Abstract
Objectives:
To study whether 60-Hz stimulation, compared with routine 130 Hz, improves swallowing function and freezing of gait (FOG) in patients with Parkinson disease (PD) who undergo bilateral subthalamic nucleus (STN) deep brain stimulation (DBS).
Methods:
We studied 7 patients with PD who experienced FOG that persisted despite routine 130-Hz stimulation and dopaminergic medication. Each patient received 3 modified barium swallow (MBS) studies in a single day under 3 DBS conditions in the medication-on state: 130 Hz, 60 Hz, or DBS off, in a randomized double-blind manner. The laryngeal penetration and aspiration events were cautiously assessed, and a swallowing questionnaire was completed. The Unified Parkinson's Disease Rating Scale, Part III motor score, axial subscore, tremor subscore, and FOG by a questionnaire and stand-walk-sit test were also assessed. The best DBS condition (60 Hz here) producing the least FOG was maintained for 3 to 8 weeks, and patients were assessed again. Changes in measurements between the 60 Hz and 130 Hz were analyzed using paired t test, with swallowing function as primary and the remainder as secondary outcomes. Changes between other DBS conditions were further explored with Bonferroni correction.
Results:
Compared with the routine 130 Hz, 60-Hz stimulation significantly reduced aspiration frequency by 57% on MBS study and perceived swallowing difficulty by 80% on questionnaire. It also significantly reduced FOG, and axial and parkinsonian symptoms. The benefits at 60-Hz stimulation persisted over the average 6-week assessment.
Conclusions:
Compared with the routine 130 Hz, the 60-Hz stimulation significantly improved swallowing function, FOG, and axial and parkinsonian symptoms in patients with PD treated with bilateral STN-DBS, which persisted over the 6-week study period.
Classification of evidence:
This study provides Class IV evidence that for patients with PD who experience FOG, STN-DBS at 60 Hz decreases aspiration events observed during MBS compared with DBS at 130 Hz.
Subthalamic nucleus (STN) deep brain stimulation (DBS) improves the levodopa responsive cardinal symptoms, and reduces motor fluctuation and dyskinesia in patients with Parkinson disease (PD).1–5 However, DBS is less effective at improving the axial symptoms of postural instability, gait disorders, and speech and swallowing dysfunction. STN-DBS might transiently improve the axial symptoms, but could make them worse over the course of 2 to 5 years.6–15 The DBS stimulation settings typically used in these studies were high frequency of 130 to 185 Hz.
Recently, a stimulation frequency of 60 Hz has been found to improve the axial symptoms of freezing of gait (FOG)16–19 and dysarthria15,19 compared with the routinely used 130-Hz stimulation.
However, whether or not the stimulation frequency could also affect another axial symptom, swallowing function, remains unknown. The answer to this question is critical, because dysphagia is frequently present in patients with mid- and late-stage PD20,21 and is associated with high risk of morbidity and mortality. Dysphagia usually does not respond to pharmacologic management.22,23
Therefore, we hypothesized that low-frequency stimulation of 60 Hz could similarly produce better swallowing function compared with the routinely used 130 Hz, as seen on other axial symptoms.15–19 This study could have critical impact on the management of dysphagia, FOG, and other axial symptoms, hence potentially decreasing the morbidity and mortality of these patients.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the institutional review board, and written informed consent was obtained from the patients. The study was listed under ClinicalTrials.gov (identification number of NCT01935011, dated August 27, 2013, titled “Effects of the stimulation frequency of STN DBS on swallowing function in patients with Parkinson's disease”). The study was conducted at the Parkinson's Disease and Movement Disorder Center Department of Neurology, Department of Radiology, Speech and Swallowing Section of Department of Surgery, and Center for Research Informatics at the University of Chicago from August 2013 to June 2014.
Participants.
