Abstract
Objective:
To assess, in a surgical biopsy cohort of active demyelinating lesions, the diagnostic utility of aquaporin-4 (AQP4) immunohistochemistry in identifying neuromyelitis optica (NMO) or NMO spectrum disorder (NMOSD) and describe pathologic features that should prompt AQP4 immunohistochemical analysis and AQP4–immunoglobulin G (IgG) serologic testing.
Methods:
This was a neuropathologic cohort study of 20 surgical biopsies (19 patients; 11 cord/9 brain), performed because of diagnostic uncertainty, interpreted as active demyelinating disease and containing 2 or more of the following additional features: tissue vacuolation, granulocytic infiltrates, or astrocyte injury.
Results:
AQP4 immunoreactivity was lost in 18 biopsies and increased in 2. Immunopathologic features of the AQP4 loss cohort were myelin vacuolation (18), dystrophic astrocytes and granulocytes (17), vascular hyalinization (16), macrophages containing glial fibrillary acid protein (GFAP)–positive debris (14), and Creutzfeldt-Peters cells (0). All 14 cases with available serum tested positive for AQP4-IgG after biopsy. Diagnosis at last follow-up was NMO/NMOSD (15) and longitudinally extensive transverse myelitis (1 each relapsing and single). Immunopathologic features of the AQP4 increased cohort were macrophages containing GFAP-positive debris and granulocytes (2), myelin vacuolation (1), dystrophic astrocytes (1), Creutzfeldt-Peters cells (1), and vascular hyalinization (1). Diagnosis at last follow-up was multiple sclerosis (MS) and both tested AQP4-IgG seronegative after biopsy.
Conclusions:
AQP4 immunohistochemistry with subsequent AQP4-IgG testing has diagnostic utility in identifying cases of NMO/NMOSD. This study highlights the importance of considering NMOSD in the differential diagnosis of tumefactive brain or spinal cord lesions. AQP4-IgG testing may avert biopsy and avoid ineffective therapies if these patients are erroneously treated for MS.
Neuromyelitis optica (NMO) is an autoimmune disease characterized by recurrent optic neuritis (ON) and transverse myelitis (TM). A circulating autoantibody (aquaporin-4 [AQP4]–immunoglobulin G [IgG]) distinguishes NMO from multiple sclerosis (MS).1 AQP4-IgG targets astrocytes, binding to the ectodomain of AQP4,2 the principal CNS water channel, resulting in primary astrocyte damage and secondary demyelination.3,4 Although originally considered a disease confined to optic nerves and spinal cord, a broad spectrum of clinical disorders referred to as NMO spectrum disorders (NMOSD) are now recognized, unified by detection of serum or CSF AQP4-IgG.5–10
Historically, a negative brain MRI was typical of NMO.11–13 However, 60% of NMO patients show MRI brain abnormalities,12 often in regions enriched for AQP4.14,15 Brain lesions may be the initial presentation and tumefactive in nature.13,16,17
Neuropathologic features at autopsy distinguishing NMO from MS include astrocytic damage, granulocytes, vascular hyalinization, vasculocentric complement deposition, myelin vacuolation, and AQP4 loss in active demyelinating lesions.3,4,18–20 Although AQP4-IgG supports NMO/NMOSD diagnosis, NMO/NMOSD may not be considered in patients presenting with mass-like or atypical lesions, leading to biopsy typically to exclude tumor.21 When the NMO/NMOSD diagnosis eludes the neurologist, the neuropathologist may suggest this diagnosis based on biopsy findings.
We describe pathologic features and clinical course in patients who underwent brain or spinal cord biopsy that was initially interpreted as active demyelinating disease, but who subsequently were clinically or serologically diagnosed with NMO/NMOSD. The recognition of specific pathologic features suggestive of NMO on biopsies should prompt AQP4 immunohistochemistry and AQP4-IgG serologic testing as part of the diagnostic workup.
METHODS
Standard protocol approvals, registrations, and patient consents.
This cohort study was approved by the Mayo Clinic Institutional Review Board (IRB 2067-99) and the University Medical Center Göttingen ethical review committee (19-9-10). Brain biopsies were obtained within the context of routine clinical care, and consent was obtained by the treating physician. No individual underwent surgery for research purposes.
Study cohort.
