Abstract
Objective:
We evaluated the association between obstructive sleep apnea (OSA) and neurocognitive function among community-dwelling Hispanic/Latino individuals in the United States.
Methods:
Cross-sectional analysis of the Hispanic Community Health Study/Study of Latinos middle-aged and older adults, aged 45 to 74 years, with neurocognitive test scores at baseline measurements from 2008 to 2011. Neurocognitive scores were measured using the Word Fluency (WF) Test, the Brief–Spanish English Verbal Learning Test (SEVLT), and the Digit Symbol Substitution (DSS) Test. OSA was defined by the apnea-hypopnea index (AHI). Multivariable linear regression models were fit to evaluate relations between OSA and neurocognitive scores.
Results:
The analysis consisted of 8,059 participants, mean age of 56 years, 55% women, and 41% with less than high school education. The mean AHI was 9.0 (range 0–142; normal AHI <5/h). There was an association between the AHI and all 4 neurocognitive test scores: Brief-SEVLT–sum (β = −0.022) and –recall (β = −0.010), WF (β = −0.023), and DSS (β = −0.050) at p < 0.01 that was fully attenuated by age. In the fully adjusted regression model, female sex was a moderating factor between the AHI and WF (β = −0.027, p < 0.10), SVELT-sum (β = −0.37), SVELT-recall (β = −0.010), and DSS (β = −0.061) at p < 0.01.
Conclusion:
OSA was associated with worse neurocognitive function in a representative sample of Hispanic/Latino women in the United States.
Proper sleep is critical for appropriate memory function.1,2 Sleep disruptions have been implicated in cognitive impairment and dementia,3 thus providing a modifiable target for prevention strategies with significant public health implications.
Obstructive sleep apnea (OSA) is a frequently underrecognized condition characterized by repetitive transient collapse of the upper airway during sleep causing hypoxemia, frequent arousals, and increased sympathetic nervous activity.4,5 Besides producing sleepiness, which may lead to impaired neurocognitive function, OSA is linked to cardiovascular disease and stroke,6 factors associated with greater cognitive decline and risk of dementia.7–9
This is of particular interest to Hispanic/Latino populations in which there is a high prevalence of obesity and cardiovascular disease risk factors10 associated with OSA.11
Hispanic/Latino populations are up to 1.5 times more likely to meet diagnostic criteria for dementia compared with non-Hispanic white populations,1 and the extent to which OSA contributes to neurocognitive dysfunction in Hispanic/Latino individuals has not been examined.
The aim of our study was to evaluate the relation between OSA and neurocognitive function among a representative sample of Hispanic/Latino individuals in the United States. We hypothesized that OSA is associated with worse neurocognitive function.12
METHODS
Study data.
We used data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a multisite, community-based, prospective cohort study of all aspects of health. The study's baseline measurements were collected from 2008 to 2011, and included self-identified Hispanic/Latino adults aged 18 to 74 years (n = 16,415), with an oversample (59%) of participants aged 45 to 74 years (n = 9,714) to facilitate examination of target outcomes of the parent study (i.e., cardiovascular mortality). The sampling frame for HCHS/SOL included 4 major US cities (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) with known substantial Latino concentrations. Data collection occurred at 4 field centers located in these cities with each site recruiting slightly more than 4,000 participants. The study used a 2-stage area probability sample design that includes clustering, stratification, and probability weighting. Detailed treatment of the HCHS/SOL sampling methods and major study aims have been published elsewhere.13,14
Study population.
The baseline HCHS/SOL examination included questionnaires administered in Spanish or English based on the participant's language preference, anthropometry, a blood draw, an oral glucose tolerance test, and other measurements as detailed previously.13,14
The baseline neurocognitive battery for HCHS/SOL was administered to respondents aged 45 years and older (n = 9,714). We excluded 91 participants who did not undergo neurocognitive testing and 665 participants who did not undergo sleep testing (n = 8,958). Finally, we excluded 899 observations with missing values on our model covariates. Our final sample consisted of 8,059 participants who were 45 to 74 years of age, had neurocognitive and sleep assessments, and self-reported a specific Hispanic/Latino background.
Standard protocol approvals, registrations, and patient consents.
The HCHS/SOL was approved by the institutional review boards at each field center where all participants gave written consent and by the study's reading and coordinating centers.
Neurocognitive outcomes.