We tested the hypothesis that swallowing function is better at the low-frequency stimulation of 60 Hz compared with the routinely used 130 Hz in patients with PD undergoing bilateral STN-DBS in the medication-on state. We initially aimed to enroll 8 patients based on the power calculation inferred from the effect of DBS frequency on speech study,15 because speech and swallowing function are often affected together,6,24 which allows 80% power to detect 25% improvement on the swallowing function at 60-Hz stimulation compared with 130 Hz at the 2-tailed α level of 0.05. Because we lost one of the key staff members performing the swallowing study after we finished 7 patients, we eliminated enrollment of the last patient to ensure quality of the study, because a substitute might cause inconsistency in swallowing assessment. All study patients had advanced PD with bilateral STN-DBS placement and medication-refractory FOG at routinely used 130-Hz stimulation. The demographics of the 7 patients were 6 men and 1 woman aged 64.0 ± 8.0 years and disease duration of 12.9 ± 4.9 years, with bilateral STN-DBS activated for 4.4 ± 4.9 years on average. Electrode position and active contact position were verified in all patients as being in the dorsal STN, by postoperative CT scans with a slice thickness of 1 mm fused with the T2-weighted stereotactic planning MRI (1 mm) depicting the STN overlaid with a digital Schaltenbrand Atlas.
Visits and measurements.
All patients completed the 2-visit study. At visit 1, each patient received 3 modified barium swallow (MBS) studies in a single day under 3 different DBS frequency conditions (bilateral DBS 130 Hz, DBS 60 Hz, or DBS off) with their usual DBS voltage (right side 3.1 ± 0.4 V; left side 3.2 ± 0.4 V), pulse width (right 81.4 ± 14.6 μs; left 90.0 ± 24.5 μs), contact setting (13 active contacts on monopolar setting and one active contact on bipolar setting), and parkinsonian medication-on state (levodopa equivalent dose of 1,007 ± 402 mg daily) regardless of the stimulation frequency used. The study was performed in a randomized and double-blind manner. The order of the DBS conditions was determined by the neurologist who randomly picked up one of the 3 folded sheets with different conditions written on them and programmed the DBS accordingly, but was not allowed to participate in any rating or evaluation. All patients, the clinical rater, the pathologists for speech and swallowing, and the radiologist were unaware of the DBS stimulation frequency condition. A single certified rater rated all of the clinical scales and questionnaires, and a single swallowing team composed of the radiologist and the speech and swallowing pathologists conducted all MBS studies and the Penetration-Aspiration Scale ratings25 to ensure consistency and quality of the evaluation.
The MBS protocol consisted of a standardized videofluorographic recording of oropharyngeal swallow in lateral and anterior-posterior views. The subjects were given radiopaque liquid, pureed contrast material, and solids coated in barium paste. The examinations were recorded on the TIMS-DICOM digital medical recording system at high resolution of 30 frames per second. Frame-by-frame analysis was used to evaluate oral, pharyngeal, laryngeal, and cricopharyngeal function. For the purposes of this study, special attention was given to aspiration. Laryngeal penetration and aspiration events were assessed using the Penetration-Aspiration Scale. The frequency of aspiration events was calculated by adding the number of swallows within each DBS condition that generated a Penetration-Aspiration Scale rating of 6 or above (6–8) for aspiration. The swallowing questionnaire was completed by each patient after the MBS study under each DBS condition.26
The parkinsonian motor, axial, and tremor symptoms as reflected by the Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III) score, axial subscore (including gait, stance, posture, postural stability, and speech), and tremor subscore, respectively, and the FOG as reflected by a FOG questionnaire score,27 and stand-walk-sit (SWS) test on FOG spell times and the time needed to finish the test (seconds) were also assessed before the MBS study under each DBS condition. The patients underwent each DBS condition for at least 30 minutes before the study. The DBS condition producing the best gait function (least FOG, which was 60 Hz for all of our patients) was continued for an additional 6 to 8 weeks for repeat MBS study and clinical assessments as in visit 1 but on 60-Hz stimulation only for follow-up visit 2. There was no change in medications between visit 1 and visit 2.
One of the patients had to return earlier at 3 weeks to resume 130-Hz simulation because of worsening of resting hand tremor, which made the average follow-up period 6 weeks (±1.4, ranging from 3 to 7.5 weeks) for all of the participants. The remaining patients stayed with the 60-Hz stimulation until completion of the study and continued that condition thereafter because of the continuous benefit received.
Analysis.
As the main purpose of the study, changes in measurements between 60 Hz and 130 Hz were analyzed using paired t test, with swallowing function (objective frequency of aspiration on the MBS study and the subjective perception of swallowing difficulty on the swallowing questionnaire) as primary outcomes, and the remainder (FOG scores on the objective SWS study and subjective questionnaire, and UPDRS-III score, axial subscore, and tremor subscore) as the secondary outcomes. Changes between other clinically relevant DBS conditions, including 60 Hz vs DBS off, 130 Hz vs DBS off, and 60 Hz vs 60 Hz FU (follow-up for 3–8 weeks), were also explored and similarly analyzed with Bonferroni correction. A 2-tailed α level of 0.05 was taken as statistically significant for the comparisons.
