Abstract
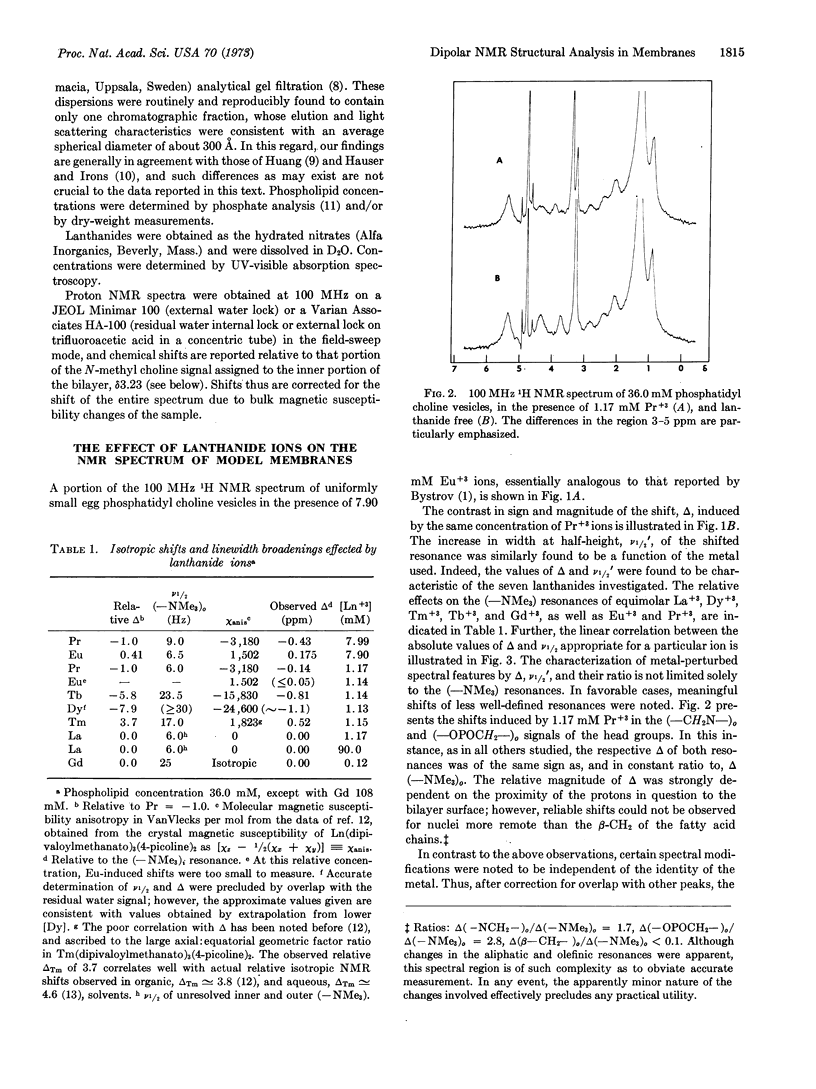
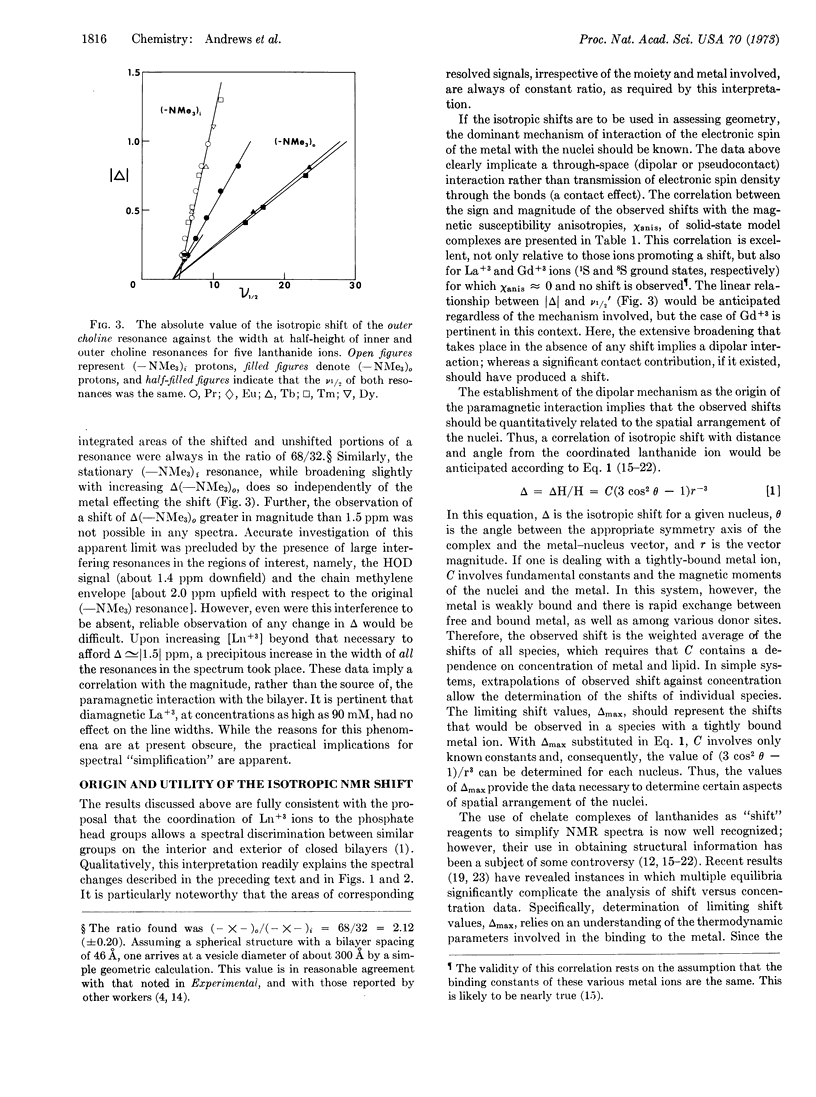
The effects of a series of aquated lanthanide ions on the nuclear magnetic resonance spectrum of phospholipid bilayer suspensions are reported. The ability of these ions to provide a spectral distinction between morphologically exterior resonances and their interior counterparts was confirmed. Measurements of the relative shifting and broadening capabilities of these ions are reported, and their correlation with solid-state magnetic susceptibility anisotropies is shown to implicate a dipolar (pseudocontact) mechanism as the source of this effect. The potential for the use of dipolar shifts as structural probes is discussed and an alternative method using dipolar shifting and broadening reagents simultaneously is presented.
Keywords: pseudocontact shift, phospholipid bilayers
Full text
PDF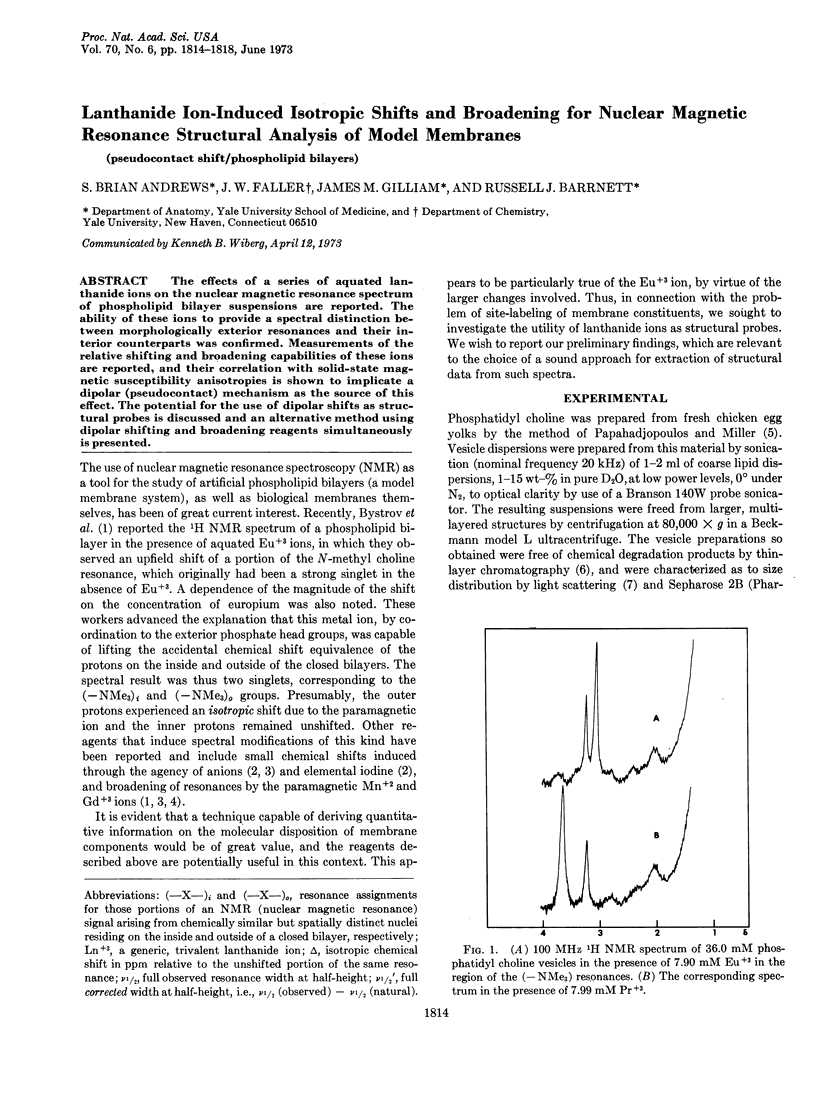


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTWOOD D., SAUNDEES L. A LIGHT-SCATTERING STUDY OF ULTRASONICALLY IRRADIATED LECITHIN SOLS. Biochim Biophys Acta. 1965 Apr 5;98:344–350. doi: 10.1016/0005-2760(65)90126-8. [DOI] [PubMed] [Google Scholar]