Three neurocognitive tests were used in this study: (1) Spanish English Verbal Learning Test (SEVLT)15,16; (2) Controlled Oral Word Association (or Word Fluency [WF]) Test of the Multilingual Aphasia Examination17,18; and (3) Digit Symbol Substitution (DSS) of the Wechsler19 Adult Intelligence Scale–Revised. A detailed description of the SEVLT and WF Test has been previously published. To reduce participant burden, the original SEVLT was abbreviated; 3 instead of 5 fifteen-word SEVLT learning trials were used. The order of SEVLT administration was fixed across the 3 learning trials. After the third trial, a 15-item distractor list was introduced in which participants were asked to repeat aloud each word. Immediately after the interference trial, a delayed free-recall trial for list A occurred. The dependent measures examined were the summed total number of items correctly recalled across the 3 learning trials (SEVLT-sum) and the memory or delayed recall trial (SEVLT-recall). The WF Test was conducted with the letters F and A for the stimulus words. The letters S and C are often pronounced similarly in Spanish, and could be a source of language bias; therefore, the letter S was omitted from the WF Test. Briefly, study participants were instructed to orally generate as many unique words as possible within 60 seconds that began with a specified letter (F and A). The sums of correctly generated words with both letters served as the dependent measure. Finally, the DSS Test administration followed previously published instructions and procedures.19 With the exception of the SEVLT, which was developed for English and Spanish use, the neurocognitive tests were translated from English to Spanish and back-translated from Spanish to English. The neurocognitive tests were administered in the participant's preferred language during face-to-face interviews by trained research assistants.
Primary exposure: OSA.
Subjective sleep data were obtained by using the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale.20 Objective testing was completed by overnight recording with the Apnea Risk Evaluation System (ARES) (Unicorder 5.2; B-Alert, Carlsbad, CA).21 The ARES is a type 3 recorder that contains a pulse oximeter, airflow, snoring sounds, and head position. The apnea-hypopnea index (AHI) is the number of respiratory events per estimated sleep hour. The variables of AHI0, AHI3, and the AHI4 were generated to denote respiratory events without a desaturation (AHI0) or with associated desaturations of ≥3% (AHI3) or ≥4% (AHI4) from the baseline oxygen levels. Respiratory events were identified as a 50% or greater reduction in airflow lasting ≥10 seconds. In the current analysis, an AHI with 3% desaturation (AHI3) was used in accordance with the current criteria of the American Academy of Sleep Medicine.22 The AHI3 measured with the ARES is in good agreement with measures obtained by polysomnography, as demonstrated in a prior validation study (ρc = 0.70, 95% confidence interval [CI] 0.47–0.92).21 Sleep records were scored at a central sleep reading center by a certified polysomnologist. Inter- and intrascorer reliability was excellent (AHI intraclass correlation coefficient: 0.99). Using the AHI3, 19% of females and 33% of males had sleep apnea in HCHS/SOL.11 When defining sleep apnea using the older definition of AHI4, the age-adjusted prevalence of sleep apnea was similar to the predominantly non-Hispanic Wisconsin cohort of middle-aged adults.11
Covariates.
We accounted for 3 sociodemographic indicators. We also evaluated age as a categorical variable divided into 3 groups by decade (45–54, 55–64, and 65–74 years). Sex-specific comparisons were made. Education was classified into 3 categories (0 = less than high school; 1 = high school or equivalent degree; 2 = more than high school). We also controlled for health conditions, mental health problems, and health behavior factors associated with neurocognitive functions. Our health conditions included prevalent stroke (0 = not prevalent; 1 = prevalent). Impaired glucose tolerance and diabetes mellitus were based on the definition of the American Diabetes Association using fasting glucose, oral glucose tolerance testing, hemoglobin A1c levels, and use of hypoglycemic medications as previously defined (0 = normal glucose regulation; 1 = impaired glucose tolerance; and 2 = diabetic). Hypertension was defined as a systolic blood pressure ≥140 and/or diastolic blood pressure ≥90 mm Hg, or self-reported use of antihypertensive medications. Mental health problems were accounted for by controlling for depressive symptoms using the shortened (10-item) Center for Epidemiological Studies–Depression Scale, and anxiety using the 10-item State-Trait Anxiety Inventory summary score. Behavioral factors included a 4-category indicator of body mass index (BMI) (<18.5, 18.5–24.9, 25–29.9, and 30+ kg/m2) and self-reported smoking status (0 = not current; 1 = current smoker). Finally, given the multicenter design of the HCHS/SOL, the final models also controlled for center effects (Bronx, Chicago, Miami, and San Diego).
Analytic procedures.
To accommodate HCHS/SOL's complex sample design, all analyses performed in this study used survey procedures that account for clustering, stratification, and unequal probability weighting in the Stata 12.1 software package (StataCorp, College Station, TX). Methods appropriate for the analyses of subpopulations were applied to generate our parameters' estimates. We used a Taylor series linearization approach to variance estimation to obtain sample design–adjusted standard errors.
Analytic approach.