Classification of evidence.
This study provides Class IV evidence that 60-Hz stimulation improves swallowing function, FOG, and overall axial and motor function compared with 130 Hz in PD with bilateral STN-DBS.
RESULTS
The results are listed in the table and figure in detail, and a brief data summary is below.
Table.
Motor, swallowing, and gait function under different DBS conditions

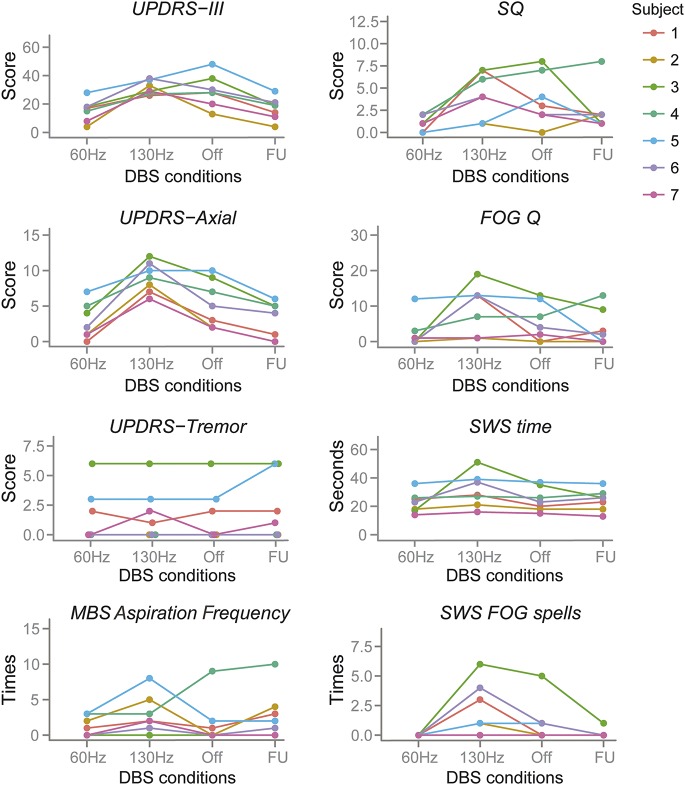
Figure. Individual response on each measurement to different DBS conditions.
DBS = deep brain stimulation; FOG = freezing of gait; FU = follow-up visit 3–8 weeks after visit 1; MBS = modified barium swallow; Q = questionnaire; SQ = swallowing questionnaire; SWS = stand-walk-sit; UPDRS = Unified Parkinson's Disease Rating Scale.
Consistent with our hypothesis on primary outcome, compared with the routinely used 130 Hz, the 60-Hz stimulation significantly reduced the aspiration frequency by 57% (p < 0.05) on objective MBS study, and significantly reduced subjective swallowing difficulty perception on questionnaire by 80% (p < 0.01) (table, 60 vs 130 Hz). The 60-Hz stimulation also significantly reduced the FOG assessed by the subjective questionnaire (p < 0.05) and objective freezing spells (p < 0.05) on SWS test, the axial symptoms (p < 0.001), and overall parkinsonian motor symptoms in UPDRS-III (p < 0.01) on our secondary outcomes, compared with 130 Hz (table, 60 vs 130 Hz). There was no significant change in tremor because tremor was largely controlled by the medications, except in one patient whose tremor was slightly worse on 60 Hz and he had to be switched to 130-Hz stimulation in 3 weeks to control resting hand tremor.
We then further explored the changes in measurements between other clinically relevant DBS conditions, including 60 Hz vs DBS off, 130 Hz vs DBS off, and 60 Hz vs 60 Hz FU (follow-up for 3–8 weeks, on average 6 weeks), with Bonferroni correction for multiple comparisons (table). Both the axial score (p < 0.05) and the UPDRS-III motor score (p < 0.001) were significantly better at 60-Hz stimulation compared with the DBS-off state. The axial score was worse at 130-Hz stimulation compared with the DBS-off state (p < 0.05). There was no statistically significant difference in any of the measurements between 60-Hz stimulation at visit 1 (60 Hz) and the follow-up visit 2 (60-Hz FU, all patients) of 6 weeks apart on average, indicating that the benefits obtained at 60 Hz remained persistent over the 6-week period studied (table).
