Abstract
Objectives:
To investigate the effects of intensive blood pressure (BP) lowering according to baseline BP levels and optimal achieved BP levels in patients with acute intracerebral hemorrhage (ICH).
Methods:
INTERACT2 was an open, blinded endpoint, randomized controlled trial in 2,839 patients with ICH within 6 hours of onset and elevated systolic BP (SBP) (150–220 mm Hg) who were allocated to receive intensive (target SBP <140 mm Hg within 1 hour, with lower limit of 130 mm Hg for treatment cessation) or guideline-recommended (target SBP <180 mm Hg) BP-lowering treatment. Outcome was physical function across all 7 levels of the modified Rankin Scale at 90 days.
Results:
Analysis of the randomized comparisons showed that intensive BP lowering produced comparable benefits on physical function at 90 days in 5 subgroups defined by baseline SBP of <160, 160–169, 170–179, 180–189, and ≥190 mm Hg (p homogeneity = 0.790). Analyses of achieved BP showed linear increases in the risk of physical dysfunction for achieved SBP above 130 mm Hg for both hyperacute (1–24 hours) and acute (2–7 days) phases while modest increases were also observed for achieved SBP below 130 mm Hg.
Conclusions:
Intensive BP lowering appears beneficial across a wide range of baseline SBP levels, and target SBP level of 130–139 mm Hg is likely to provide maximum benefit in acute ICH.
Classification of evidence:
This study provides Class I evidence that the effect of intensive BP lowering on physical function is not influenced by baseline BP.
Acute intracerebral hemorrhage (ICH) is the most lethal and disabling type of stroke, affecting several million people worldwide each year,1–3 most of whom are residing in central and eastern Asia.1 Early blood pressure (BP) elevation is common after ICH,4,5 with multiple observational studies showing strong associations of increasing BP levels with hematoma growth6–8 and subsequent poor outcomes.7,9–13 The main phase of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial 2 (INTERACT2) demonstrated safety of early intensive BP lowering (target systolic BP [SBP] <140 mm Hg) on mortality and serious adverse events.14 Although the trial showed a nonsignificant 4% absolute treatment effect (p = 0.06) on the primary outcome of death or major disability, key secondary ordinal analysis of the primary outcome measure, the modified Rankin Scale (mRS),15 indicated improved functional outcomes with intensive BP lowering. Herein, we provide more detailed information about the effects of randomized treatment in relation to baseline BP, and report new observational analyses designed to identify the optimum BP target for maximum beneficial outcome.
METHODS
Primary research questions.
The primary research questions are (1) whether the effects of randomized intensive BP lowering on physical function are influenced by baseline SBP (Class I), and (2) what is the optimal achieved SBP level with the best physical function (Class IV) in acute ICH.
Trial design.
INTERACT2 was an international, multicenter, open, blinded endpoint, randomized controlled trial, the details of which are outlined elsewhere.14,16 Briefly, 2,839 patients with spontaneous ICH within 6 hours of onset and elevated SBP between 150 and 220 mm Hg were included from 21 countries. Excluded were patients with a definite indication for, or contraindication to, intensive BP-lowering treatment; a structural cause for the ICH; deep coma (scores 3–5 on the Glasgow Coma Scale) or massive hematoma with a poor prognosis; or if early surgery to evacuate the hematoma was planned.
Patients were centrally randomized to intensive or guideline-recommended BP-lowering treatment; the former involved administration of IV treatment and therapy with oral agents according to prespecified treatment protocols based on locally available agents, with a target SBP level of <140 mm Hg to be achieved within 1 hour after randomization and to be sustained for the next 7 days. An SBP of 130 mm Hg was regarded as the lower limit for the cessation of IV BP-lowering therapy. In patients assigned to guideline-recommended treatment, BP lowering was to be initiated if their SBP was higher than 180 mm Hg.
Measurements.
BP levels were recorded in the nonparetic arm in a supine position using an automated device or a manual sphygmomanometer with an appropriate size cuff. Baseline BP was measured twice with an interval of ≥2 minutes and the mean of the 2 measurements was used. Achieved postrandomization BP in the hyperacute phase was measured at 1, 6, 12, 18, and 24 hours postrandomization, and the means of these 5 measurements were calculated. Likewise, achieved postrandomization BP in the acute phase was measured twice daily during 2 to 7 days, and the means of these 12 measurements were calculated.
The outcome measures were physical function across 7 levels of the mRS,15 as determined by an ordinal analysis,17 and a poor outcome, defined as death or major disability (mRS scores 3–615) at 90 days postrandomization.
Standard protocol approvals, registrations, and patient consents.
The trial was registered at http://clinicaltrials.gov (NCT00716079). The study complied with the Declaration of Helsinki, ethics committee at each site approved the research protocol, and written informed consent was obtained from all participants or relevant surrogates.
Statistical analysis.
In the first part of the analysis, participants were divided into 5 subgroups defined by their baseline SBP (<160, 160–169, 170–179, 180–189, and ≥190 mm Hg). The effects of randomized treatment on physical function were estimated in each subgroup using proportional odds regression models. Likewise, the effects of randomized treatment on death or major disability were estimated using logistic regression models. The heterogeneity of treatment effects across the 5 baseline SBP groups were ascertained by adding interaction terms to the statistical models. Analyses were performed according to the principle of intention-to-treat.
The second part of the analysis evaluated the relationship of achieved postrandomization BP levels with outcomes. Proportional odds and logistic regression models were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) of physical function and death or major disability, adjusting for baseline variables of age, sex, region, time from onset to randomization, NIH Stroke Scale (NIHSS) score, hematoma volume and location, intraventricular extension, and randomized treatment. The associations between achieved postrandomization SBP levels as continuous variables and outcomes were estimated using linear splines with knots at 130, 140, 150, and 160 mm Hg. An achieved SBP of 130 mm Hg was taken as the reference point for estimation of ORs.
Two-sided p values were reported and p < 0.05 was considered statistically significant. The SAS version 9.3 (SAS Institute, Cary, NC) was used for the analysis.
RESULTS
Effects of randomized intensive BP lowering by baseline BP levels.