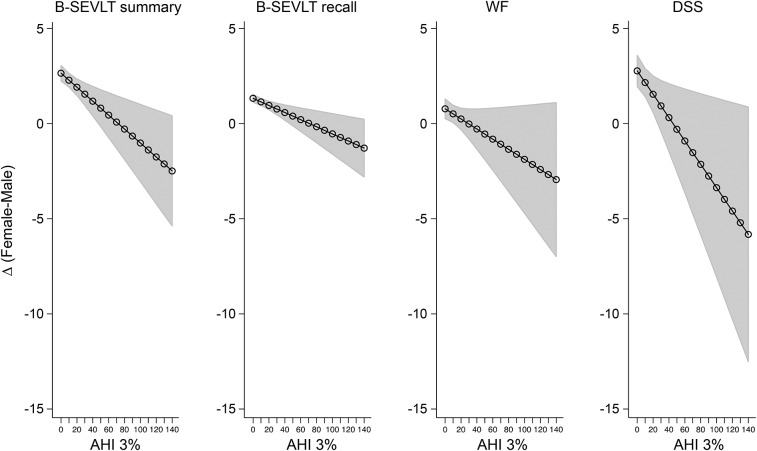
The analyses for this study were conducted in 4 steps. First, we generated bivariate descriptive statistics for the overall analytic sample and the 3 age groups of interest (table 1). Second, we calculated population- and age-stratified means and proportions for the AHI3 (table 2). Third, we fit linear regression models to examine the relationship between the AHI and the neurocognitive outcomes. For each neurocognitive outcome, we estimated 4 survey linear regression models incrementally adjusting for our model covariates (table 3). Subsequently, we fit age-stratified linear regression models to examine the AHI within the 3 age cohorts of interest (results not shown in tables). Fourth, we fit age-stratified fully adjusted (i.e., controlling for all model covariates) linear models to examine the interaction between sex and the AHI (table 4). For all of our models, we used survey design–adjusted, 2-tailed t tests to adjudicate the statistical significance of the estimated parameters. We followed standard procedures to evaluate violations of modeling assumptions.23 To facilitate the interpretation of our results, we generated plots for adjusted estimates of sex differences in neurocognitive performance as a function of AHI scores and their 95% CI24 (figure). Daytime sleepiness, by the Epworth Sleepiness Scale, was not included in the statistical models because it was not associated with neurocognitive function (data not shown) and with the AHI in HCHS/SOL.11
Table 1.
Overall and age-stratified descriptive statistics for Hispanic/Latino adults aged 45 to 74 years
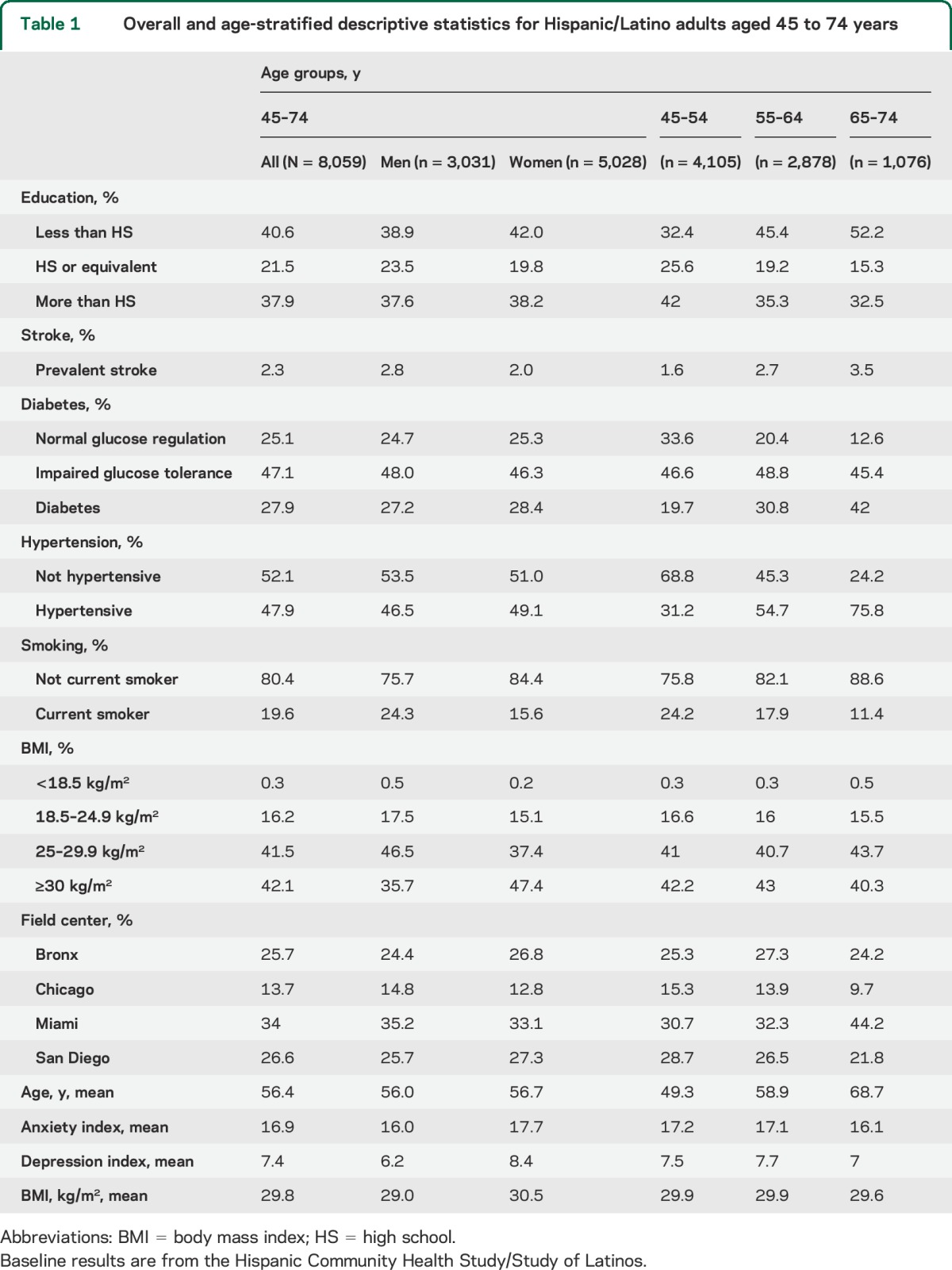
Table 2.