The individual patient's response on each measurement to different frequency of stimulations was also plotted in the figure.
DISCUSSION
We assessed the effect of low-frequency stimulation of 60 Hz compared with the routinely used high-frequency stimulation of 130 Hz on swallowing function as primary outcome, and on FOG and overall axial and motor symptoms as secondary outcomes, in patients with PD undergoing bilateral STN-DBS in the medication-on state, using both objective and subjective measurements. We found that the low-stimulation frequency of DBS at 60 Hz improved swallowing function compared with 130 Hz, by both the objective MBS study (significantly decreasing aspiration frequency by 57%) and the subjective questionnaire (significantly decreasing swallowing difficulty by 80%), a conclusion of significant clinical impact. This study also confirmed previous reports that 60-Hz stimulation improved FOG and axial symptoms16–19 compared with 130 Hz, and further extended to the medication-on state, which means that 60-Hz DBS could potentially provide benefit to axial symptoms even beyond the medication effects, and to some nonaxial symptoms as well, because the overall parkinsonian motor symptoms also improved. The tremor was not worse in our patients because tremors largely responded well to the medications, except in one patient who had to return to 130-Hz stimulation in 3 weeks because of slight worsening of hand tremor on 60 Hz. The benefits obtained from 60-Hz stimulation on swallowing function, FOG, and overall axial and motor symptoms persisted over the 3- to 8-week study period (6 weeks on average). Six of the 7 patients continued on 60-Hz stimulation even after the study was completed because of the sustained benefit on swallowing, gait, and overall axial and other motor symptoms.
Our study was designed in a randomized double-blind manner to objectively assess the measurements and offset possible carryover effects between different DBS settings. We also completed the study under 3 different DBS conditions in a single day to avoid day-to-day variations and ensure a fair comparison. We performed the study in medication-on state (including medication-refractory FOG), which is different from a previous study on FOG assessed in the medication-off state,16 and could make the data more applicable to further improve clinical symptoms beyond what current medication and the routine DBS setting could provide. Our study has significant clinical impact on improving the axial symptoms in PD, which usually are difficult to manage and often are associated with increased risk of aspiration pneumonia, falls, and death in advanced PD. Hence, our study results could potentially decrease the morbidity and mortality in patients with advanced PD treated with bilateral STN-DBS, at least in the subset of the patients with medication-refractory FOG.
Nevertheless, our study has limitations. First, the sample size is small, although a statistical significance has been reached to support our primary hypothesis in comparing 60- with 130-Hz stimulation on swallowing function and other axial and general motor symptoms. The small sample size makes it difficult to compare the baseline characteristics to check for a potential carryover effect. However, our randomized double-blind design with at least 30 minutes apart on each condition minimizes the carryover effect, because the tremor and FOG are often immediately changed within a second of the DBS frequency switch, and bradykinesia and rigidity within seconds to minutes per observation, in contrast to the GPi (globus pallidus interna) DBS in patients with dystonia or a medication trial, which often has more prominent carryover effect. Second, all of the patients enrolled had tremors that were responding well to medications. For those with medication-refractory tremor as the reason for DBS, the benefit of the 60-Hz stimulation could be offset by the usual worsening of the tremors at 60 Hz compared with that at 130–180 Hz (our unpublished observation), as also suggested by one of our patients in this study. Third, only laryngeal penetration/aspiration was evaluated for the purpose of this study. Fourth, we do not know whether the dysphagia would still be reduced at 60 Hz for those without FOG because we only enrolled those with medication-refractory FOG, the most challenging symptom. Further studies with a larger sample size in patients with or without FOG, with more in-depth analysis of swallow physiology and a longer follow-up period, is needed to corroborate and extend our conclusions.
GLOSSARY
- DBS
deep brain stimulation
- FOG
freezing of gait
- FU
follow-up
- MBS
modified barium swallow
- PD
Parkinson disease
- STN
subthalamic nucleus
- SWS
stand-walk-sit
- UPDRS-III
Unified Parkinson's Disease Rating Scale, Part III
AUTHOR CONTRIBUTIONS
Design or conceptualization of the study: T.X. Analysis or interpretation of the data: T.X., J.V., E.M., A.G., J.Y., W.K., U.J.K. Drafting or revising the manuscript: T.X., J.V., E.M., A.G., J.Y., W.K., J.B., P.W., U.J.K.
