Abstract
Objective:
To investigate whether an increasing load of β-amyloid and/or neuritic plaques influences the phenotype, and thus the clinical diagnostic accuracy, of dementia with Lewy bodies (DLB).
Methods:
A series of 64 subjects with autopsy-proven DLB was studied. Last diagnosis before death was used to determine the clinical diagnostic accuracy of DLB in relation to Lewy body distribution and extent of Alzheimer β-amyloid and/or neuritic pathology. DLB pathologic diagnosis was made according to consensus criteria, using α-synuclein immunostaining for Lewy body identification. β-Amyloid immunostaining was used for quantifying β-amyloid deposits. The Consortium to Establish a Registry for Alzheimer's Disease criteria and Braak stage were applied for semiquantitative grading of neuritic plaque and neurofibrillary tangle pathology.
Results:
Overall clinical diagnostic accuracy for the entire DLB cohort was high (80%), reflecting the high prevalence of core clinical features (fluctuations [81%], parkinsonism [77%], visual hallucinations [70%]). Lower frequencies of core clinical features of DLB, resulting in lower accuracy of its clinical diagnosis, were associated with decreasing Lewy body distribution (p < 0.0001) and with increasing neuritic plaque pathology (p = 0.035), but not with the number of β-amyloid plaque deposits.
Conclusions:
The likelihood of occurrence of the DLB clinical syndrome is positively related to the extent of Lewy body pathology and negatively related to the severity of Alzheimer neuritic pathology, while β-amyloid load has no effect.
Dementia with Lewy bodies (DLB) is the most common form of dementia in the elderly after Alzheimer disease (AD). Besides cognitive impairment, the consortium on DLB originally described 3 core clinical features of DLB (fluctuations, visual hallucinations [VHs], and spontaneous parkinsonism),1 but perhaps because these features are not always correctly recognized, sensitivity of clinical diagnosis in clinicopathologic studies has, with one notable exception,2 been poor.3,4 Although the subsequent addition of REM behavior disorder in the diagnostic algorithm has further improved the identification of DLB,5 its differentiation from AD is still a challenge, even for experienced clinicians.
Pathologic diagnosis of DLB requires the presence of Lewy bodies (LBs), but also permits other pathologies, including Alzheimer changes in the form of neuritic and amyloid plaques and neurofibrillary tangles.1 Because such heterogeneous pathologic features are allowed in the pathologic diagnosis of DLB, it has been hypothesized, and successfully demonstrated, that the extent of concurrent AD neurofibrillary pathology can obscure the typical clinical characteristics of DLB, making its recognition more difficult.3 Whether an increasing amyloid and/or neuritic plaque load has the same masking effect on the DLB clinical phenotype is unclear. The aim of this study was therefore to investigate the possible confounding effects of the 2 types of plaque pathology in DLB.
METHODS
Subjects.
The patients included in this series were followed clinically at the University of Newcastle and represent all cases who came to autopsy between 1989 and 2011 with a clinical diagnosis of dementia (DSM-III-R, DSM-IV, or DSM-IV-TR) and a pathologic diagnosis of DLB (n = 89). Of these cases, 22 were excluded because of the concomitance of other confounding pathologic diagnoses (cerebrovascular disease [n = 14], hippocampal sclerosis [n = 2], large tumors [n = 2], progressive supranuclear palsy [n = 2], frontotemporal degeneration (n = 1), and corticobasal degeneration [n = 1]), and 3 were excluded because of insufficient clinical data. Thus, 64 subjects (52% males) with autopsy-proven DLB were included in final analyses. Pathologically, all DLB cases had brainstem and cortical LBs. Cases with LBs restricted to the amygdala were not regarded as DLB. Based on LB distribution, 2 had brainstem LB disease (LBD), 9 had limbic LBD, and the remaining 53 were labeled as having diffuse neocortical LBD. Fifty-one (80%) of the DLB cases also had moderate to frequent β-amyloid deposits, but only 23 (36%) had enough neuritic plaques to meet CERAD (Consortium to Establish a Registry for Alzheimer's Disease) criteria for AD and only 10 (16%) had high Braak neurofibrillary tangle stages (5–6).