- Barry C. D., North A. C., Glasel J. A., Williams R. J., Xavier A. V. Quantitative determination of mononucleotide conformations in solution using lanthanide ion shift and broadenine NMR probes. Nature. 1971 Jul 23;232(5308):236–245. doi: 10.1038/232236a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972 Nov 28;71(3):523–528. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Kirschner D. A. Myelin membrane structure at 10 A resolution. Nat New Biol. 1971 May 12;231(19):46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Seiter C. H., Feigenson G. W. Anisotropic and restricted molecular motion in lecithin bilayers. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1488–1492. doi: 10.1016/0006-291x(72)90775-9. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. Mechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particles. Biochim Biophys Acta. 1972 Jan 27;260(1):49–58. doi: 10.1016/0005-2760(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hauser H., Irons L. The effect of ultrasonication on the chemical and physical properties of aqueous egg yolk lecithin dispersions. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1579–1590. doi: 10.1515/bchm2.1972.353.2.1579. [DOI] [PubMed] [Google Scholar]
- Horrocks W. D., Jr, Sipe J. P., 3rd Lanthanide complexes as nuclear magnetic resonance structural probes: paramagnetic anisotropy of shift reagent adducts. Science. 1972 Sep 15;177(4053):994–996. doi: 10.1126/science.177.4053.994. [DOI] [PubMed] [Google Scholar]
- Horwitz A. F., Horsley W. J., Klein M. P. Magnetic resonance studies on membrane and model membrane system: proton magnetic relaxation rates in sonicated lecithin dispersions. Proc Natl Acad Sci U S A. 1972 Mar;69(3):590–593. doi: 10.1073/pnas.69.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Charlton J. P. Studies on the state of phosphatidylcholine molecules before and after ultrasonic and gel-filtration treatments. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1660–1666. doi: 10.1016/0006-291x(72)90800-5. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Jendrasiak G. L. Halide interaction with phospholipids: proton magnetic resonance studies. Chem Phys Lipids. 1972 Oct;9(2):133–146. doi: 10.1016/0009-3084(72)90009-6. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]