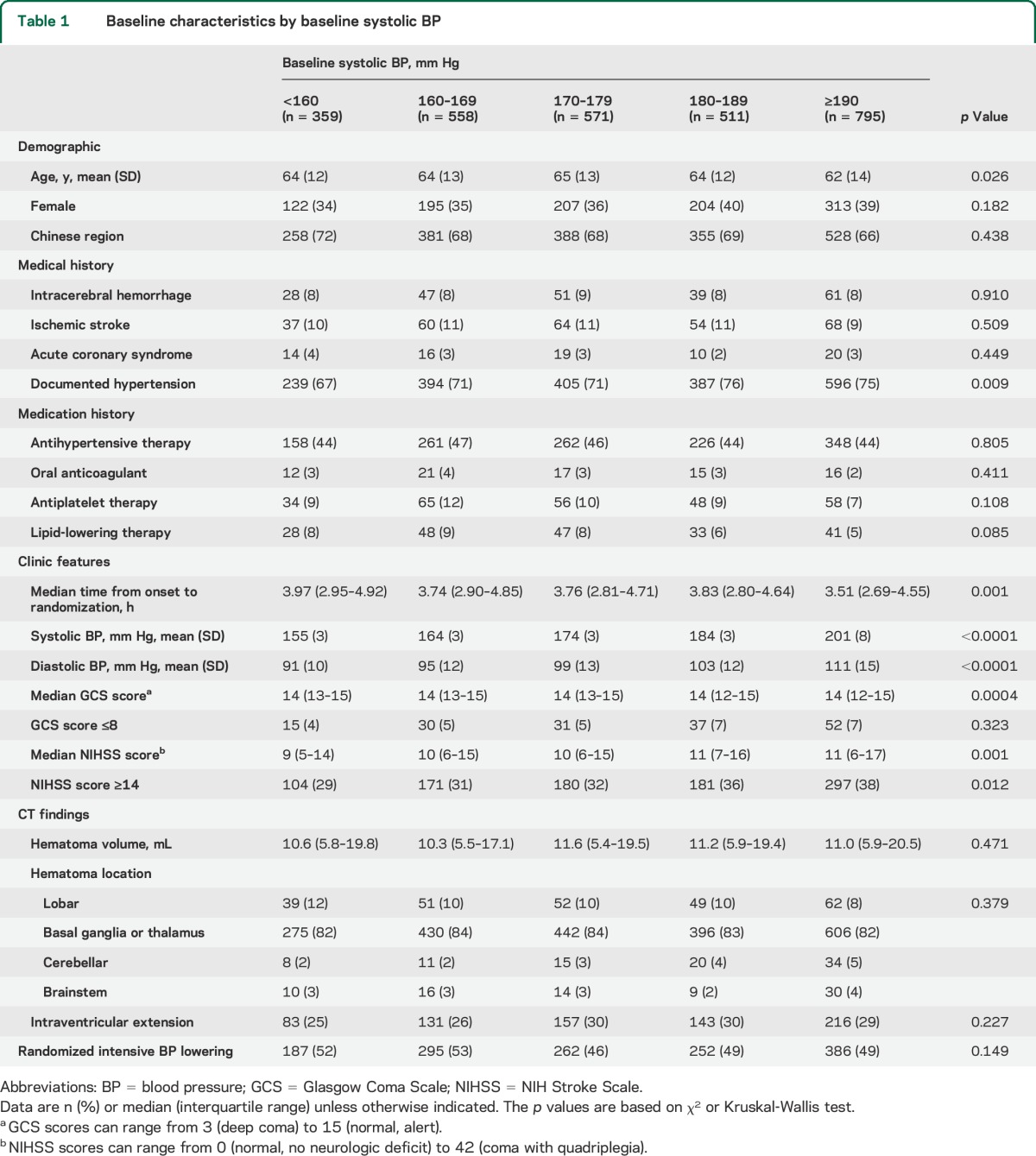
After exclusion of patients with missing information on outcomes, 2,794 (98.4%) were included in the analysis of the effects of randomized intensive BP lowering. Baseline characteristics of the 5 groups defined by baseline SBP of <160, 160–169, 170–179, 180–189, and ≥190 mm Hg are shown in table 1. Subjects with higher baseline SBP had higher NIHSS scores.
Table 1.
Baseline characteristics by baseline systolic BP
Achieved SBP levels in the hyperacute phase (1–24 hours) for the 5 baseline SBP groups were 137 (95% CI 135–138), 139 (137–140), 141 (139–142), 144 (142–146), and 147 (145–148) mm Hg, respectively, in the intensive group, and 145 (143–147), 150 (148–151), 154 (153–156), 156 (155–158), and 163 (161–164) mm Hg in the guideline group. The mean BP differences between randomized groups for the 5 baseline SBP groups were 9 (95% CI 7–12), 11 (9–13), 14 (12–16), 12 (10–15), and 16 (13–18) mm Hg, respectively (p = 0.002 for homogeneity). Likewise, achieved SBP levels in the acute phase (2–7 days) were 135 (95% CI 133–136), 137 (136–139), 140 (138–141), 142 (141–144), and 144 (143–146) mm Hg in the intensive group, and 142 (140–143), 144 (143–146), 148 (146–149), 150 (148–152), and 154 (153–156) mm Hg in the guideline group. The mean BP differences between randomized groups for the 5 baseline SBP groups were 8 (95% CI 5–10), 7 (5–9), 8 (6–10), 8 (6–10), and 10 (8–12) mm Hg, respectively (p = 0.463 for homogeneity).
There were comparable effects of intensive BP lowering on physical function (p = 0.790 for homogeneity; table e-1 on the Neurology® Web site at Neurology.org, and figure 1) and death or major disability (p = 0.934 for homogeneity; figure e-1) across the 5 subgroups defined by baseline SBP.
Figure 1. Effects of randomized intensive blood pressure–lowering treatment on modified Rankin Scale scores at 90 days by baseline SBP risk reductions were estimated using ordinal analyses.