Subjects had been evaluated annually with medical, neurologic, neuropsychological, and laboratory examinations. Each of the 3 DLB core clinical features (VHs, parkinsonism, and fluctuations) was rated as present or absent on the basis of its appearance at any point along the disease course. Clinical methods used for their identification have been described previously.2
For analysis of overall clinical diagnostic accuracy, the last clinical diagnosis before death, as agreed by 2 independent assessors, was recorded for all 64 subjects. For subsequent analyses, the subjects with autopsy-proven DLB were stratified by the extent of α-synuclein pathology, number of β-amyloid deposits, neuritic plaque density, and Braak stage. The clinical diagnostic accuracy for subjects stratified according to these pathologic characteristics was determined.
Neuropathologic methods.
Neuropathologic methods used to diagnose DLB and AD cases and to quantify plaques, tangles, and LBs have been described previously.6 Based on DLB consortium recommendations,5 LBs were additionally quantified by α-synuclein immunostaining (antibody NCL-L-ASYN). Neocortical β-amyloid deposits were quantified using BA4 immunohistochemistry (antibody BA4 [M0872]).7 Each brain was also staged for the degree of neuritic plaque pathology according to CERAD criteria,8 using the Gallyas silver technique, and for the degree of neurofibrillary pathology by the method of Braak,9 using AT8 antibody.
Statistical analysis.
Group comparisons were performed using Student t test for continuous data and Fisher exact test for categorical data (software JMP Pro, v.11.2.0; SAS Institute Inc., Cary, NC). The significance level was set at p < 0.05.
Standard protocol approvals, registrations, and patient consents.
This retrospective clinical-pathologic study was approved by the local ethics committee and appropriate informed consent was obtained for brain autopsy.
RESULTS
Eighty percent of the subjects were correctly clinically diagnosed as having DLB, whereas 17% were diagnosed with AD and 3% with mixed dementia (AD with additional cerebrovascular disease). Demographic and clinical characteristics for subjects clinically diagnosed with DLB vs those diagnosed with AD are summarized in table 1. They did not differ significantly in any of the demographic variables, but the subjects diagnosed with AD had a considerably lower frequency of core clinical features.
Table 1.
Demographic and clinical features of DLB cohort with clinical diagnoses of DLB or AD
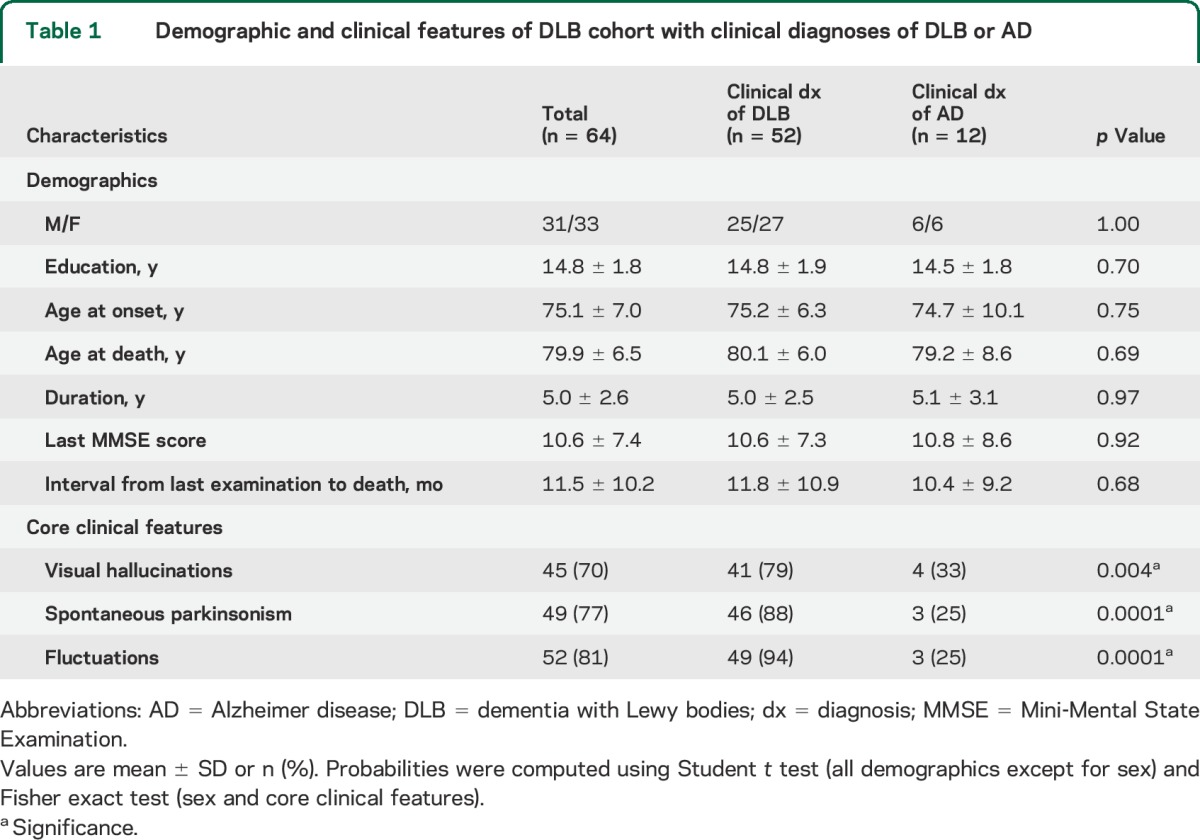
