Summary of recommendations
Erectile dysfunction (ED) is the preferred clinical term describing the persistent or recurrent inability to achieve and maintain a penile erection of sufficient rigidity to permit satisfactory sexual activity for at least 3 months.
The initial diagnosis and treatment of ED is most commonly performed in Canada by primary care physicians (PCPs).
PCPs, urologists, internists, psychiatrists, and other treating healthcare professionals should be encouraged to initiate an open dialogue of sexual issues to identify men with ED who may not otherwise volunteer their sexual concerns.
Frequently a careful history, physical exam, serum glucose or hemoglobin A1C, lipid profile and optional hormonal testing facilitate the diagnosis of ED and effective therapy. Patient history can differentiate ED from other male sexual dysfunctions, including ejaculatory disorders (premature ejaculation and other abnormalities), hypogonadism, disorders of orgasm, and Peyronie’s disease.
Organic (physical) causes of ED are present in most men, but situational or psychosocial contributing factors often play a contributory role. Addressing these issues may enhance treatment efficacy.
Underlying risk factors associated with ED are common to cardiovascular disease in general, and should be identified during evaluation as they may represent the initial clinical sign of generalized endothelial disease (vascular insufficiency). Evaluation of family history, nicotine use, blood pressure, lipid profile, and glucose is required or should be documented if previously performed. Active management of identified cardiac risk factors should be instituted (i.e., smoking cessation, blood pressure treatment).
Once reversible causes of ED are ruled out, a trial of oral medication is recommended as first-line therapy, based on treatment efficacy, side effect profile, and minimal invasiveness. Specialized testing and referral are generally reserved for cases where oral first-line treatments fail or are not appropriate, of if greater insight into the etiology is desired by the patient/physician.
Second-line therapies, although more invasive than oral agents, are generally well-tolerated and effective.
Surgery remains an important option for men refractory to medical management, offering effective and durable ED treatment outcomes.
Background
Erectile dysfunction (ED) is a highly prevalent condition, which affects the physical and psychosocial well-being and quality of life (QoL) for thousands of Canadian men, their partners, and families. The Canadian Study of Erectile Dysfunction identified 49.4% of men over 40 with ED, with other studies showing that 5% to 20% of men have moderate to severe ED.1–3 Contemporary treatment options include highly effective, minimally invasive therapeutic agents – most commonly oral therapies using phosphodiesterase type 5-(PDE5) inhibitors. Second-line self-injection with vasoactive agents, vacuum erection devices, and surgical approaches with inflatable penile prostheses offer ED management with high potential for patient and partner treatment satisfaction.
Most cases of ED in Canada are identified and effectively treated by primary care physicians (PCPs).4 Evidence-based diagnostic and therapeutic approaches, including effective oral agents like the PDE5-inhibitors, has allowed for a shift of ED management from a historical surgical approach to contemporary medical management. Family physicians, urologists, internists, endocrinologists, cardiologists, and other medical specialists are approached by couples with ED requesting treatment more frequently. In many cases longstanding relationships exist between the couple and their treating physician, fostering an important therapeutic alliance which may translate into improved clinical response to the selected treatment approach. A shared-care model for the treatment of ED is a valid concept and also may reflect optimal utilization of healthcare resources in the Canadian healthcare environment.4,5 This shared-care model is one in which PCPs initially identify and treat patients with ED and refer primarily those individuals who have incomplete responses or require more invasive or specialized testing and treatment. The combined experience and knowledge of PCPs coupled with the diverse ED knowledge of the specialist can ideally result in optimal care for the patient.
In the contemporary model of ED care delivery, urologists remain an essential resource for several important reasons:
Referral requested for the difficult-to-treat, oral-refractory cases.
Second-line intracavernous and intraurethral vasoactive therapy may be outside of the practice pattern of some PCPs and therefore require urologic care.
In some cases anatomical penile deformity (Peyronie’s disease or post-trauma) may play an important role in the ED and more frequently require operative correction.
In a small but definable population (often men with severe vascular disease or poorly controlled diabetes), a trial of nonsurgical approaches may not succeed, requiring surgical options in the difficult-to-treat group.
Patients with congenital venous leak as the cause of their ED require urologic care. These patients are usually young and do not respond to PDE5-inhibitors.
Specific tests performed by urologists may be indicated at the request of the patient or his partner or for medico-legal issues.