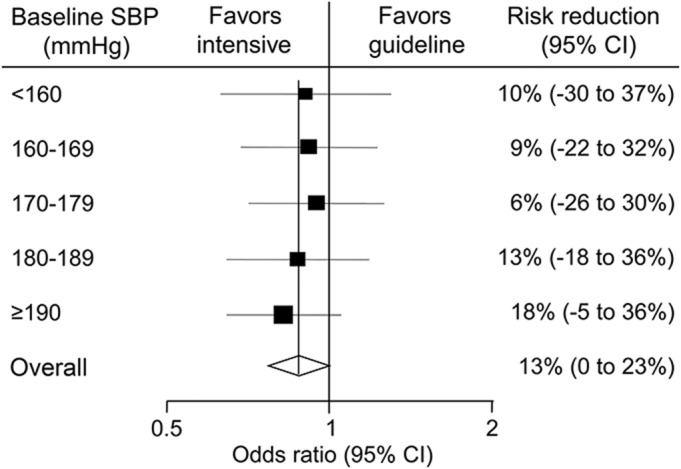
The p value for homogeneity, which tested the consistency of the treatment effects among subgroups, was 0.790. Solid boxes represent estimates of treatment effect on the risk of outcomes. Centers of the boxes are placed at the estimates of the effect; areas of the boxes are proportional to the reciprocal of the variance of the estimates. Horizontal lines represent 95% CIs. Diamonds represent estimates and 95% CI for overall effects in total subjects. CI = confidence interval; SBP = systolic blood pressure.
Associations of achieved postrandomization BP levels with outcomes.
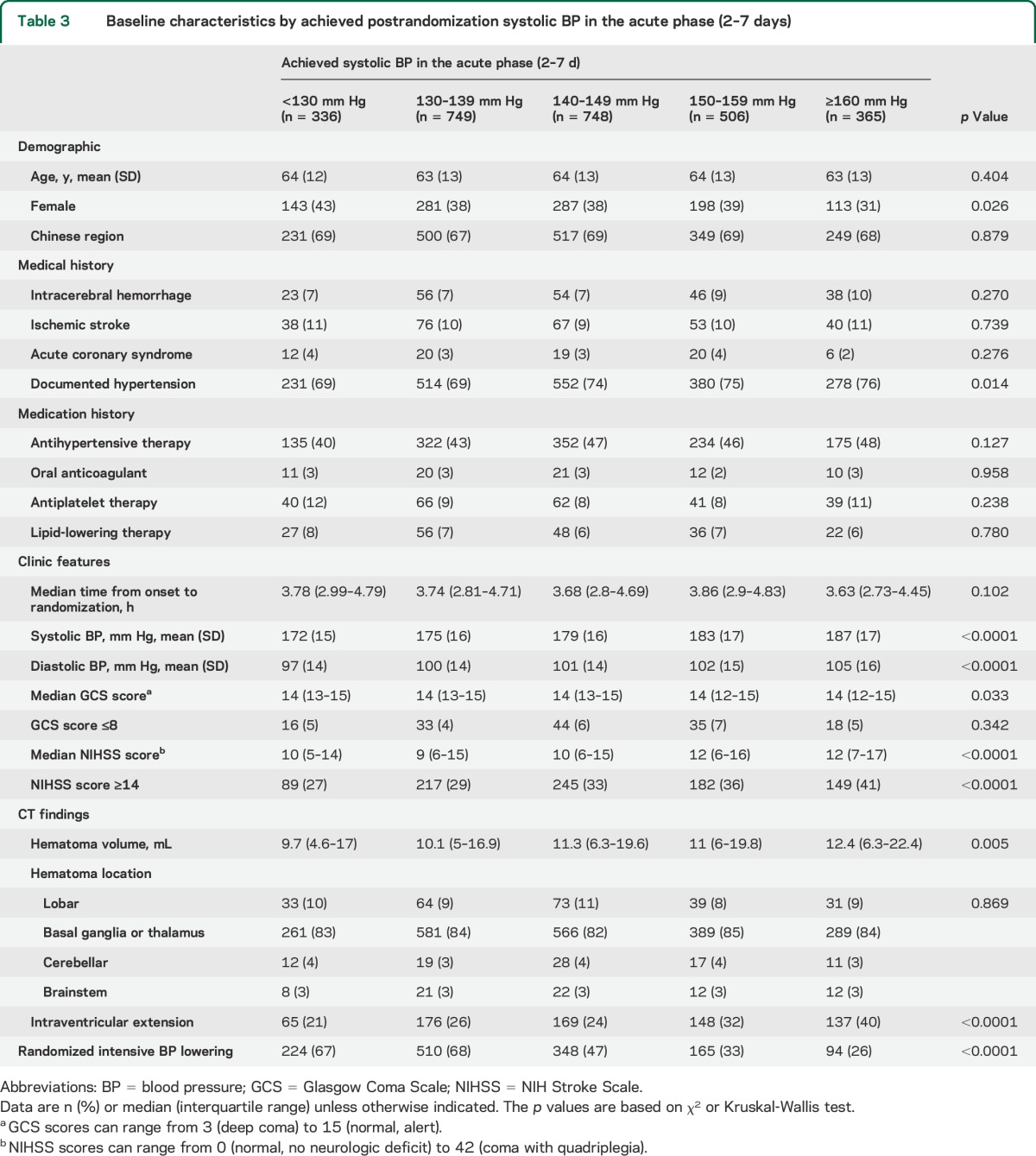
After further exclusion of patients with missing information on achieved postrandomization SBP or outcomes, 2,781 (98.0%) were included in the analysis of achieved SBP in the hyperacute phase (1–24 hours) and 2,704 (95.2%) were included in that in the acute phase (2–7 days). Baseline characteristics of participant groups defined by achieved SBP levels were summarized separately for the hyperacute phase (1–24 hours, table 2) and the acute phase (2–7 days, table 3). The participant subgroups with higher achieved SBP levels had higher NIHSS scores and were less likely to be assigned intensive BP lowering.
Table 2.
