Abstract
Introduction:
Microsurgical spermatic cord denervation (MSCD) is an effective surgical technique to manage chronic orchalgia, but it has not been readily adopted by Canadian urosurgeons. This paper reviews the early experience of a single urosurgeon in Canada.
Methods:
Nine consecutive testicular units underwent MSCD over a 24 month period. These patients underwent MSCD after ruling out reversible causes and after a successful diagnostic spermatic cord block.
Results:
Of these patients, 77% (7/9) had a complete resolution and 22% (2/9) had a partial resolution of their pain symptoms following MSCD. There were no failures or complications.
Conclusions:
MSCD is an effective, safe, and reproducible surgical technique that should be included in the treatment armamentarium for chronic orchalgia.
Introduction
Chronic pain is the most common long-term disability reported in North America. It affects more than 100 million people and results in significant loss of work productivity and a significant increase in direct and indirect healthcare costs. In a systematic review by the International Association for the Study of Pain, chronic pain studies around the world were analyzed and the prevalence of chronic pain varies from 10.1% to 55.2%.1 In the United States, the conservative estimate of prevalence is 30%.2
The pathogenenis of chronic pain is not completely understood, but is felt to represent neural changes to the peripheral and central nervous system that allow persistent stimulation of pain centres in the absence of a threshold stimulation.3 As such, chronic pain syndromes can occur anywhere, including the testicle or scrotum. Although data on the prevalence of chronic orchalgia are difficult to find, up to 52% of men have described chronic post-vasectomy pain, although a much lower percentage actually seek treatment. Since as many as 50% of cases of chronic orchalgia are idiopathic, its true prevalence rates remain unknown.4,5
However, virtually all family practitioners and certainly all urologists have seen patients with chronic scrotal or testicular pain. These complaints can be very frustrating and demanding for the patient and the physician given the difficulties in establishing a diagnosis, and the absence of any well-established or accepted treatment approach.
Microsurgical spermatic cord denervation (MSCD) has been described since the late 1970s, but has not been widely adopted in Canada. Over the last 2 decades, articles have been published supporting this technique, its efficacy, and its safety in the management of chronic orchialgia.6–10
The goal of MSCD is to transect, disrupt, and separate all spermatic cord structures that carry neural elements. MSCD also preserves arterial inflow to the testicle and several veins and lymphatics to prevent postoperative testicular congestion and hydrocele formation. The rationale for this approach is that by interrupting the neural pathways to and from the scrotal contents, this decreases afferent nerve stimulation and downregulates pain centres.9
Recent evidence based on spermatic cord biopsies have reinforced the physiologic rationale behind MSCD by identifying dozens of microscopic (<1 mm) nerve fibres which travel within the cremasteric muscle fibres, perivasal tissues, lipomatous tissue, and the perivascular tissues of the spermatic cord. These nerves also show Wallerian degeneration, which supports their role in the perpetuation of chronic pain syndrome. This recent evidence serves to support use of MSCD to manage chronic orchalgia.11
The advantages of microsurgical spermatic cord denervation include complete response rates ranging from 71% to 100% – significantly better compared to current medical management and traditional surgical approaches of orchiectomy and epididymectomy. The testis-sparing nature of the procedure reduces the physical and psychological morbidity associated with extirprative surgery.6–10 This paper describes the early experience of MSCD at a single centre by a single surgeon.
Methods
All prospective patients required a minimum of 3 months of either unilateral or bilateral hemiscrotal pain. Reversible causes were ruled out with urinalysis, urine culture, sexually transmitted infection screen, and detailed scrotal ultrasound. All patients also must have been deemed unresponsive or intolerant to medical management.
All patients completed a visual analog scale (VAS) and numeric pain scale prior to and after any intervention. All patients also required a diagnostic spermatic cord block to demonstrate at least a 50% reduction in their pain scores to be considered candidates for MSCD.