Ongoing research into the basic and clinical consequences of ED is performed in urologic laboratories and clinical practices
As presented in this document, the Canadian Urological Association (CUA) Guidelines Committee has updated the CUA Erectile Dysfunction Guidelines using a Canadian perspective. Suggestions were based on peer-reviewed literature through 2015, and the ED recommendations from the World Health Organization (WHO)-endorsed 2010 International Consultation on Sexual Medicine, the International Society for Sexual Medicine, the Sexual Medicine Society of North America, and evolving research on new approaches to ED management.4,6–7
Global management objectives
To help the patient and partner establish their objectives of treatment.
To select diagnostic tests based on presenting complaints and goals of therapy.
To use diagnostic tests in a cost-effective and meaningful manner which would affect choice of treatment as well as help to identify and disqualify certain contributory health problems.
To provide a diagnosis and understanding of the likely etiology of the ED to the patient and partner.
To identify ED etiologies which may affect patient morbidity and mortality (if not previously identified), including screening for vascular risk, and facilitate access to the most appropriate healthcare providers for definitive management.
To offer treatment choices with comprehensive information on cost, likelihood of success and common side effects.
To initiate therapy with the least invasive option which would satisfy the patient and partner goals of treatment.
To provide patients with information and ongoing support to maximize treatment success.
To re-establish the couples’ ability to achieve and maintain sexual intimacy in the most natural manner possible.
To choose approaches which are reversible whenever possible.
Management approach: Diagnosis
Overview
The CUA supports the view that the general framework for the evaluation of patients with any type of sexual dysfunction should follow the same basic principles.3,4,6 The sexual history should ascertain the severity, onset, and duration of the problem, concomitant medical or psychosocial factors, and bother to the patient and partner (if applicable). In-person interview is often supplemented with questionnaires or potential web-based methods. The manner of sexual inquiry is important and should reflect a high level of sensitivity and regard for each individual’s unique ethnic, cultural, and personal background.
Determine that the problem is ED versus other aspects of the sexual response cycle (desire, ejaculation, orgasm) or from other causes (Peyronie’s disease, lifestyle factors including illicit drug use, quality of partners relationship).
Determine the timing of onset, nature of the problem, and significance to the partner (if applicable). The patient (with or without their partner) will guide whether treatment is desired.
Identify whether a potentially reversible cause to the ED exists (medications), stress, depression, hormonal abnormalities including androgen, thyroid and pituitary, tobacco, excessive alcohol use, drug abuse, and partner-specific issues). Testosterone profile is appropriate at the ED diagnosis if hypogonadism suspected, but screening is not recommended for all ED patients.
Establish a likely underlying etiology based on history, physical exam, and lab testing. Obtaining or confirming recent blood pressure measurements, lipid profile, and glucose/HgBA1C are highly recommended.
A commonly used schema is:
Vascular
Endocrine
Neurological
Situational
End organ (penile deformity – Peyronie’s disease or trauma)
Mixed (Most cases have an underlying organic cause or causes; similarly most men will subsequently report anxiety, stress, and depression as a consequence of ED.)
Methodology
History and clinical questioning (most important component of the ED evaluation).
Focused physical examination (directed at anatomic, vascular and neural systems essential for erections).
Use of formalized questionnaire instruments (e.g., Sexual Health Inventory for Men [SHIM], Appendix-http://journals.sfu.ca/cuaj/index.php/journal/article/view/2699/2022) is recommended but not mandatory, as the questionnaires are useful in establishing baseline function, ED severity, evaluate treatment response, and in most cases questionnaire results do not add significantly to duration of the doctor-patient encounter.
Laboratory investigations: serum glucose, lipid profile, and in select cases hormonal screening (total testosterone/bioavailable testosterone).
Consultation with subspecialists (endocrinology, psychology, cardiology).
- Specialized tests:
- Combined injection and stimulation test (CIS)
- Duplex ultrasound with vasoactive penile injection
- Nocturnal penile tumescence testing (NPTR) (Rigiscan)
- Dynamic infusion cavernosography and cavernosometry (DICC)
- Penile and pelvic angiogram
Diagnosis history
Obtaining a diagnostic history is the cornerstone of the evaluation of sexual dysfunction and ED. The history will provide the likely diagnosis in most cases.4,6,8 A supportive healthcare professional allows the couple to relate their concerns and express their goals of treatment in an unhurried manner. A monogamous, heterosexual relationship should not be assumed. Potential etiologies for sexual dysfunction include a wide range of organic and medical factors, but multiple psychological or interpersonal factors (i.e., anxiety, depression, relationship distress) can be causal or contributory.