Baseline characteristics by achieved postrandomization systolic BP in the hyperacute phase (1–24 hours)
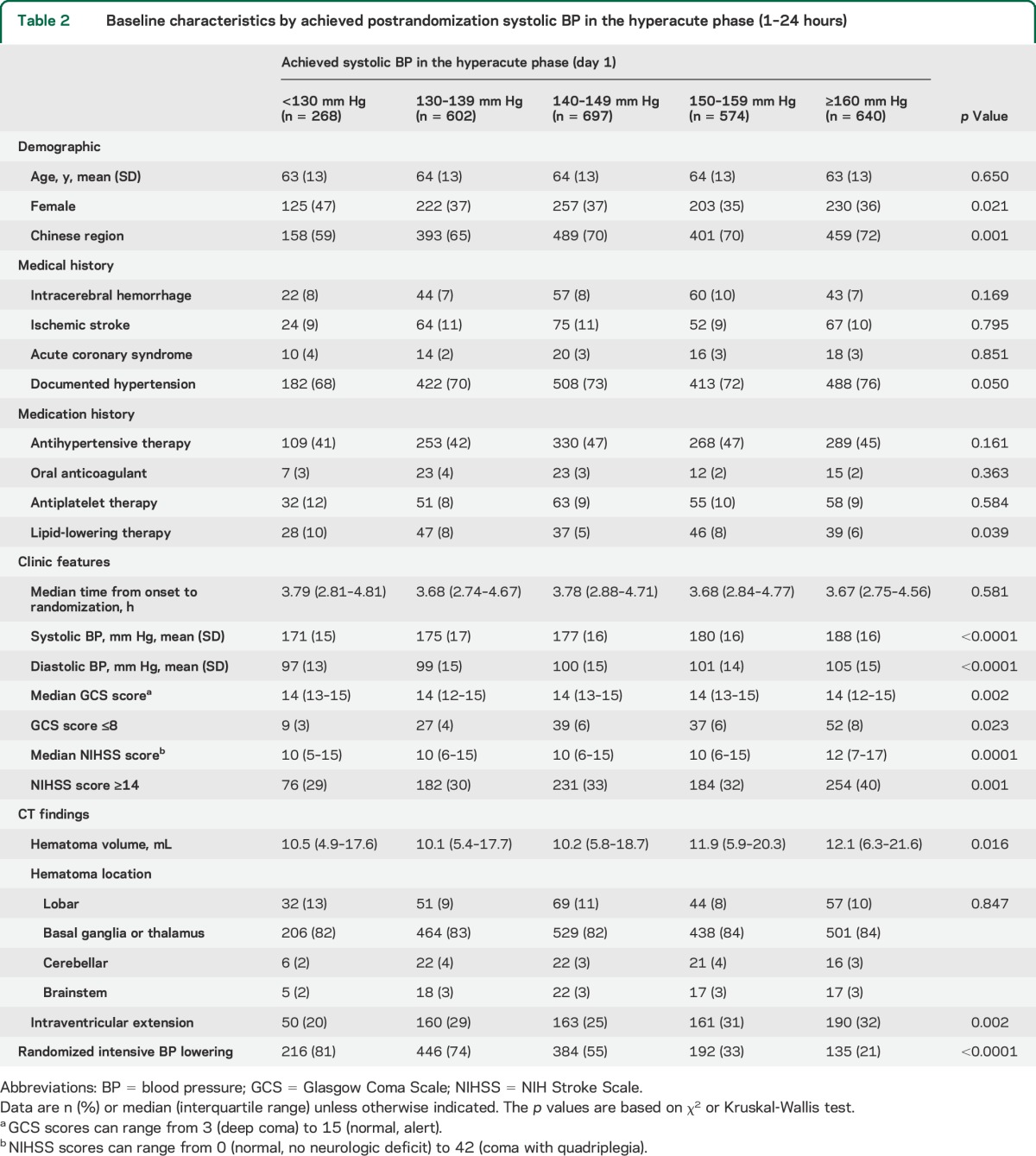
Table 3.
Baseline characteristics by achieved postrandomization systolic BP in the acute phase (2–7 days)
Estimates of adjusted ORs for physical function according to categories of achieved SBP in the hyperacute phase (1–24 hours) and the acute phase (2–7 days) are shown in figure 2. Risk of physical dysfunction increased fairly constantly in the range of achieved SBP of ≥130 mm Hg. Compared with achieved SBP of 130 mm Hg, adjusted ORs for poor outcome were 1.21 (95% CI 0.88–1.66, p = 0.242) and 0.94 (0.70–1.25, p = 0.663) at 140 mm Hg, 1.42 (1.07–1.89, p = 0.016) and 1.16 (0.89–1.52, p = 0.265) at 150 mm Hg, 1.57 (1.17–2.12, p = 0.003) and 1.47 (1.09–1.97, p = 0.011) at 160 mm Hg, 2.19 (1.66–2.88, p < 0.0001) and 1.88 (1.41–2.51, p < 0.0001) at 170 mm Hg, and 3.04 (2.21–4.17, p < 0.0001) and 2.42 (1.59–3.68, p < 0.0001) at 180 mm Hg for the hyperacute (1–24 hours) and the acute (2–7 days) phases, respectively. Compared with achieved SBP of 130 mm Hg, modest increase in the risk of death or major disability was seen at 120 mm Hg (OR 1.30 [95% CI 0.92–1.83], p = 0.144 for hyperacute, and OR 1.52 [1.14–2.02], p = 0.005 for acute phase). The p values for changes in slopes around knots were >0.05 for all knots. There were comparable associations of achieved SBP and poor outcomes between randomized groups (p homogeneity = 0.641 for the hyperacute phase and 0.487 for the acute phase). Similar associations were observed for the effects of achieved SBP in the hyperacute (1–24 hours, A) and the acute (2–7 days, B) phases on major disability (figure e-2). Likewise, there were broadly similar effects on physical function for diastolic BP (figure e-3), mean BP (figure e-4), achieved SBP at 1 hour (figure e-5), the lowest SBP during the first 24 hours (figure e-6), and in the area under the curve assessment of SBP during the first 24 hours (figure e-7).
Figure 2. Effects of achieved SBP on modified Rankin Scale score at 90 days.