STUDY FUNDING
This study was funded by the Michael J. Fox Foundation under the Rapid Response Innovation Award program to Dr. Tao Xie.
DISCLOSURE
T. Xie was funded by the Michael J. Fox Foundation under the Rapid Response Innovation Award program. He is also supported by NIH and GE Healthcare for research. J. Vigil, E. MacCracken, A. Gasparaitis, J. Young, W. Kang, and J. Bernard report no disclosures relevant to the manuscript. P. Warnke serves as associate editor of the Journal of Neurology, Neurosurgery & Psychiatry and is supported by NIH for research. U. Kang is supported by NIH, MJFF, and PDF for research, and is on the medical advisory board of CVS/Caremark. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 2.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- 4.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG Trial): a randomised, open-label trial. Lancet Neurol 2010;9:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368:610–622. [DOI] [PubMed] [Google Scholar]
- 6.Kraus M, Fogel W, Mayer P, et al. Chronic inhibition of the subthalamic nucleus in Parkinson's disease. J Neurol Sci 2004;219:119–124. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain 2005;128:2240–2249. [DOI] [PubMed] [Google Scholar]
- 8.Østergaard K, Aa Sunde N. Evolution of Parkinson's disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord 2006;21:624–631. [DOI] [PubMed] [Google Scholar]
- 9.Romito LM, Contarino MF, Vanacore N, et al. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson's disease: long-term observation. Mov Disord 2009;24:557–563. [DOI] [PubMed] [Google Scholar]
- 10.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 2010;25:578–586. [DOI] [PubMed] [Google Scholar]
- 11.St. George RJ, Nutt JG, Burchiel KJ, et al. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 2010;75:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagbami OY, Donato AA. Stridor and dysphagia associated with subthalamic nucleus stimulation in Parkinson's disease. J Neurosurg 2011;115:1005–1006. [DOI] [PubMed] [Google Scholar]
- 13.Robertson LT, St. George R, Carlson-Kuhta P, et al. Site of deep brain stimulation and jaw velocity in Parkinson's disease. J Neurosurg 2011;115:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripoliti E, Zrinzo L, Martinez-Torres I, et al. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology 2011;76:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau C, Pennel-Ployart O, Pinto S, et al. Modulation of dysarthropneumophonia by low-frequency STN DBS in advanced Parkinson's disease. Mov Disord 2011;26:659–663. [DOI] [PubMed] [Google Scholar]
- 16.Moreau C, Defebvre L, Destee A, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2008;71:80–84. [DOI] [PubMed] [Google Scholar]
- 17.Brozova H, Barnaure I, Alterman RL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2009;72:770. [DOI] [PubMed] [Google Scholar]
- 18.Moreau C, Defebvre L, Destee A, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease: reply from the authors. Neurology 2009;72:770–771.19237711 [Google Scholar]
- 19.Xie T, Kang UJ, Warnke P. Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:1015–1017. [DOI] [PubMed] [Google Scholar]
- 20.Hely MA, Morris JG, Reid WG, et al. Sydney Multicenter Study of Parkinson's disease: non-l-dopa-responsive problems dominate at 15 years. Mov Disord 2005;20:190–199. [DOI] [PubMed] [Google Scholar]
- 21.Hely MA, Reid WG, Adena MA, et al. The Sydney Multicenter Study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 22.Fuh JL, Lee RC, Wang SJ, et al. Swallowing difficulty in Parkinson's disease. Clin Neurol Neurosurg 1997;99:106–112. [DOI] [PubMed] [Google Scholar]
- 23.Hunter PC, Crameri J, Austin S, et al. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry 1997;63:579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg 2008;16:205–210. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbeck JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;11:93–98. [DOI] [PubMed] [Google Scholar]
- 26.Manor Y, Giladi N, Cohen A, et al. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson's disease. Mov Disord 2007;22:1917–1921. [DOI] [PubMed] [Google Scholar]
- 27.Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord 2009;24:655–661. [DOI] [PubMed] [Google Scholar]