve 2014;50:366–371. [DOI] [PubMed] [Google Scholar]
- 23.Pazzaglia C, Vollono C, Ferraro D, et al. Mechanisms of neuropathic pain in patients with Charcot-Marie-Tooth 1 A: a laser-evoked potential study. Pain 2010;149:379–385. [DOI] [PubMed] [Google Scholar]
- 24.Trapp BD, Pfeiffer SE, Anitei A, Kidd GJ. Cell biology and myelin assembly. In: Lazzarini RA, ed. Myelin Biology and Disorders. San Diego: Elsevier Academic Press; 2003:29–56. [Google Scholar]
- 25.Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature 1989;342:430–433. [DOI] [PubMed] [Google Scholar]
- 26.Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L. Myelinated nerve endings in human skin. Muscle Nerve 2007;35:767–775. [DOI] [PubMed] [Google Scholar]
- 27.Nolano M, Provitera V, Crisci C, et al. Small fibers involvement in Friedreich's ataxia. Ann Neurol 2001;50:17–25. [DOI] [PubMed] [Google Scholar]
- 28.Pan CL, Tseng TJ, Lin YH, Chiang MC, Lin WM, Hsieh ST. Cutaneous innervation in Guillain-Barré syndrome: pathology and clinical correlations. Brain 2003;126:386–397. [DOI] [PubMed] [Google Scholar]
- 29.Ruts L, van Doorn PA, Lombardi R, et al. Unmyelinated and myelinated skin nerve damage in Guillain-Barré syndrome: correlation with pain and recovery. Pain 2012;153:399–409. [DOI] [PubMed] [Google Scholar]
- 30.Haney C, Snipes GJ, Shooter EM, et al. Ultrastructural distribution of PMP22 in Charcot-Marie-Tooth disease type 1A. J Neuropathol Exp Neurol 1996;55:290–299. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol 2013;47:673–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–207. [DOI] [PubMed] [Google Scholar]
- 33.Gardner ED, Bunge RP. Gross anatomy of the peripheral nervous system. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy, 4th ed Philadelphia: Elsevier; 2005:11–34. [Google Scholar]
- 34.Ingall TJ, McLeod JG. Autonomic function in hereditary motor and sensory neuropathy (Charcot-Marie-Tooth disease). Muscle Nerve 1991;14:1080–1083. [DOI] [PubMed] [Google Scholar]
- 35.Nolano M, Provitera V, Perretti A, et al. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain 2006;129:2119–2131. [DOI] [PubMed] [Google Scholar]
- 36.Ellaway PH, Kuppuswamy A, Nicotra A, Mathias CJ. Sweat production and the sympathetic skin response: improving the clinical assessment of autonomic function. Auton Neurosci 2010;155:109–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.