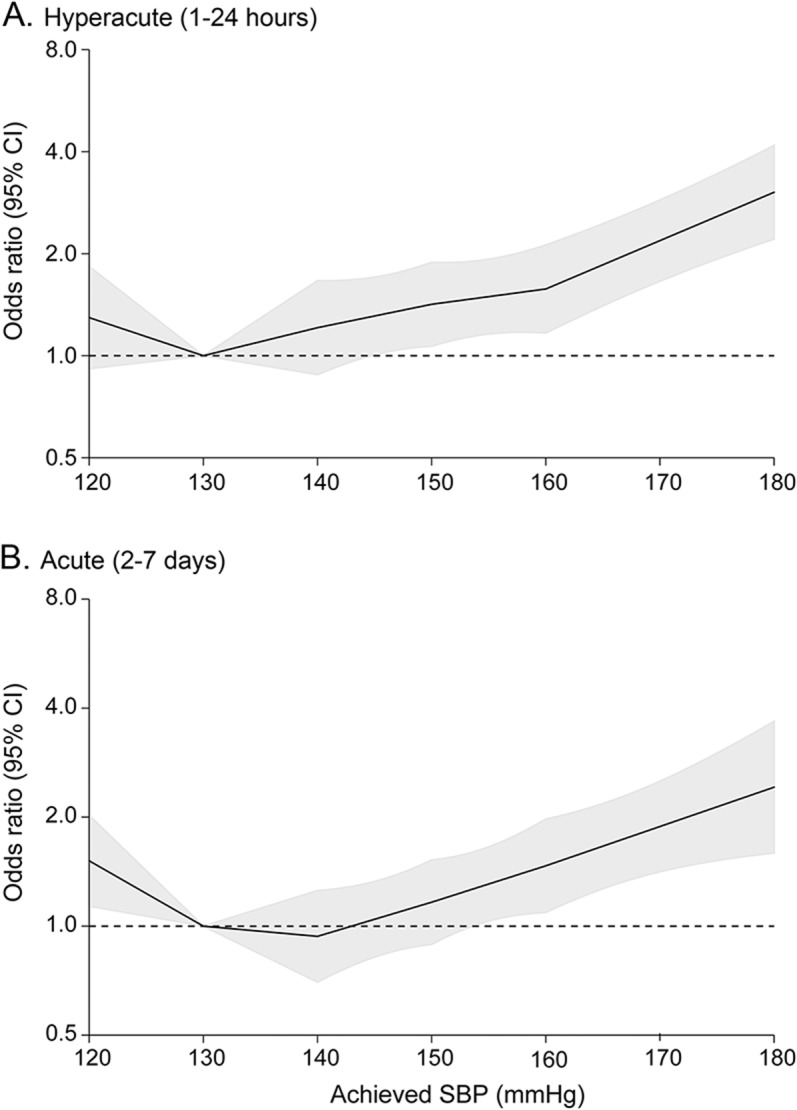
(A) 1–24 hours; (B) 2–7 days. Odds ratios and 95% CIs (shaded areas) were estimated using ordinal analyses and were shown according to achieved SBP after adjustment for age, sex, region, time from onset to randomization, NIH Stroke Scale score, volume and location of hematoma, intraventricular extension, and randomized treatment. The reference was achieved SBP of 130 mm Hg. CI = confidence interval; SBP = systolic blood pressure.
DISCUSSION
The main results of INTERACT2 showed that early intensive BP lowering toward a target SBP level of <140 mm Hg was safe and improved functional outcomes in acute ICH.14 In addition, a sensitivity analysis indicated that the benefits of the treatment were similar for patients with baseline SBP levels above and below ≥180 mm Hg.14 These subsequent analyses expand on these prior reports and suggest a net benefit of intensive BP lowering across a wide range of baseline SBP. The likely validity of these findings is supported by observational analyses of the relationship of achieved postrandomization BP with physical dysfunction, which clearly showed that the achieved SBP of approximately 130 mm Hg was associated with better outcomes. These results suggest that in patients with acute ICH, lowering SBP to 130–139 mm Hg, the per-protocol goal for the intensive treatment group in INTERACT2, is likely to be maximally beneficial.
In patients with acute ICH, the risk of death or disability has been shown to increase in the range of SBP above 130–140 mm Hg.7,12,13 However, a few studies propose a J-shaped association, whereby increased mortality was observed for both the highest and lowest SBP.9,11 A hospital-based study from Greece reported a modest increase in 1-year mortality among patients with admission SBP ≤140 mm Hg compared to those with 141–161 mm Hg. Another study from Japan demonstrated 35% increased risk of 30-day mortality among patients with admission SBP <150 mm Hg compared to those with 150–169 mm Hg. However, increased mortality observed in the lowest SBP level in these studies did not reach statistical significance. Regarding randomized evidence, the first Antihypertensive Treatment of Acute Cerebral Hemorrhage dose-escalation study demonstrated a higher mortality in patients who were assigned to the lowest treatment target SBP of 110–140 mm Hg, although relatively few subjects were studied.18 In the present analysis of INTERACT2, there was an approximately linear increase in the risk of physical dysfunction observed in the range of achieved SBP of 130 mm Hg or higher. INTERACT2 also suggests modest increases in the risks of physical dysfunction in the range of achieved SBP of <130 mm Hg, which is the protocol-defined level for cessation of IV BP-lowering treatment. However, this finding could represent confounding due to reverse causality, whereby severe ICH directly lowers BP as a preterminal event, because the current analysis of the effects of randomized treatment did not reveal such adverse effects of intensive BP lowering across a wide range of baseline SBP. On the totality of the current evidence, patients with acute ICH would have the lowest risks of physical dysfunction if their SBP could be controlled between 130 and 140 mm Hg.
One important mechanism underlying the beneficial effects of early intensive BP lowering is likely to be attenuation of hematoma growth.14,19 In fact, hematoma growth, most of which occurs in the first few hours after onset of ICH, has been shown to be a strong modifiable predictor of poor prognosis.20,21 In the present analysis, however, achieved BP levels in the acute phase (after 24 hours) were also associated with increased risks of physical dysfunction. These findings suggest that other effects of intensive BP lowering, such as reduction in cerebral edema, limiting further vascular injury, and minimizing the likelihood of early recurrence, may also have important roles in the observed improvement in functional outcomes.
Strengths of this study involve the large sample size and precise estimates of association. Furthermore, the wide range of patients who were included from many countries, along with the use of a range of BP-lowering regimens, supports the generalizability of these findings. Limitations include post hoc subgroup analyses that were not prespecified and selection bias from using a clinical trial population in which patients with a poor prognosis because of massive hematoma or deep coma, and patients in whom early surgery was planned, were excluded. In addition, the heterogeneity of treatments used creates uncertainty as to the most desirable agent and BP-lowering dosing protocol.