Inclusion criteria were (1) surgical spinal cord or brain biopsy with a pathologic diagnosis of active inflammatory demyelinating disease confirmed by a Mayo neuropathologist; (2) sufficient tissue (≥1 mm2); (3) reliable tissue staining; (4) presence of active demyelinating lesions as defined by the presence of macrophages containing myelin debris immunoreactive for myelin proteins (proteolipid protein [PLP], myelin oligodendrocyte glycoprotein [MOG], or myelin-associated glycoprotein [MAG]; figure 1)22; and (5) at least 2 of 3 neuropathologic features considered unusual for inflammatory demyelination consistent with MS: (a) tissue vacuolation23; (b) granulocytic parenchymal or perivascular inflammatory infiltrates19; or (c) evidence of astrocyte injury (dystrophic astrocytes defined as small glial fibrillary acid protein [GFAP]–positive astrocytes with few short and blunted processes,18 or macrophages containing GFAP-positive degradation products20).
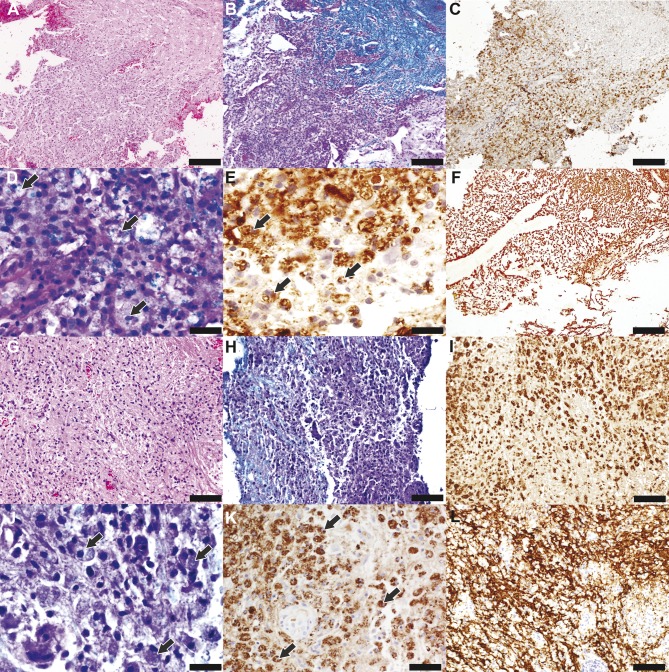
Figure 1. General histopathologic features in neuromyelitis optica and multiple sclerosis biopsies.
(A–F) Neuromyelitis optica (NMO). (G–L) Multiple sclerosis (MS). (A) Examination of the NMO spinal cord biopsy reveals a hypercellular lesion and a region of normal-appearing white matter (upper right corner) (hematoxylin & eosin [H&E], scale bar = 200 µm). (B) The lesion is demyelinated (Luxol fast blue [LFB]/periodic acid–Schiff [PAS], scale bar = 200 µm) and (C) infiltrated by activated macrophages (CD68, scale bar = 200 µm). (D, E) Macrophages within the demyelinated lesion contain (D) LFB-positive (arrows) (LFB/PAS, scale bar = 33 µm) and (E) proteolipid protein (PLP)–positive (arrows) (scale bar = 33 µm) myelin degradation products consistent with active demyelination. (F) Axons are preserved within the demyelinating lesion (neurofilament protein [NF], scale bar = 200 µm). (G) The MS brain biopsy reveals a hypercellular lesion (H&E, scale bar = 100 µm). (H) Myelin loss is evident (LFB/PAS, scale bar = 100 µm) with (I) macrophages/microglia (CD68, scale bar = 100 µm) containing (J) LFB-positive (arrows) (LFB/PAS, scale bar = 33 µm) and (K) PLP-positive (arrows) (scale bar = 50 µm) myelin degradation products consistent with active demyelination. (L) Axons are preserved within the lesion (NF, scale bar = 100 µm).
In the context of Mayo Clinic's extramural consultative pathologic practice, 20 biopsies (19 patients) were diagnosed with active inflammatory demyelination by a Mayo neuropathologist over the past 4 years and met study inclusion. A single patient had 2 biopsies (1 brain/1 spinal cord). Eleven of the 20 biopsies were from spinal cord (6 cervical, 3 thoracic, 2 not specified), and 9 from brain (3 parietal, 1 occipital, 1 temporal, 2 frontal, 1 cerebellum, and 1 not specified). Five biopsies were initially misinterpreted at the local institutions as spinal cord tumor (1), arteriovenous malformation (1), brain astrocytoma (1), or nondiagnostic (2).
Histopathology.