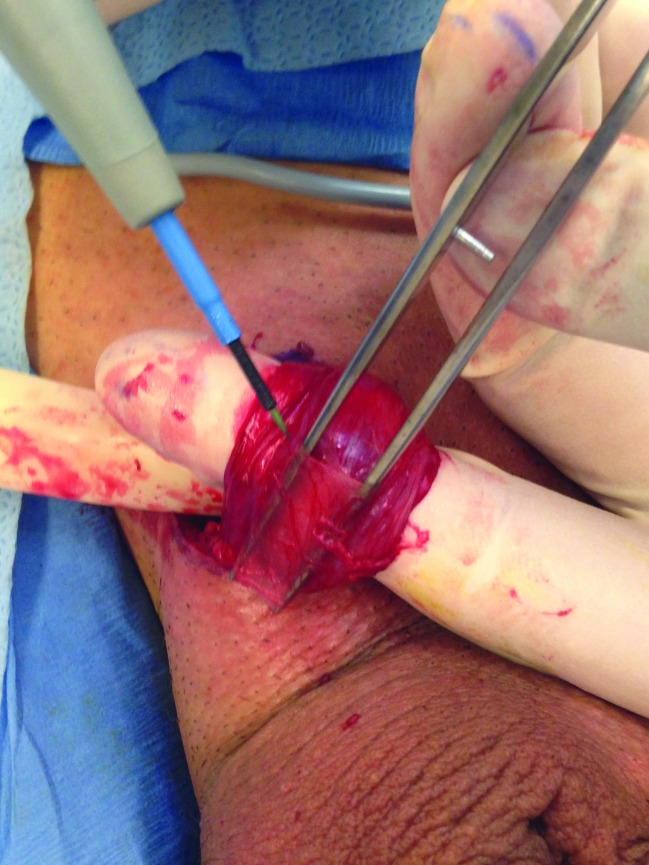
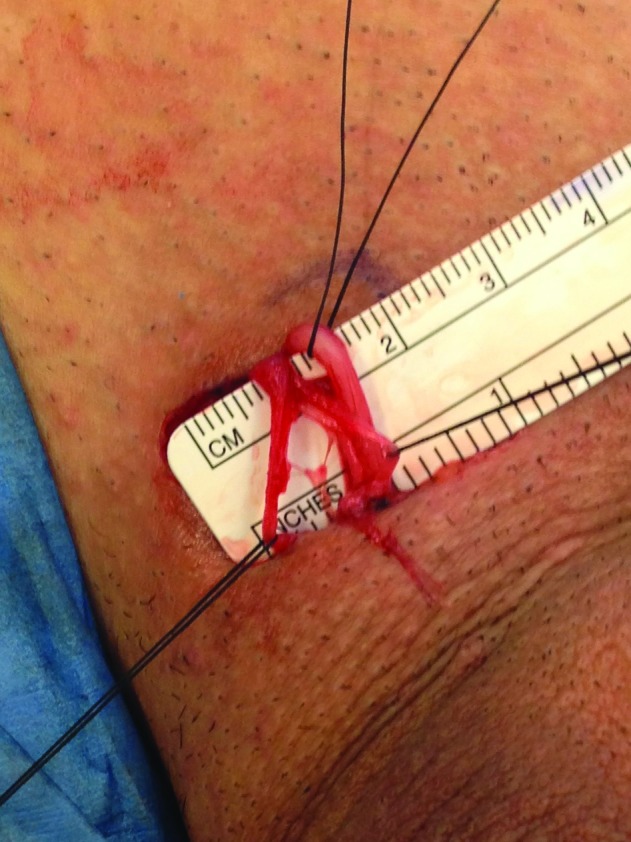
The procedure itself is carried out similar to a subinguinal microsurgical varicocelectomy. A 2-cm skin crease incision is made in the sublingual area and dissection is carried out to the spermatic cord (Fig. 1). The spermatic cord is then mobilized with its fascial layers intact and supported with a Penrose drain (Fig. 2) The ilioinguinal nerve is identified, divided, and then separated by about 2 cm. Using operative magnification (×5–10), the fascial layers of the spermatic cord are then opened, divided, and separated (Fig. 3). The underlying spermatic cord contents are similarly divided between small titanium clips or 4-0 silk ligatures. Using a micro-surgical Doppler ultrasound, we identified the arterial branches, secured them with a vessel loop, and preserved along with several veins and lymphatics (Fig. 4). The vas deferens is also divided and separated if a previous vasectomy had been performed; if the patient had not had a vasectomy, the vas deferens was stripped of its perivasal tissues for about 2 cm based on the description by Levine and his group at the University of Chicago.6
Fig. 1.