Intensive BP lowering appears to be beneficial across a wide range of baseline BP, and target SBP of 130–139 mm Hg is likely to provide maximum benefits in acute ICH.
Supplementary Material
GLOSSARY
- BP
blood pressure
- CI
confidence interval
- ICH
intracerebral hemorrhage
- INTERACT2
Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial 2
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- SBP
systolic blood pressure
Footnotes
Editorial, page 444
Supplemental data at Neurology.org
Contributor Information
Collaborators: C.S. Anderson, J. Chalmers, H. Arima, S. Davis, E. Heeley, Y. Huang, P. Lavados, B. Neal, M.W. Parsons, R. Lindley, L. Morgenstern, T. Robinson, C. Stapf, C. Tzourio, J.G. Wang, Y. Huang, S. Chen, X.Y. Chen, L. Cui, Z. Liu, C. Lu, J. Wang, S. Wu, E. Xu, Q. Yang, C. Zhang, J. Zhang, R. Beer, E. Schmutzhard, P. Redondo, M. Kaste, L. Soinne, T. Tatlisumak, C. Stapf, K. Wartenberg, S. Ricci, K. Klijn, E. Azevedo, A. Chamorro, M. Arnold, U. Fischer, S. Kaul, J. Pandian, H. Boyini, S. Singh, A.A. Rabinstein, C. Estol, G. Silva, P. Lavados, V.V. Olavarria, T.G. Robinson, R.J. Simes, M.-G. Bousser, G. Hankey, K. Jamrozik, S.C. Johnston, S. Li, E. Heeley, C.S. Anderson, K. Bailey, J. Chalmers, T. Cheung, C. Delcourt, S. Chintapatla, E. Ducasse, T. Erho, J. Hata, B. Holder, E. Knight, R. Lindley, M. Leroux, T. Sassé, E. Odgers, R. Walsh, Z. Wolfowicz, C.S. Anderson, G. Chen, C. Delcourt, S. Fuentes, R. Lindley, B. Peng, H.-M. Schneble, M.-X. Wang, H. Arima, L. Billot, S. Heritier, Q. Li, M. Woodward, C. Delcourt, S. Abimbola, S. Anderson, E. Chan, G. Cheng, P. Chmielnik, J. Hata, S. Leighton, J.-Y. Liu, B. Rasmussen, A. Saxena, S. Tripathy, M. Armenis, M.A. Baig, B. Naidu, G. Starzec, S. Steley, C.S. Anderson, E. Heeley, M. Leroux, C. Delcourt, T. Sassé, E. Knight, K. Bailey, T. Cheung, E. Odgers, E. Ducasse, B. Holder, Z. Wolfowicz, R. Walsh, S. Chintapatla, T. Erho, C. Estol, A. Moles, A. Ruiz, M. Zimmermann, J. Marinho, S. Alves, R. Angelim, J. Araujo, L. Kawakami, P. Lavados, V.V. Olavarria, C. Bustos, F. Gonzalez, P. Munoz Venturelli, Y. Huang, X. Chen, Y. Huang, R. Jia, N. Li, S. Qu, Y. Shu, A. Song, J. Sun, J. Xiao, Y. Zhao, J.-G. Wang, Q. Huang, C. Stapf, E. Vicaut, A. Chamam, M.-C. Viaud, C. Dert, U. Fiedler, V. Jovis, S. Kabla, S. Marchand, A. Pena, V. Rochaud, K. Mallikarjuna, H. Boyini, N. Hasan, E. Berge, E.C. Sandset, A. S. Forårsveen, T. Robinson, D. Richardson, T. Kumar, S. Lewin, N. Poulter, J. Field, A. Anjum, A. Wilson, H. Perelmuter, A.M. Agarie, A.G. Barboza, L.A. Recchia, I.F. Miranda, S.G. Rauek, R.J. Duplessis, H. Dewey, L. Walker, S. Petrolo, C. Bladin, J. Sturm, D. Crimmins, D. Griffiths, A. Schutz, V. Zenteno, M.W. Parsons, F. Miteff, N. Spratt, E. Kerr, C.R. Levi, T.G. Phan, H. Ma, L. Sanders, C. Moran, K. Wong, S. Read, R. Henderson, A. Wong, R. Hull, G. Skinner, S. Davis, P. Hand, B. Yan, H. Tu, B. Campbell, C.S. Anderson, C. Delcourt, D.J. Blacker, T. Wijeratne, M. Pathirage, M. Jasinararchchi, Z. Matkovic, S. Celestino, F. Gruber, M.R. Vosko, E. Diabl, S. Rathmaier, R. Beer, E. Schmutzhard, B. Pfausler, R. Helbok, F. Fazekas, R. Fischer, B. Poltrum, B. Zechner, U. Trummer, M.P. Rutgers, A. Peeters, A. Dusart, M.-C. Duray, C. Parmentier, S. Ferrao-Santos, R. Brouns, S. De Raedt, A. De Smedt, R.-J. Van Hooff, J. De Keyser, S.C.O. Martins, A.G. de Almeida, R. Broudani, N.F. Titton, G.R. de Freitas, F.M. Cardoso, L.M. Giesel, N.A. Lima Junior, A.C. Ferraz de Almeida, R.B. Gomes, T.S. Borges dos Santos, E.M. Veloso Soares, O.L.A. Neto, G.S. Silva, D.L. Gomes, F.A. de Carvalho, M. Miranda, A. Marques, V.F. Zétola, G. de Matia, M.C. Lange, J. Montes, A. Reccius, P. Munoz Venturelli, V.V. Olavarria, A. Soto, R. Rivas, C. Klapp, S. Illanes, C. Aguilera, A. Castro, C. Figueroa, J. Benavides, P. Salamanca, M.C. Concha, J. Pajarito, P. Araya, F. Guerra, Y. Li, G. Liu, B. Wang, J. Zhang, Y. Chong, M. He, L. Wang, J. Liu, X. Zhang, C. Lai, H. Jiang, Q. Yang, S. Cui, Q. Tao, Y. Zhang, S. Yao, M. Xu, Y. Zhang, Z. Liu, H. Xiao, J. Hu, J. Tang, J. Sun, H. Ji, M. Jiang, F. Yu, Y. Zhang, X. Yang, X. Guo, Y. Wang, L. Wu, Z. Liu, Y. Gao, D. Sun, X. Huang, Y. Wang, L. Liu, Y. Li, P. Li, Y. Jiang, H. Li, H. Lu, J. Zhou, C. Yuan, X. Qi, F. Qiu, H. Qian, W. Wang, J. Liu, Y. Huang, W. Sun, F. Li, R. Liu, Q. Peng, Z. Ren, C. Fan, Y. Zhang, H. Wang, T. Wang, F. Shi, C. Duan, S. Chen, J. Wang, Z. Chen, X. Tan, Z. Zhao, Y. Gao, J. Chen, T. Han, S. Wu, L. Zhang, L. Wang, Q. Hu, Q. Hou, X. Zhao, L. Wang, G. Zeng, L. Ma, F. Wang, S. Chen, L. Zeng, Z. Guo, Y. Fu, Y. Song, L. Tai, X. Liu, X. Su, Y. Yang, R. Dong, Y. Xu, S. Tian, S. Cheng, L. Su, X. Xie, T. Xu, D. Geng, X. Yan, H. Fan, N. Zhao, S. Wang, J. Yang, J. Zhang, M. Yan, L. Li, Z. Li, X. Xu, F. Wang, L. Wu, X