Overall, spontaneous parkinsonism was exhibited by 77% of the patients, while fluctuations and VHs were respectively reported for 81% and 70% of the patients (table 1). Four-fifths of the subjects had at least 2 core features and approximately half displayed all of the 3 core features. Only 7% of the subjects had no core features. Notably, this minority of cases were all pathologically diagnosed with brainstem or limbic LBD, which implies relatively restricted LB distributions.
After stratifying patients for the extent of α-synuclein pathology, clinical diagnostic accuracy improved as LB distribution increased (p < 0.0001). Ninety-two percent of the patients with diffuse neocortical LBD (n = 53) were correctly clinically diagnosed with DLB compared with only 36% of those with brainstem or limbic LBD (n = 11).
After stratifying patients for the number of β-amyloid deposits, there were no significant group differences in either the frequency of core clinical features or accuracy of clinical diagnosis (table 2A).
Table 2.
Influence of Alzheimer pathology on clinical diagnostic accuracy in DLB

Conversely, when the patients with DLB were stratified by neuritic plaque density, the frequency of core clinical features was lower in those with moderate to frequent neuritic plaques than in those with no or sparse neuritic plaques (fluctuations 50% vs 87%, VHs 40% vs 76%, spontaneous parkinsonism 40% vs 81%), resulting in a lower clinical diagnostic accuracy with increasing neuritic plaque density (table 2B). Clinical diagnostic accuracy also decreased with increasing Braak stage, but this relationship did not reach statistical significance (table 2C).
DISCUSSION
The aim of the present study was to examine to what extent concomitant AD neuritic and β-amyloid pathologies influenced the clinical characteristics, and consequently the clinical diagnostic accuracy, of DLB. Despite the recent publication of several clinical-pathologic studies on the field,10 evaluation of clinical diagnostic accuracy of DLB in relation to β-amyloid deposits has not, to our knowledge, been described previously.
Several significant points emerge from this study. First, consistent with a prior study from our institution,2 only one-fifth of our pathologically proven DLB cases went clinically unrecognized.
Second, the likelihood that the typical DLB phenotype was fully expressed during the disease course was positively related to the extent of LB pathology and weakly negatively related to the severity of AD neuritic pathology. In our series, LB distribution emerged as the primary predictor because, in the presence of extensive α-synuclein pathology (diffuse neocortical LBD), clinical diagnostic accuracy of DLB remained high (≥75%) even with advanced neuritic/neurofibrillary pathology (C neuritic plaque score; Braak stage 6).