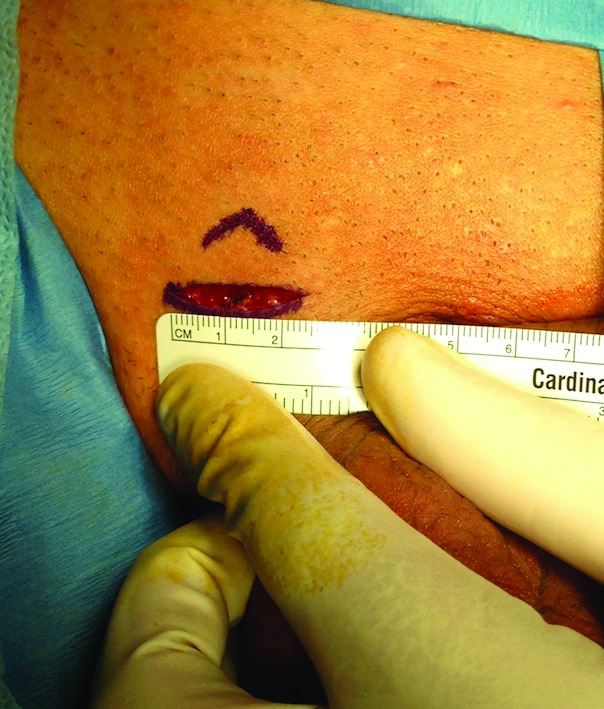
An approximate 2-cm skin crease incision in the sublingual area.
Fig. 2.
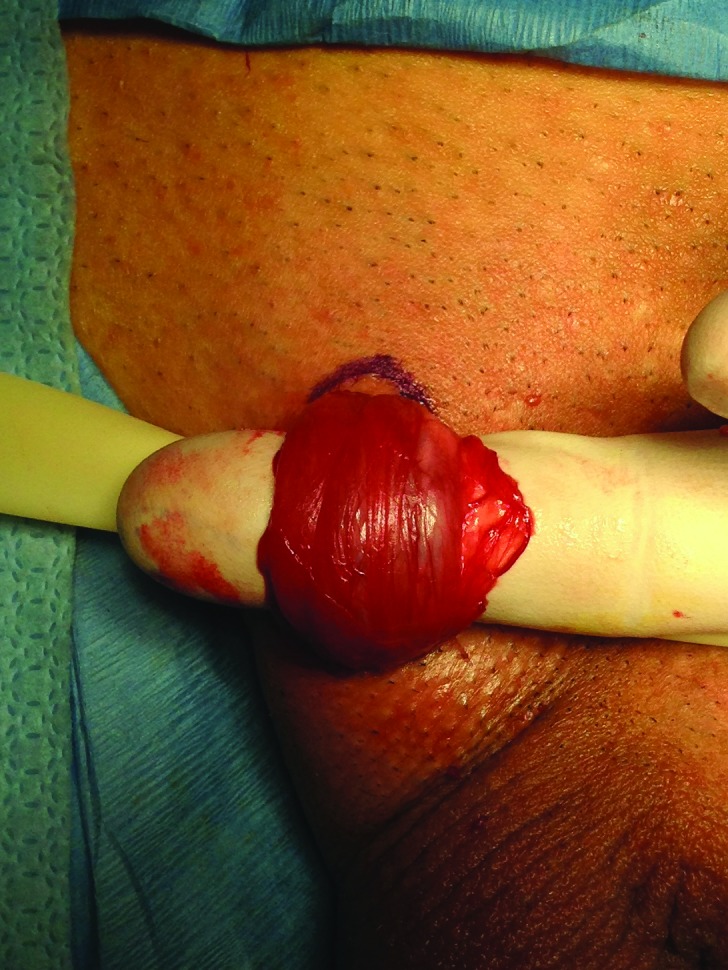
The spermatic cord mobilized with its fascial layers intact and supported with a Penrose drain.