General domains of the history
Determine specifics related to ED (onset, severity, significance and situations) and desire, arousal, orgasm, and ejaculation.
Sexual desire, relationship issues, stressors at home and work.
Genital pain or altered shape.
Lifestyle factors: smoking, substance use/abuse, sedentary lifestyle.
Comorbid conditions: hypertension, peripheral vascular disease, diabetes, obesity, and renal disease.
Pelvic surgery, radiation or trauma.
Medications.
Psychiatric illness or conditions.
Questionnaires
Use of validated questionnaires may be beneficial. These tools can be patient self-administered and provide much of the above information in an efficient non-threatening manner, while being time-saving and cost-effective.6 There are validated instruments designed to evaluate sexual and erectile function. The greatest utility of these questionnaires may be in establishing a response to therapy and determining overall satisfaction with drug use over a specified length of time (i.e., 4 weeks). The most common questionnaire is the SHIM (Appendix-http://journals.sfu.ca/cuaj/index.php/journal/article/view/2699/2022).9
Physical exam
The aim of a focused physical examination in men with ED is to examine genital anatomy and identify any related abnormalities (e.g., Peyronie’s plaques), endocrine signs, and possible comorbidities (neurological, vascular, and possible life-threatening conditions).10 An association exists between erectile dysfunction and peripheral vascular disease, as well as ED and the potential development of coronary artery disease.11 Assessment should include body habitus (secondary sexual characteristics), peripheral circulation (ED is a predictor of cardiovascular morbidity and mortality, findings consistent when controlled for confounders), neurological and genitourinary systems.10 Testicular examination is important to ensure testes/testis presence and to examine consistency of the testicle (i.e., atrophy, hypogonadism). The identification of penile deformities may be best achieved in the erect state, but is most commonly examined by stretching the penis to make the Peyronie’s plaque(s) more pronounced. The physical examination can also be a source of embarrassment or discomfort for some patients; therefore, every effort should be made to ensure privacy and personal comfort.
Laboratory testing
Overview
The recommendations by the International Society for Sexual Medicine’s International Consultation on Sexual Medicine suggest that laboratory tests for men with sexual problems may include fasting glucose, lipid profile and, in select cases, a hormone profile. Hormone profiles are used to identify or confirm specific etiologies (e.g., hypogonadism) or to assess the role of potential medical comorbidities or concomitant illnesses.6,12
Assessment for occult diabetes may be performed with a fasting glucose or HbA1c. Although recommended by the WHO consensus panel, the lipid screen is considered an optional component of the Canadian ED assessment but is suggested as a valuable addition to the evaluation and good general practice.2
Hormonal profile screening remains a controversial aspect of the routine evaluation of ED. There is a general guideline agreement that in a man with ED and hypoactive desire, incomplete response to PDE5-inhibitor treatment, and in all men with known diabetes (as suggested by 2013 Canadian Diabetes Association guidelines)2 testing and potential treatment for low levels of testosterone is appropriate. In men with normal desire and ED the need for global testing is controversial and currently undetermined. In the appropriate patient, once treatment with exogenous testosterone is initiated, ongoing follow-up is mandatory according to published guidelines.12,13
For men with diabetes, the 2013 Canadian Diabetes Association guidelines also support annual review of sexual function and determination of testosterone levels.
Optional testing such as thyroid-stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH), prolactin, complete blood count (CBC), and urinalysis are considered complementary and not felt to be mandatory in the evaluation of ED in most cases, but are added when dictated by clinical context.3,6,10
Specialized testing
1. Psychological/psychiatric assessment
These assessments often provide important complementary insight into relationships and situational causes to ED. The lack of widespread availability and cost limit their use in most cases of ED treatment. The primary goals of psychotherapy are to reduce or eliminate performance anxiety, to understand the context in which men or a couple function sexually, to implement psycho-education in order to modify sexual scripts, and to reduce premature discontinuation of pharmacotherapy.3,6,14
2. NPTR
NPTR is a minimally invasive means to measure and record nighttime erectile events (nocturnal penile tumescence), but has limited availability in Canada and costs are not covered by most provinces. Nocturnal penile tumescence and rigidity testing using Rigiscan should take place for at least 2 nights, measuring 2 to 5 overnight erections. A functional erectile mechanism is indicated by an erectile event of 60% rigidity recorded on the tip of the penis lasting for 10 minutes. NPTR’s greatest utility is in medico-legal cases and in investigative pharmacological studies to assess treatment impact.15
3. Vascular testing
A variety of vascular tests exist. Penile duplex cavernous artery flow determination after corporal injection of vaso-active agents is commonly performed.16 Use of ultrasound to localize and measure the size and flow through the cavernous vessels, pre- and post-vasoactive injection allow a more refined assessment of penile circulation. This test is performed less frequently in Canada since the advent of effective oral medications. In cases where indicated, further vascular investigation is unnecessary if duplex ultra-sound is normal, as indicated by a peak systolic blood flow >30 cm/sec and a resistance index >0.8. If the ultrasound is abnormal, however, arteriography and dynamic infusion cavernosometry and cavernosography should be performed only in patients who are potential candidates for vascular reconstructive surgery – these tests though are rarely used in current Canadian ED treatment.