Overall, sex-, and age-stratified means for apnea-hypopnea index scores
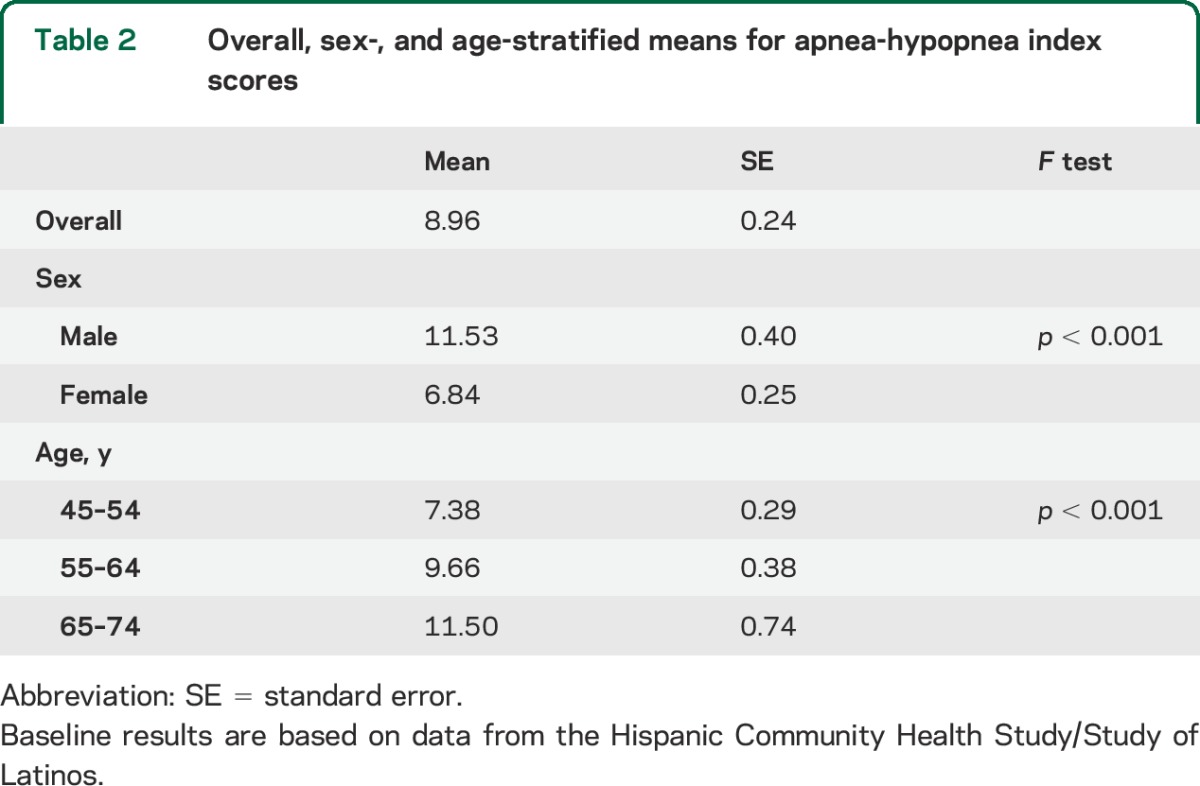
Table 3.