Unlike others,3 we did not find a significant influence of Braak stage on clinical accuracy, but this failure may have several explanations, including an overall lower rate of misdiagnosis and a relatively smaller sample size. Our observation of an inverse relationship between neuritic plaque density and the likelihood of the DLB clinical syndrome remains nonetheless consistent with the notion that concomitant AD neuritic pathology influences the clinical characteristics, and thus the clinical diagnostic accuracy, of DLB.
Finally, the extent of β-amyloid pathology had no confounding effect on DLB clinical phenotype, as indicated by the high concordance (≥75%) between the clinical and pathologic diagnoses of DLB at any level of β-amyloid deposition.
The molecular relationship between LB and AD-type pathology remains obscure. It may be that β-amyloid deposition is the primary event in both AD and DLB and that preferential downstream effects are either tau aggregation, resulting in a clinical phenotype more like that of AD, or α-synuclein aggregation, resulting in a clinical phenotype more like that of DLB. In some individuals, these overlapping pathologies result in an ambiguous clinical profile. This study suggests that superimposed neuritic plaques can obscure the clinical characteristics of DLB, making its recognition more difficult, but diffuse β-amyloid plaques do not have this confounding effect.
GLOSSARY
- AD
Alzheimer disease
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- DLB
dementia with Lewy bodies
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision)
- LB
Lewy body
- LBD
Lewy body disease
- VH
visual hallucination
AUTHOR CONTRIBUTIONS
Study concept and design: Tiraboschi, McKeith. Data acquisition: Tiraboschi, Brown, Jaros, Lett, Ossola, Perry, Ramsay, Walker. Data analysis/interpretation: Tiraboschi, Attems, Brown, Lett, Ossola, Ramsay, Walker, McKeith. Manuscript preparation: Tiraboschi. Manuscript critical revision for important intellectual content: Tiraboschi, Attems, Thomas, Jaros, Perry, McKeith. Study supervision: Tiraboschi, McKeith.
STUDY FUNDING
The current study was supported by National Institute for Health Research (NIHR) Newcastle Biomedical Research Unit and Italian Minister of Health (Ricerca Corrente).
DISCLOSURE
P. Tiraboschi receives research funding from the Italian Minister of Health (Ricerca Corrente). J. Attems receives research funding from the Medical Research Council (MRC) and the Dunhill Medical Trust. A. Thomas receives research funding from the MRC, NIHR, and Alzheimer's Society and Alzheimer's Brain Bank UK. He also receives support from GE Healthcare for an investigator-led imaging study. A. Brown, E. Jaros, D. Lett, M. Ossola, R. Perry, L. Ramsay, and L. Walker report no disclosures relevant to the manuscript. I. McKeith receives research funding from the MRC and NIHR, and consultancy fees from GE Healthcare and Nutricia. Go to Neurology.org for full disclosures.
REFERENCES
- 1.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Ballard CG, Perry RH, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050–1058. [DOI] [PubMed] [Google Scholar]
- 3.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 2003;60:1586–1590. [DOI] [PubMed] [Google Scholar]
- 4.Verghese J, Crystal HA, Dickson DW, Lipton RB. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology 1999;53:1974–1982. [DOI] [PubMed] [Google Scholar]
- 5.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 6.Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type: a clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci 1990;95:119–139. [DOI] [PubMed] [Google Scholar]
- 7.Bruce CV, Clinton J, Gentleman SM, Roberts GW, Royston MC. Quantifying the pattern of beta/A4 amyloid protein distribution in Alzheimer's disease by image analysis. Neuropathol Appl Neurobiol 1992;18:125–136. [DOI] [PubMed] [Google Scholar]
- 8.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Halliday G. Can we clinically diagnose dementia with Lewy body yet? Transl Neurodegener 2013;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]