DICC aims to define how well the penile blood-trapping mechanism (the veno-occlusive mechanism) works.17 In brief, dye and fluid are delivered into the penis to induce an erection. Measurement of the rise and fall of intra-penile pressure with radiologic visualization of the veins draining the penis determine whether a competent or incompetent veno-occlusive mechanism exists.
The most invasive diagnostic test is an arteriography. It is reserved generally for cases of high-flow priapism or planned vascular bypass. A penile angiogram allows visualization of the penile circulation and directs embolization for the unusual case of penile injury induced high-flow priapism.
4. Endocrinological tests
There is still controversy on the ideal endocrine workup for men with ED.3,6 A morning total testosterone or bioavailable testosterone is logical in men with: decreased sexual interest, delayed ejaculation, reductions in ejaculate volume, failure of PDE5-inhibitor treatment, and men with ED and diabetes.13 Free testosterone measurements have significant intra-assay variability which limits their clinical utility in Canada and is not recommended. Bioavailable testosterone is clinically useful and recommended, but is not available in all areas of Canada; as well, patients may incur a cost for a bioavailable testosterone assay. Calculated bioavailable testosterone (which requires a morning total testosterone, albumin and sex-hormone binding globulin) is an acceptable substitute for measured bioavailable testosterone if it is not available or cost-prohibitive.
5. Neuro-physiological testing
This form of testing generally allows us to measure the sacral reflex arc, an indirect measure of the perineal neural integrity, and has limited clinical availability and utility.18
Treatment options
Overview
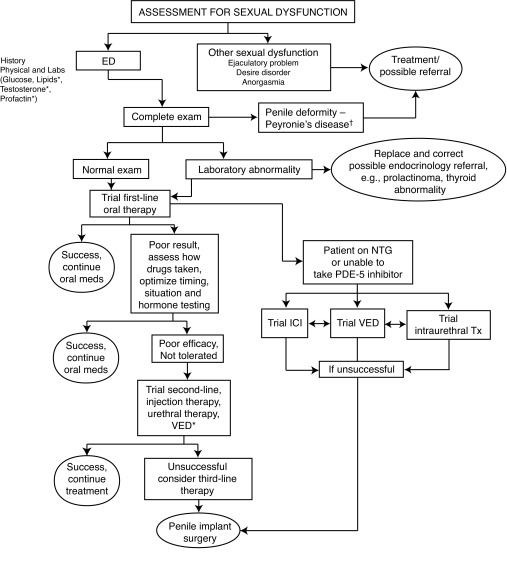
Management of ED most often will occur concurrently with lifestyle modification and treatment of organic or psycho-sexual dysfunctions. Patients and partners are made aware of efficacy, risks and benefits of appropriate treatments, taking into consideration preferences and expectations. Oral therapy failure may often be salvaged by patient re-education on PDE5-inhibitor use and optimization of dosing. Stepwise progression from oral agents through second- and third-line therapies occurs as needed (Fig. 1).
Oral therapy (on-demand or daily dosing). Given the differences between oral agents, the choice of which initiating PDE5-inhibitor you should use may be influenced by several factors, including timing or frequency of intercourse, and interactions with food or alcohol (Table 1).3,6,19–21
Testosterone replacement therapy in men with documented hypogonadism is an option. Testosterone may be used alone for hypogonadism, or in combination with oral PDE5-inhibitor therapy when ED is present.20–21
Sexual counselling (this may represent a spectrum of approaches from a simple open discussion with the PCP to psychologist, sexual therapists and/or psychiatrists).3,6
Local therapy (intracavernous or intraurethral agents).2,3,6,22
Vacuum erection device therapy.23
Fig. 1.