Association between AHI at 3% desaturation and neurocognitive function
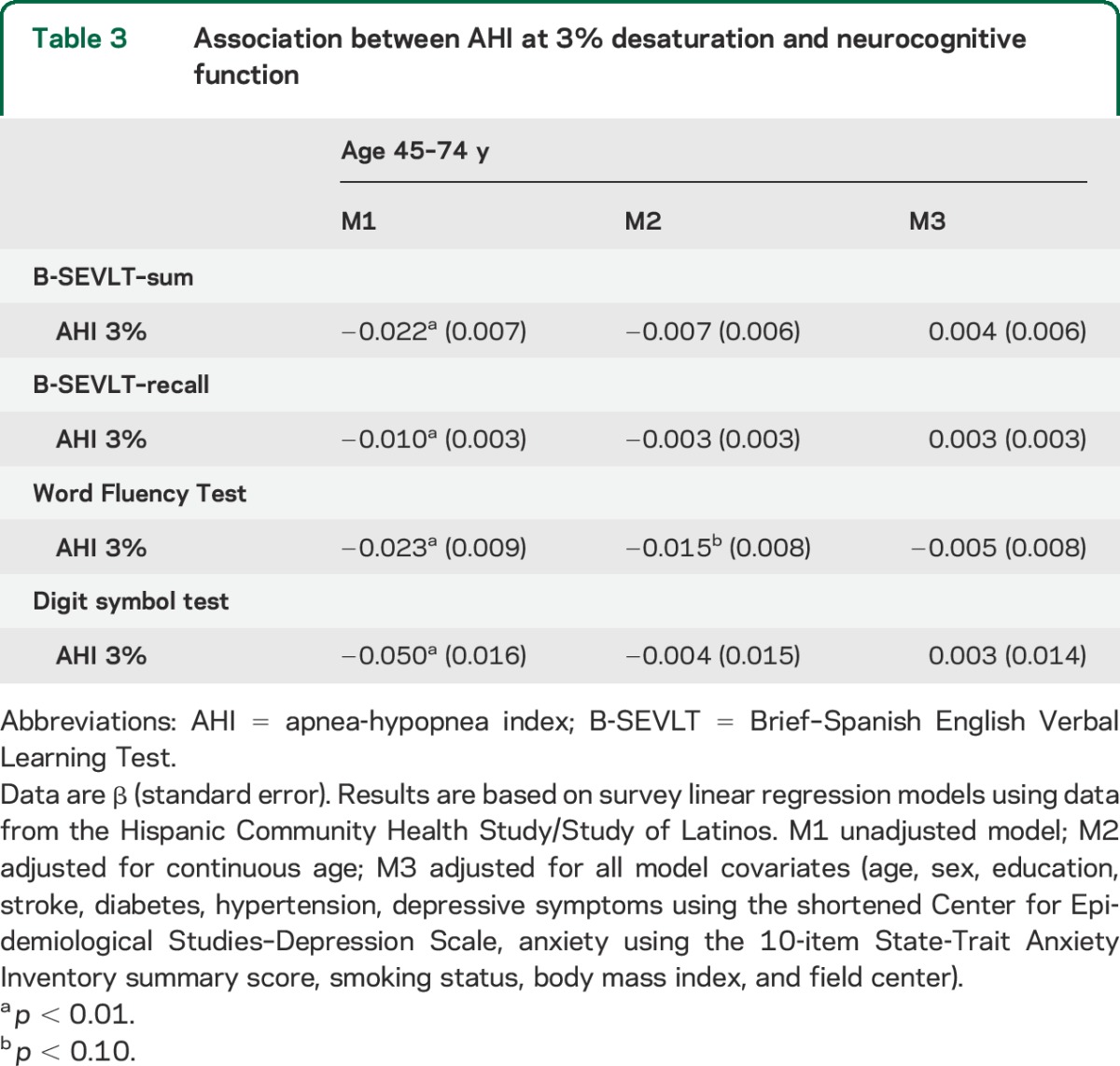
Table 4.
Sex moderation effects on the association between 3% saturation AHI and neurocognitive function
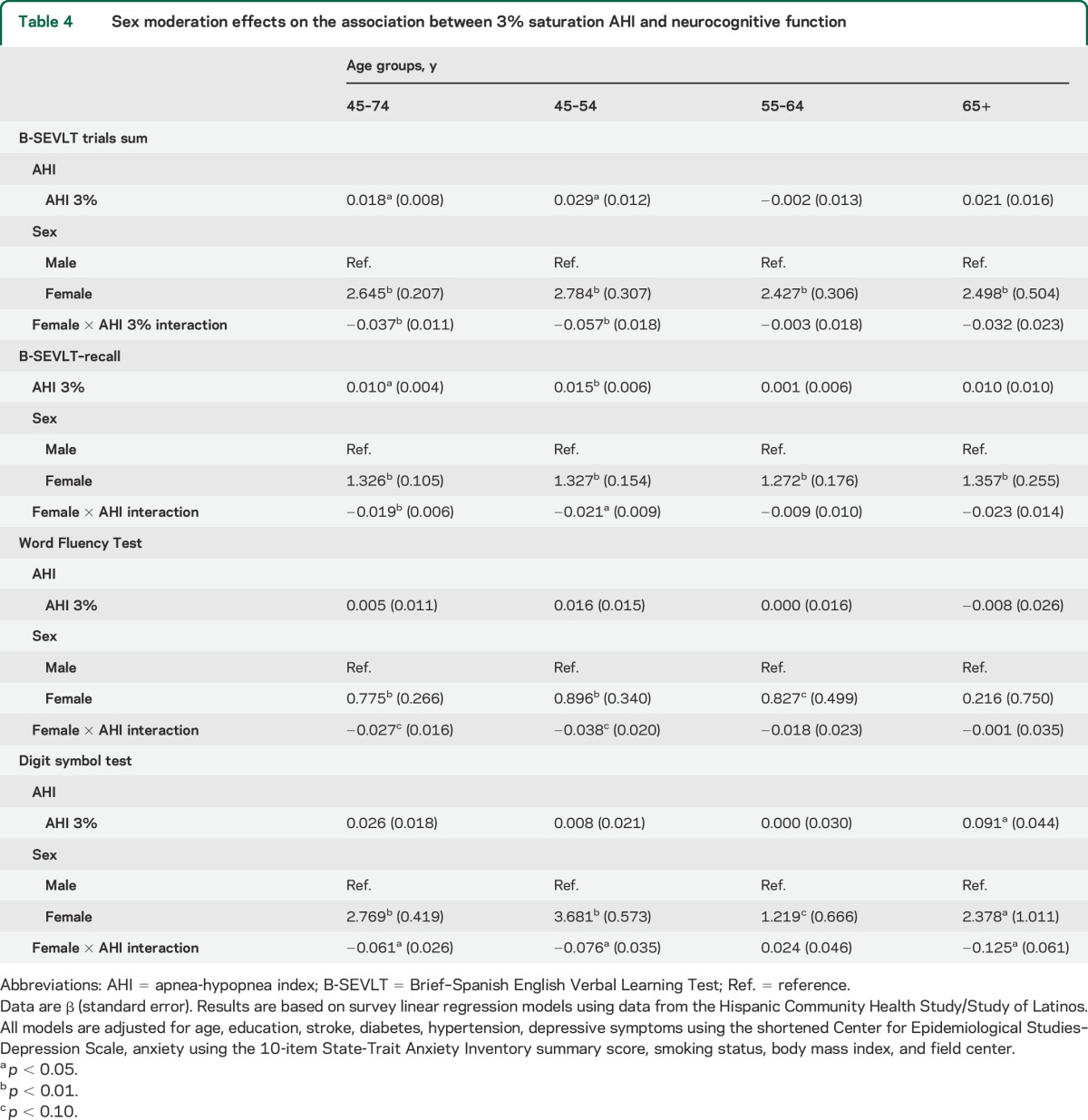
Figure. Estimated differences in cognitive scores between Hispanic women and men over the AHI scores.
Estimates and their 95% confidence bounds are derived from sex by AHI 3% index interactions in survey linear regression models using data from the Hispanic Community Health Study/Study of Latinos. Positive values indicate advantageous cognitive scores for women. Estimates are adjusted for age, sex, education, stroke, diabetes, hypertension, depressive symptoms using the shortened Center for Epidemiological Studies–Depression Scale, anxiety using the 10-item State-Trait Anxiety Inventory summary score, smoking status, body mass index, and field center. The x-axis represents the AHI with 3% desaturation. The y-axis represents the average estimated difference in neurocognitive test score between female and male respondents. AHI = apnea-hypopnea index; B-SEVLT = Brief–Spanish English Verbal Learning Test; DSS = Digit Symbol Substitution of the Wechsler Adult Intelligence Scale–Revised; WF = Word Fluency or Controlled Oral Word Association Test of the Multilingual Aphasia Examination.
Sensitivity analyses.
To ensure the validity of our findings, we checked the sensitivity of the results to different operationalizations of the AHI. As such, we grouped respondents' scores into deciles and refit our regression models treating the generated variable as both a continuous and a categorical indicator. Doing so allowed us to test the