. Guo, Y. Lian, H. Sun, D. Liu, N. Wang, Q. Tang, Z. Han, L. Feng, Y. Cui, J. Tian, H. Chang, X. Sun, J. Wang, C. Liu, Z. Wen, E. Xu, Q. Lin, X. Zhang, L. Sun, B. Hu, M. Zou, Q. Bao, X. Lin, L. Zhao, X. Tian, H. Wang, X. Wang, X. Li, L. Hao, Y. Duan, R. Wang, Z. Wei, J. Liu, S. Ren, H. Ren, Y. Wang, Y. Dong, Y. Cheng, M. Zou, W. Liu, J. Han, C. Zhang, Z. Zhang, J. Zhu, Y. Wang, Q. Li, J. Qian, Y. Sun, K. Liu, F. Long, X. Peng, Q. Zhang, Z. Yuan, C. Wang, M. Huang, J. Zhang, F. Wang, P. He, Y. You, X. Wang, Q. Yang, H. Wang, J. Xia, L. Zhou, Y. Hou, Y. Wang, L. Liu, Y. Qi, L. Mei, R. Lu, G. Chen, L. Liu, L. Ping, W. Liu, S. Zhou, J. Wang, L. Wang, H. Li, S. Zhang, L. Wang, R. Zou, J. Guo, M. Li, W. Wei, L. Soinne, S. Curtze, M. Saarela, D. Strbian, F. Scheperjans, T. De Broucker, C. Henry, R. Cumurciuc, N. Ibos-Augé, A.-C. Zéghoudi, F. Pico, O. Dereeper, M.-C. Simian, C. Boisselier, A. Mahfoud, S. Timsit, F.M. Merrien, B. Guillon, M. Sevin, F. Herisson, C. Magne, A. Ameri, C. Cret, S. Stefanizzi, F. Klapzcynski, C. Denier, M. Sarov-Riviere, C. Stapf, P. Reiner, J. Mawet, D. Hervé, F. Buffon, E. Touzé, V. Domigo, C. Lamy, D. Calvet, M. Pasquini, S. Alamowitch, P. Favrole, I.-P. Muresan, S. Crozier, C. Rosso, C. Pires, A. Leger, S. Deltour, C Cordonnier, H. Henon, C. Rossi, M. Zuber, M. Bruandet, R. Tamazyan, C. Join-Lambert, E. Juettler, T. Krause, S. Maul, M. Endres, G.J. Jungehulsing, M. Hennerici, M. Griebe, T. Sauer, K. Knoll, R. Huber, K. Knauer, C. Knauer, S. Raubold, H. Schneider, H. Hentschel, C. Lautenschläger, E. Schimmel, I. Dzialowski, C. Foerch, M. Lorenz, O. Singer, I.M. R. Meyer dos Santos, A. Hartmann, A. Hamann, A. Schacht, B. Schrader, A. Teíchmann, K.E. Wartenberg, T.J. Mueller, S. Jander, M. Gliem, C. Boettcher, M. Rosenkranz, C. Beck, D. Otto, G. Thomalla, B. Cheng, K.S. Wong, T.W. Leung, Y.O.Y. Soo, S. Prabhakar, S.R. Kesavarapu, P.K. Gajjela, R.R. K. Ummer, M. Basheer, A. Andipet, A.U.R. Mohammed, V.G. Pawar, S.S.K. Eranki, J. Pandian, Y. Singh, N. Akhtar, N.C. Borah, M. Ghose, N. Choudhury, N.R. Ichaporia, J. Shendge, S. Khese, V. Pamidimukkala, P. Inbamuthaiah, S.R. Nuthakki, N.M.R. Tagallamudi, A.K. Gutti, D. Khurana, P. Kesavarapu, V. Jogi, A. Garg, D. Samanta, G.R.K. Sarma, R. Nadig, T. Mathew, M.A. Anandan, E. Caterbi, A. Zini, M. Cavazzuti, F. Casoni, R. Pentore, F. Falzone, S. Ricci, T. Mazzoli, L.M. Greco, C. Menichetti, F. Coppola, S. Cenciarelli, E. Gallinella, A. Mattioni, R. Condurso, I. Sicilia, M. Zampolini, F. Corea, M. Barbi, C. Proietti, D. Toni, A. Pieroni, A. Anzini, A. Falcou, M. Demichele, C.J.M. Klijn, A. Tveiten, E.T. Thortveit, S. Pettersen, N. Holand, B. Hitland, S.H. Johnsen, A. Eltoft, A. Kamal, A. Iqrar, L. Ali, D. Begum, G. Gama, E. Azevedo, L. Fonseca, G. Moreira, L.M. Veloso, D. Pinheiro, L. Paredes, C. Rozeira, T. Gregorio, T. Segura Martin, O. Ayo, J. Garcia-Garcia, I. Feria Vilar, I. Gómez Fernández, A. Chamorro, S. Amaro, X. Urra, V. Obach, A. Cervera, Y. Silva, J. Serena, M. Castellanos, M. Terceno, C. Van Eendenburg, U. Fischer, M. Arnold, A. Weck, O. Findling, R. Lüdi, E.A. Warburton, D. Day, N. Butler, E. Bumanlag, S. Caine, A. Steele, M. Osborn, E. Dodd, P. Murphy, B. Esisi, E. Brown, R. Hayman, V. K.V. Baliga, M. Minphone, J. Kennedy, I. Reckless, G. Pope, R. Teal, K. Michael, D. Manawadu, L. Kalra, R. Lewis, B. Mistry, E. Cattermole, A. Hassan, L. Mandizvidza, J. Bamford, H. Brooks, C. Bedford, R. Whiting, P. Baines, M. Hussain, M. Harvey, K. Fotherby, S. McBride, P. Bourke, D. Morgan, K. Jennings-Preece, C. Price, S. Huntley, V.E. Riddell, G. Storey, R.L. Lakey, G. Subramanian, D. Jenkinson, J. Kwan, O. David, D. Tiwari, M. James, S. Keenan, H. Eastwood, L. Shaw, P. Kaye, D. Button, B. Madigan, D. Williamson, A. Dixit, J. Davis, M.O. Hossain, G.A. Ford, A. Parry-Jones, V. O'Loughlin, R. Jarapa, Z. Naing, C. Lovelock, J. O'Reilly, U. Khan, A. Bhalla, A. Rudd, J. Birns, D.J. Werring, R. Law, R. Perry, I. Jones, R. Erande, C. Roffe, I. Natarajan, N. Ahmad, K. Finney, J. Lucas, A. Mistri, D. Eveson, R. Marsh, V. Haunton, T. Robinson, A.A. Rabinstein, J.E. Fugate, and S.W. Lepore
AUTHOR CONTRIBUTIONS
H.A. contributed to the design of the study, analysis of data, and drafting the manuscript. Y.H. contributed to analysis of data. J.C. and C.S.A. contributed to the design of the study and drafting the manuscript. E.H., C.D., X.W., M.W., T.R., C.S., M.P., P.M.L., Y.H., and J.W. contributed to revising the manuscript.
STUDY FUNDING
The INTERACT2 study was supported by program grant (571281) and project grants (512402 and 1004170) from the National Health and Medical Research Council (NHMRC) of Australia. H.A. holds an Australian Research Council (ARC) Future Fellowship. C.S.A. holds an NHMRC Senior Principal Research Fellowship. All authors had full access to data.