Fig. 3.
The fascial layers of the spermatic cord opened, divided, and separated.
Fig. 4.
The arterial branches are identified, secured with a vessel loop, and preserved as are several veins and lymphatics.
Results
A total of 9 patients underwent MSCD over 24 months. The average patient age was 41.8 (range: 25–73). The average initial VAS score was 7.5 cm (range: 4.6–9) and the average initial numeric pain scale was 7.7/10 (range: 5–8.5) – consistent with severe to very severe pain. The average VAS pain score following a diagnostic spermatic cord block was 1 cm (range: 0–2) and the average numeric pain score was 1/10 (range: 0–3). Postoperatively, the average VAS pain score was 0.11 cm (range: 0–0.5) and the average postoperative numeric pain score was 0.3/10 (range: 0–2).
Overall, 77% (7/9) of patients demonstrated complete response and 22% (2/9) had minimal, non-bothersome discomfort postoperatively. The follow-up ranged from 3 to 9 months. There have been no long-term complications and all patients have demonstrated a durable response to date. There were no failures in this early group and no significant postoperative complications were reported.
Discussion
These results are consistent with the literature for MSCD in the management of chronic orchalgia. The initial response rates were excellent and there were no complications, although the number of patients is small and follow-up to date is short.
Conclusion
Based on this experience and the published literature, MSCD should be considered part of the armamentarium to manage chronic orchalgia.
Footnotes
Competing interests: Authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Harstall C, Ospina M. How prevalent is chronic pain. Pain Clinical Updates. 2003;2:1–4. [Google Scholar]
- 2.Institute of Medicine of the National Academies Report. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Neumann S, Doubell TP, Leslie T, et al. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen CG, Sandlow JI. Testicular pain following vasectomy: A review of postvasectomy pain syndrome. J Andrology. 2003;24:293–7. doi: 10.1002/j.1939-4640.2003.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 5.McMahon AJ, Buckley J, Taylor A, et al. Chronic testicular pain following vasectomy. Br J Urol. 1993;69:188–91. doi: 10.1111/j.1464-410X.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 6.Strom KH, Levine L. Microssurgical denervation of the spermatic cord (MSCD) for chronic orchalgia: Long-term results from a single center. J Urol. 2008;180:949–53. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol. 2002;41:392–7. doi: 10.1016/S0302-2838(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 8.Levine L, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical therapy for the treatment of chronic orchalgia. J Urol. 2001;165:1927–30. doi: 10.1016/S0022-5347(05)66244-1. [DOI] [PubMed] [Google Scholar]
- 9.Levine L, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: A surgical alternative in the treatment of chronic orchalgia. J Urol. 1996;155:1005–7. doi: 10.1016/S0022-5347(01)66369-9. [DOI] [PubMed] [Google Scholar]
- 10.Devine CJ, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchalgia. Trans Amer Ass Genito-Uro Surg. 1978;70:149. [PubMed] [Google Scholar]
- 11.Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: Potential anatomic basis for microsurgical denervation of the spermatic cord for chronic orchalgia. J Urol. 2013;190:265–70. doi: 10.1016/j.juro.2013.01.045. [DOI] [PubMed] [Google Scholar]