robustness of our results to the empirical skew in the distribution of the AHI. Categorizing a continuous scale is normally not recommended because it risks losing important information embedded in the original scale of the variable.25,26 In our case, it served as a sensitivity test. The results of these analyses (available from the authors) were qualitatively equivalent to the findings from the original scale.
RESULTS
Overall and age-stratified descriptive statistics of the sample are presented in table 1. Women were on average slightly older than men (Δ = 0.7 years; p = 0.017), had higher average BMI levels (Δ = 1.5; p < 0.001), and had higher scores on the anxiety (Δ = 1.8; p < 0.001) and depression (Δ = 2.2; p < 0.001) indices. Men were more likely to report being current smokers (p < 0.001).
Age-stratified analyses indicated that older age groups were more likely to include women (p = 0.0237) and to report lower levels of education (p < 0.001). We found no age differences in depression scores or BMI levels. Older adults (65–74 years) had significantly lower anxiety scores compared with the youngest age group (p < 0.001). Older adults were less likely to be current smokers (p < 0.001) and had higher prevalence of hypertension (p < 0.001), diabetes (p < 0.001), and prevalent stroke (p = 0.0087).
Overall, sex, and age group specific descriptive statistics of the distribution of the AHI scores are included in table 2. Men had a higher average AHI (Δ = 4.68; p < 0.001). Slightly more than 50% of the sample had a normal AHI (<5 per hour of sleep); older adults (65–74 years) had increased AHI compared with middle-aged (55–64 years) and young middle-aged (45–54 years) adults.