Management of erectile dysfunction. †Consider first-line ED treatment for men with ED and Peyronie’s disease. *Optional testing.
ICI: intracavernosal injections; VED: vacuum erection device; NTG: nitrates/nitroglycerine; ED: erectile dysfunction.
Table 1.
Comparison of the properties of PDE5-inhibitors
| Property | Sildenafil | Tadalafil | Vardenafil |
|---|---|---|---|
| >TMAX | 30–120 minutes (median 60 minutes) | 30–360 minutes (median 120 minutes) | 30–120 minutes (median 60 minutes) |
| T ½ | 4 hours | 17.5 hours | 4 hours |
| Absorption | Fatty meals cause a mean delay in TMAX of 60 minutes | Not affected by food | Fatty meals cause a reduction in CMAX |
| Available Doses | 25 mg, 50 mg, 100 mg PRN | 2.5 mg, 5 mg daily 5 mg, 10 mg, 20 mg PRN |
10 mg oral dissolvable tablet 2.5 mg, 5 mg, 10 mg, 20 mg PRN |
| Maximum Dose | 100 mg daily | 20 mg daily | 20 mg daily |
| Efficacy | Each of the PDE5 inhibitors offers similar efficacy. | ||
| Dose adjustments may be needed |
|
|
|
| Contraindications |
|
|
|
| Use with alpha blockers | Concomitant use of selective alpha blockers does not present a risk for significant hypotension. There is a potential risk of significant hypotension when using non-selective alpha blockers. | ||
| Side effects (5 most common in order of frequency when compared to placebo) | Headache, flushing, dyspepsia, nasal congestion, alteration in colour vision | Headache, dyspepsia, backpain, myalgia, nasal congestion | Headache, flushing, rhinitis, dyspepsia, sinusitis |
Please consult the individual product monographs for additional information.
Conclusions
A careful history and physical exam are the essential elements of the ED workup in most cases.
Basic screening tests include the identification of cardiac risk factors and blood tests. The following tests are recommended: serum fasting glucose, lipids and testosterone (if indicated).
A stepwise treatment approach using the least invasive option is suggested.
In some cases where greater detailed information is desired or failure of the initial oral medication is encountered, trials of more invasive second-line treatment or investigations may be appropriate.
Surgery should be reserved for men in whom less invasive reversible treatment has not succeeded or is contraindicated.
Treatment should be individualized and patient follow-up should be arranged to assess the efficacy of treatment.
Appendix
Footnotes
Competing interests: Dr. Bella is a member of the advisory boards for Lilly, Actavis, American Medical Systems, and Coloplast. He is also a member of the Speakers’ Bureau for Lilly and CUA (accredited CME speaker) and a co-principal investigator and cofounder of the PROPPER (Prospective Registry of Outcomes with Penile Prosthesis for Erectile Restoration) registry. Dr. Lee is a member of the advisory boards for Abbott and Lilly. He has also received grants from Abbott, Lilly and Actavis and is participating in a clinical trial with Lilly. Dr. Carrier is a member of the advisory boards for Astellas, Eli Lilly Canada, Pfizer Canada, Abbott, Novartis, and Actavis. He is also a member of the Speakers’ bureau for Eli Lilly Canada, Pfizer Canada, Abbott, Merck, and Actavis. He is participating in clinical trials with Astellas and Eli Lilly Canada. Dr. Bénard is a member of the advisory boards and part of the Speakers’ Bureau for Abbott, Astellas, Pfizer, Lilly, Paladin, and Actavis. He has received payment for talks from Abbott, Astellas, Pfizer, Lilly, Paladin, and Actavis. He also has investments in many pharmaceutical companies through his diversified retirement plan. He is also currently participating in a clinical trial with Allergan. Dr. Brock is a member of the advisory boards and part of the Speakers’ Bureau for Lilly, Coloplast, AMS, GSK, Abbott, and Actavis. He has also received payments and grants from these same companies.
This paper has been peer-reviewed.