DISCLOSURE
H. Arima reports grants from National Health and Medical Research Council of Australia during the conduct of the study. E. Heeley reports grants from National Health and Medical Research Council of Australia during the conduct of the study. C. Delcourt, Y. Hirakawa, and X. Wang report no disclosures relevant to the manuscript. M. Woodward reports personal fees from Novartis outside the submitted work. T. Robinson reports consultancy payments from Boehringer Ingelheim and Daiichi Sankyo, and his institution has received grant funding from the National Institute of Health Research, British Heart Foundation, Stroke Association, and the Engineering and Physical Sciences Research Council, on application where he is listed as principal or coapplicant. C. Stapf reports grants and nonfinancial support from The George Institute, Sydney, Australia, during the conduct of the study. M. Parsons reports no disclosures relevant to the manuscript. P. Lavados reports grants from The George Institute during the conduct of the study, personal fees from Bristol-Myers Squibb, atrial fibrillation and stroke advisory board, grants from Lundbeck unrestricted research grant, grants from The George Institute as an ENCHANTED regional leader, personal fees from AstraZeneca as SOCRATES national leader, grants from The George Institute as a HEADPOST main study coinvestigator, grants and nonfinancial support from Clínica Alemana de Santiago as a HEADPOST Pilot coinvestigator outside the submitted work. Y. Huang and J. Wang report no disclosures relevant to the manuscript. J. Chalmers reports grants from National Health and Medical Research Council of Australia during the conduct of the study, grants from Servier International outside the submitted work, and being a chief or cochief investigator for other large stroke trials, including the PROGRESS, INTERACT, and ENCHANTED trials. C. Anderson reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010), GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007;6:456–464. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66:1182–1186. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TG, Potter JF. Blood pressure after stroke. Age Ageing 2004;33:6–12. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007;25:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 1997;28:2370–2375. [DOI] [PubMed] [Google Scholar]
- 7.Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke 1997;28:1396–1400. [DOI] [PubMed] [Google Scholar]
- 8.Arima H, Anderson CS, Wang JG, et al. ; INTERACT Investigators. Lower treatment blood pressure is associated with greatest reduction in hematoma growth after acute intracerebral hemorrhage. Hypertension 2010;56:852–858. [DOI] [PubMed] [Google Scholar]
- 9.Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med 2004;255:257–265. [DOI] [PubMed] [Google Scholar]
- 10.Ohwaki K, Yono E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 2004;35:1364–1367. [DOI] [PubMed] [Google Scholar]
- 11.Okumura K, Ohya Y, Maehara A, Wakugami K, Iseki K, Takishita S. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens 2005;23:1217–1223. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Reilly KH, Tong W, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens 2008;26:1446–1452. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto Y, Koga M, Yamagami H, et al. Systolic blood pressure after intravenous antihypertensive treatment and clinical outcomes in hyperacute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement–Intracerebral Hemorrhage Study. Stroke 2013;44:1846–1851. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CS, Heeley E, Huang Y, et al. ; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 15.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [DOI] [PubMed] [Google Scholar]
- 16.Delcourt C, Huang Y, Wang JG, et al. ; INTERACT2 Investigators. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT2). Int J Stroke 2010;5:110–116. [DOI] [PubMed] [Google Scholar]
- 17.Bath PM, Lees KR, Schellinger PD, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke 2012;43:1171–1178. [DOI] [PubMed] [Google Scholar]
- 18.Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) Investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med 2010;38:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson CS, Huang Y, Wang JG, et al. Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;7:391–399. [DOI] [PubMed] [Google Scholar]
- 20.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 21.Delcourt C, Huang Y, Arima H, et al. ; INTERACT1 Investigators. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 Study. Neurology 2012;79:314–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