Our unadjusted regression models showed that increments in the AHI were associated with worse neurocognitive scores in the population aged 45 years and older (table 3). However, age adjustment in the models completely attenuated the relationship between the AHI and neurocognitive performance (table 3). The lack of statistical association was maintained after controlling for our model covariates (table 3).
We found that sex was a moderating factor in the relationship between the AHI and neurocognitive function (table 4). Multiple linear regression models accounting for the interaction between sex and the AHI indicated that women had better neurocognitive performance, but when compared with men, an increased AHI among women was associated with a steeper decrease in neurocognitive performance. These relationships were resilient to covariate adjustments and consistent across all 4 neurocognitive tests considered. The figure presents the covariate-adjusted cognitive score differences between men and women over the AHI score continuum and their 95% confidence bounds.
Age-stratified regression models, however, indicated that the moderating effects of sex were mostly specific to younger middle-aged (45–54 years) Latinos (table 4). The interactions between sex and the AHI were not statistically significant in the 55–64 and 65–74 age groups. These results were consistent for the SEVLT trials sum, SEVLT-recall, and WF tests. Furthermore, we found a statistically significant interaction between sex and AHI among older age (65–74 years) Hispanic/Latino participants for the DSS Test.
DISCUSSION
Among middle-aged and older Hispanic/Latino participants, we observed that increases in OSA were associated with worse neurocognitive function. The associations between decreased episodic learning and memory and executive function and OSA were mostly evident among middle-aged women. Among older women, the association between cognitive function and OSA was restricted to the DSS Test, a measure of processing speed. No such relationships were observed in men. To our knowledge, this is the largest study of the relationship between neurocognitive function and OSA in a Hispanic/Latino population. While the associations between OSA and neurocognitive function were relatively modest, these findings may have important implications for the prevention of neurocognitive disorders (e.g., Alzheimer disease).1
Epidemiologic studies demonstrate an increased prevalence of OSA and increased AHI in males,5,27 but some suggest that females are more symptomatic at lower AHI levels.28 Cross-sectional analyses of population-based and clinical cohorts have revealed associations between OSA and impairments in attention, executive function, and psychomotor speed29,30; however, there are no available studies describing sex differences in neurocognitive function and OSA. In accordance with our study, the Osteoporotic Fractures Study followed elderly women (mean age of 82 years) with and without OSA for a median of 5 years.31 Those with moderate to severe OSA (AHI ≥15 events per hour of sleep) had greater odds of mild cognitive impairment and dementia (31.1% vs 44.8%) in a multivariable analysis (adjusted odds ratio = 1.85, 95% CI 1.11–3.08). This association was mediated by the levels of hypoxemia, as opposed to sleep fragmentation.31
The Sacramento Area Latino Study on Aging, which included 1,789 community-dwelling older Mexican Americans (60–101 years), revealed sex differences in cardiovascular disease risk and neurocognitive function in a 10-year prospective analysis. The higher cardiovascular disease risk was significantly associated with greater decline in SEVLT scores in Mexican women but not in men.32 Mild levels of OSA have also been associated with reduced vascular endothelial function in females.33 These findings suggest an early vulnerability to cardiovascular disease, but the mechanism by which OSA might be related to worse neurocognitive function in women is unknown. Alterations of white matter integrity in diffusion tensor imaging (DTI) have been associated with impaired neurocognitive function and dementia in non-OSA populations.34 Sex differences in white matter integrity by DTI were described in middle-aged patients with OSA.35 Women, with AHI levels similar to men, had increased daytime sleepiness, reduced sleep quality, and showed greater extent of white matter injury with a reduction of fraction anisotropy on DTI.35
It is plausible that early white matter alterations associated with OSA could lead impairments in neurocognitive function in women. Our findings suggest that further studies for the early detection and treatment of OSA in this population are warranted.
Except for the processing speed, we did not observe a relation between the AHI and neurocognitive function in the older age groups. In our sample, age and education explained most of the relationship between neurocognitive function and OSA in those older than 55 years. In accordance with our study,36,37 education was the variable that mostly explained neurocognitive performance in the Apnea Positive Pressure Long-Term Efficacy Study, a multicenter randomized controlled trial of 1,204 adults (mean age 51 years) that evaluated continuous positive airway pressure and neurocognitive function in OSA.37
The results of our study could be partly explained by our sample and methodologic differences. Impaired attention-vigilance (i.e., psychomotor vigilance test), working memory (i.e., digit span), and executive function are described in OSA using measures that differ from the neurocognitive test used in our sample.30,38,39 In addition, impaired neurocognitive function has been described in clinical cohorts in which the majority of patients have moderate to severe OSA.30,31,38 In contrast, our population is mainly composed of participants with mild OSA. Some of the neurocognitive measures obtained in our study may not discern subtle cognitive differences related to mild OSA.