References
- 1.Grover SA, Lowensteyn I, Kaouache M, et al. The prevalence of erectile dysfunction in the primary care setting: Importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 2.Brock G, Harper W, for Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Erectile dysfunction. Can J Diabetes. 2013;37:S150–2. doi: 10.1016/j.jcjd.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Urological Guidelines Committee. Erectile dysfunction practice guidelines. Can J Urol. 2002;9:1583–87. [PubMed] [Google Scholar]
- 5.Costa P, Grandmottet G, Mai HD, et al. Impact of a first treatment with phosphodiesterase inhibitors on men and partners’ quality of sexual life: Results of a prospective study in primary care. J Sex Med. 2013;10:1850–60. doi: 10.1111/jsm.12186. [DOI] [PubMed] [Google Scholar]
- 6.Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7:3572–88. doi: 10.1111/j.1743-6109.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- 7.Porst H, Burnett A, Brock G. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med. 2013;10:130–71. doi: 10.1111/jsm.12023. [DOI] [PubMed] [Google Scholar]
- 8.Brock G. Diagnosing erectile dysfunction could save your patient’s life. Can Urol Assoc J. 2014;8:S151–2. doi: 10.5489/cuaj.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanathan R, Mulhall J, Rao S, et al. Predictive correlation between the International Index of Erectile Function (IIEF) and Sexual Health Inventory for Men (SHIM): Implications for calculating a derived SHIM for clinical use. J Sex Med. 2007;4:1336–44. doi: 10.1111/j.1743-6109.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem HM, Salonia A, Martin-Morales A. SOP: Physical examination and laboratory testing for men with erectile dysfunction. J Sex Med. 2013;10:108–10. doi: 10.1111/j.1743-6109.2012.02734.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Prac. 2013;67:1163–72. doi: 10.1111/ijcp.12200. [DOI] [PubMed] [Google Scholar]
- 12.Meuleman EJ, Hatzichristou D, Rosen RC, et al. Diagnostic tests for male erectile dysfunction revisited. Committee Consensus Report of the International Consultation in Sexual Medicine. J Sex Med. 2010;7:2375–81. doi: 10.1111/j.1743-6109.2010.01841.x. [DOI] [PubMed] [Google Scholar]
- 13.Morales A, Bella AJ, Chun S, et al. A practical guide to diagnosis, management and treatment of testosterone deficiency for Canadian physicians. Can Urol Assoc J. 2010;4:269–75. doi: 10.5489/cuaj.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt HM, Munder T, Gerger H, et al. Combination of psychological intervention and phosphodisterase-5 inhibitors for erectile dysfunction: A narrative review and meta-analysis. J Sex Med. 2014;11:1376–91. doi: 10.1111/jsm.12520. [DOI] [PubMed] [Google Scholar]
- 15.Nocturnal penile erections. The role of RigiScan in the diagnosis of vascular erectile dysfunction. J Sex Med. 2010;9:3219–26. doi: 10.1111/j.1743-6109.2012.02954.x. [DOI] [PubMed] [Google Scholar]
- 16.Sikka SC, Hellstrom WJ, Brock G, et al. Standardization of vascular assessment of erectile dysfunction: Standard operating procedures for duplex ultrasound. J Sex Med. 2013;10:120–9. doi: 10.1111/j.1743-6109.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- 17.Glina S, Ghanem H. SOP: corpus cavernosum assessment (cavernosography/cavernosometry) J Sex Med. 2013;10:111–4. doi: 10.1111/j.1743-6109.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano F, Rowland DL. Standard operating procedures for neurophysiologic assessment of male sexual dysfunction. J Sex Med. 2013;10:1205–11. doi: 10.1111/jsm.12164. [DOI] [PubMed] [Google Scholar]
- 19.Smith WB, McCaslin IR, Gokce A, et al. PDE5 inhibitors: Considerations for preference and long-term adherence. Int J Clin Pract. 2013;67:768–80. doi: 10.1111/ijcp.12074. [DOI] [PubMed] [Google Scholar]
- 20.Alhathal N, Elshal AM, Carrier S. Synergistic effect of testosterone and phosphodiesterase-5 inhibitors in hypogonadal men with erectile dysfunction: A systematic review. Can Urol Assoc J. 2012;6:269–74. doi: 10.5489/cuaj.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: A systematic review and meta-analysis. Ann Intern Med. 2009;151:650–61. doi: 10.7326/0003-4819-151-9-200911030-00150. [DOI] [PubMed] [Google Scholar]
- 22.Coombs PG, Heck M, Guhring P, et al. A review of an intracavernosal injection therapy program. BJU Int. 2012;110:1787–91. doi: 10.1111/j.1464-410X.2012.11080.x. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom WJ, Montague DK, Moncada I, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7:501–23. doi: 10.1111/j.1743-6109.2009.01626.x. [DOI] [PubMed] [Google Scholar]