Initial biopsy diagnostic pathologic evaluation involved histochemical staining of formalin-fixed paraffin-embedded sections with hematoxylin & eosin, Luxol fast blue (LFB)/periodic acid–Schiff or LFB/hematoxylin & eosin, and Bielschowsky silver impregnation and immunohistochemical staining by avidin–biotin or alkaline phosphatase/antialkaline phosphatase technique without modification,24 using primary antibodies specific for GFAP (mouse monoclonal 1:4,000; Dako Denmark, Glostrup), neurofilament protein (mouse monoclonal 1:800; Dako Denmark), T lymphocytes (CD3, rat monoclonal 1:400; Serotec, Oxford, UK), cytotoxic T lymphocytes (CD8, mouse monoclonal 1:50; Dako Denmark), B lymphocytes (CD20, mouse monoclonal; Dako Denmark), plasma cells (CD138, mouse monoclonal; Dako Denmark), and macrophages/microglial cells (CD68, mouse monoclonal 1:100; Dako Denmark).
Additional immunohistochemical analyses on a research basis were performed using primary antibodies specific for PLP (rabbit polyclonal 1:500; Serotec), 2′3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase, mouse monoclonal 1:2,000; Covance, MD), MOG (mouse monoclonal 1:200; gift from Dr. Sarah Piddlesden, Cardiff, UK), MAG (mouse monoclonal 1:10; Chemicon, Billerica, MA), macrophages/microglial cells (KiM1P, mouse monoclonal 1:1,000; gift from Dr. Radzun, University of Göttingen, Germany), and 3 different anti-C9neo antibodies (complement C9neo antigen, activated terminal complement: [1] clone B7; [2] pc, directed against human complement; and [3] pc, directed against rat complement) (1:200; antibodies from Prof. Morgan, Cardiff, UK).
Because AQP4 loss or decrease in active demyelinating lesions is a neuropathologic hallmark of NMO/NMOSD and can distinguish in autopsy tissues NMO/NMOSD from MS in which AQP4 is increased,3,4,25 we performed AQP4 immunohistochemistry to determine its pattern of immunoreactivity (affinity-purified rabbit polyclonal 1:250; Sigma-Aldrich, St. Louis, MO). Tissues were exposed to primary antibodies overnight, at 4°C. Primary antibodies were omitted in control staining. Antigen retrieval was performed as previously described.4,19
Clinical characteristics.
Clinical information was obtained via multiple sources: medical record review (n = 20), face-to-face encounter and examination (n = 10), and family or physician contact (n = 9). The following details were recorded: sex, age at disease onset and biopsy, presenting symptoms, date of relapses, nature and timing of relapses relative to biopsy (i.e., unilateral or bilateral ON, TM, intractable nausea, vomiting or hiccups, brain syndrome), disease duration, diagnosis at last follow-up according to established criteria (i.e., NMO, NMOSD, MS, or other), and if applicable, date of death.
Serologic AQP4-IgG testing.
No patient was tested for AQP4-IgG prior to biopsy. Postbiopsy testing was performed by the Mayo Neuroimmunologic laboratory in 16 patients meeting inclusion criteria by mouse tissue-based indirect immunofluorescence, ELISA, or AQP4-transfected cell-binding assay (Euroimmun; Lübeck, Germany).
RESULTS
AQP4 immunohistochemistry.
Autopsy studies have previously described the loss of AQP4 in active demyelinating NMO lesions. In contrast, active MS lesions show increased AQP4 immunoreactivity, particularly on astrocyte surface membranes, relative to the normal AQP4 baseline expression.4 Among the 20 surgical biopsies with active demyelination meeting inclusion criteria, perivascular and astrocyte surface AQP4 immunoreactivity was lost in 18 (figure 2, A, B, D–E). Five of these 18 biopsies demonstrated occasional astrocytes (figure 2, E and F, arrows) and astrocytic profiles (figure 2, E and F, arrowheads) with AQP4-immunoreactive foci, and a single biopsy had AQP4-immunoreactive degradation products within macrophages (figure 2E, arrow). In contrast, in 2 biopsies, AQP4 immunoreactivity was increased within the active demyelinated lesion, outlining the astrocytic surface membranes (figure 2, G and H, arrows) with preservation of the rim and rosette vasculocentric distribution pattern of normal AQP4 immunoreactivity (figure 2, H and I, asterisks).