The strengths of our study are the large sample size and representative sample of Hispanic/Latino individuals in the United States. In addition, there were strict quality-control procedures and use of standardized measurements.11 The neurocognitive assessments were chosen based on published validity studies and were availability in Spanish and English.15–17 The sleep and neurocognitive data were respectively analyzed by a central reading center with high scorer reliability.11 Despite these strengths, there are certain weaknesses in the study. First, our cross-sectional analysis does not allow us to evaluate causality or cumulative exposure to OSA. As such, our findings should be interpreted as associations that warrant additional examination. Second, we did not identify dementia cases in our study. However, given the cohort's younger mean age, dementia cases would likely be few. Third, our data were obtained with a type 3 portable sleep study that has a reduced number of signals compared with polysomnography.11 Finally, the AHI with 3% desaturation (AHI3) criterion used in our study differed from the AHI definitions used in previous studies, which could partly explain differences between our study and previous work. Readers should be cognizant of the differing criteria when comparing study results.11
The result of this large study provides evidence of a relation between worse neurocognitive function and OSA across diverse Hispanic/Latino groups. Women were more likely than men to have lower neurocognitive function associated with OSA.
ACKNOWLEDGMENT
The authors thank the staff and participants of HCHS/SOL for their important contributions. Investigators Web site: http://www.cscc.unc.edu/hchs.
GLOSSARY
- AHI
apnea-hypopnea index
- ARES
Apnea Risk Evaluation System
- BMI
body mass index
- CI
confidence interval
- DSS
Digit Symbol Substitution
- DTI
diffusion tensor imaging
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- OSA
obstructive sleep apnea
- SEVLT
Spanish English Verbal Learning Test
- WF
Word Fluency
AUTHOR CONTRIBUTIONS
Alberto Ramos: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Wassim Tarraf: statistical analysis, drafting/revising the manuscript for content, including medical writing for content, study concept or design. Tatjana Rundek: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Susan Redline: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. William K. Wohlgemuth: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Jose Loredo: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Ralph L. Sacco: study concept or design, analysis or interpretation of data. David J. Lee: study concept or design, analysis or interpretation of data. Raanan Arens: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Patricia Lazalde: study concept or design, analysis or interpretation of data. James Choca: study concept or design, analysis or interpretation of data. Thomas Mosley: study concept or design, analysis or interpretation of data. Hector M. González: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data.
STUDY FUNDING
The Hispanic Community Health Study/Study of Latinos was performed as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes/centers/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and NIH Office of Dietary Supplements. The project described was also supported by grant 1KL2TR000461, Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement 2010;6:158–194. [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci 2011;34:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 2009;29:320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea–hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010;182:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelick PB, Pantoni L. Advances in vascular cognitive impairment. Stroke 2013;44:307–308. [DOI] [PubMed] [Google Scholar]
- 9.de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer's disease. J Alzheimers Dis 2012;32:553–567. [DOI] [PubMed] [Google Scholar]
- 10.Daviglus ML, Talavera GA, Aviles-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds: the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014;189:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–497. [DOI] [PubMed] [Google Scholar]
- 13.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc 2001;7:544–555. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol 2002;16:439–451. [DOI] [PubMed] [Google Scholar]
- 17.Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 18.Benton AL, Hamsher K. Multilingual Aphasia Examination, 2nd ed. Iowa City: AJA Associates; 1989. [Google Scholar]
- 19.Wechsler D. WAIS-R Manual. San Antonio: Psychological Corporation; 1981. [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 21.Westbrook PR, Levendowski DJ, Cvetinovic M, et al. Description and validation of the Apnea Risk Evaluation System: a novel method to diagnose sleep apnea–hypopnea in the home. Chest 2005;128:2166–2175. [DOI] [PubMed] [Google Scholar]
- 22.Grigg-Damberger MM. The AASM Scoring Manual four years later. J Clin Sleep Med 2012;8:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox J. Applied Regression Analysis and Generalized Linear Models. Thousand Oaks: Sage; 2008. [Google Scholar]
- 24.Mitchell MN. Interpreting and Visualizing Regression Models Using Stata. College Station: Stata Press; 2012. [Google Scholar]
- 25.Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Method 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology 1995;6:450–454. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893–900. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis: are women missed because they have different symptoms? Arch Intern Med 1996;156:2445–2451. [PubMed] [Google Scholar]
- 29.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res 2009;18:36–48. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep 2012;12:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeki Al Hazzouri A, Haan MN, Neuhaus JM, et al. Cardiovascular risk score, cognitive decline, and dementia in older Mexican Americans: the role of sex and education. J Am Heart Assoc 2013;2:e004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randby A, Namtvedt SK, Hrubos-Strom H, Einvik G, Somers VK, Omland T. Sex-dependent impact of OSA on digital vascular function. Chest 2013;144:915–922. [DOI] [PubMed] [Google Scholar]
- 34.Wen W, Zhu W, He Y, et al. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci 2011;31:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep 2012;35:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res 2002;11:265–272. [DOI] [PubMed] [Google Scholar]
- 37.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kielb SA, Ancoli-Israel S, Rebok GW, Spira AP. Cognition in obstructive sleep apnea–hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment. Neuromolecular Med 2012;14:180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc 2001;49:1622–1627. [DOI] [PubMed] [Google Scholar]