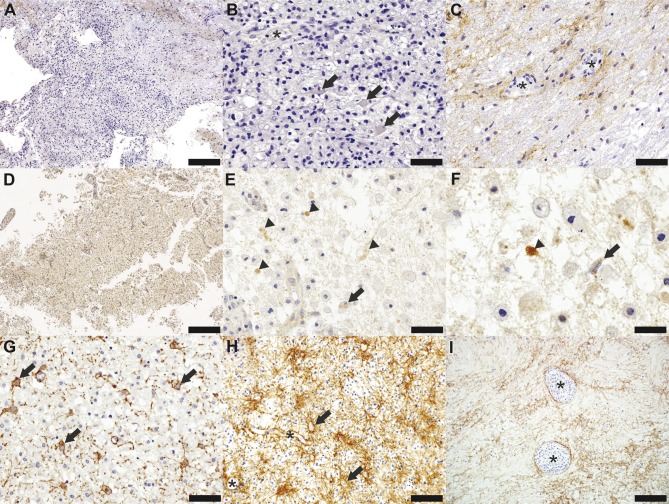
Figure 2. Aquaporin-4 immunohistochemistry in neuromyelitis optica and multiple sclerosis.
(A–F) Neuromyelitis optica (NMO). (G–I) Multiple sclerosis (MS). (A) Loss of aquaporin-4 (AQP4) extends beyond the area of active demyelination in an NMO spinal cord biopsy (AQP4, scale bar = 200 µm). (B) Reactive astrocytes within the lesion (arrows) exhibit loss of the astrocytic AQP4 membrane surface immunoreactivity; of the perivascular rim or rosette AQP4 normal staining pattern is also lost within the lesion (AQP4, scale bar = 50 µm). (C) AQP4 immunoreactivity is preserved within the normal-appearing white matter (AQP4, scale bar = 50 µm). (D) AQP4 is lost within an active demyelinating NMO brain lesion (AQP4, scale bar = 500 µm). (E) Astrocytic profiles show AQP4-positive vacuoles (arrowheads) consistent with AQP4 internalization and occasional macrophages containing AQP4-positive degradation products (arrow) (AQP4, scale bar = 50 µm). (F) Higher magnification shows AQP4 internalization within astrocytic profiles (arrowhead) and reactive astrocytes (arrow) (glial fibrillary acid protein, scale bar = 25 µm). (G) Increased AQP4 expression on the membrane of dystrophic astrocytes (arrows) is present in an active demyelinating MS lesion (AQP4, scale bar = 50 µm). (H) AQP4 immunoreactivity is increased on both the cytoplasmic membrane of reactive astrocytes (arrows) and perivascularly (asterisks) in an MS biopsy that displays a reactive astrocytic morphology more typical of MS. The perivascular rim/rosette pattern of AQP4 expression is preserved (asterisk; AQP4, scale bar = 100 µm). (I) AQP4 immunoreactivity is increased in the MS periplaque white matter and around vessels (asterisks) surrounded by inflammatory infiltrates (AQP4, scale bar = 200 µm).
Periplaque white matter (PPWM) was present in 14 biopsies. AQP4 immunoreactivity was increased in the PPWM in 12 biopsies (11 with lesional AQP4 loss, 1 with lesional AQP4 increase) (figure 2C) and was decreased in 3 biopsies.
Astrocyte pathology.
We observed dystrophic astrocytes characterized by short and blunted processes in 18 of the 20 biopsies (figure 3, A–G). Two biopsies lacked dystrophic astrocytes: 1 AQP4 loss biopsy displayed pronounced fibrillary gliosis; the other biopsy had increased AQP4 immunoreactivity and numerous reactive gemistocytic astrocytes (figure 3, H and I). Macrophages in active demyelinating regions contained GFAP-immunoreactive astrocytic degradation products in 16 biopsies (14 with AQP4 loss; 2 with increased AQP4 immunoreactivity) (figure 3, C and F, white arrows).
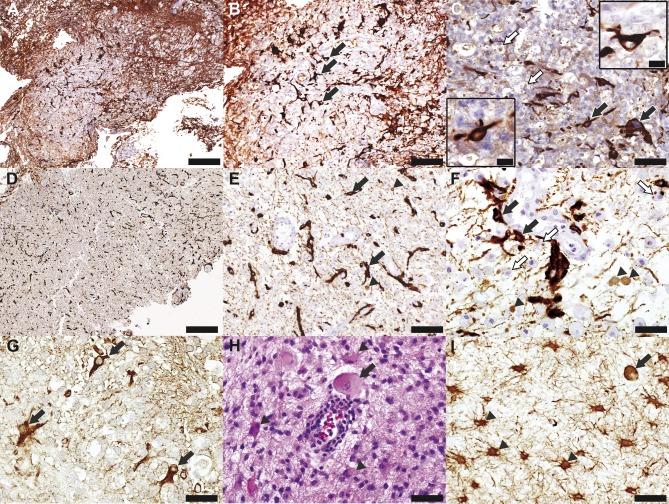
Figure 3. Astrocyte pathology in neuromyelitis optica and multiple sclerosis.
(A–F) Neuromyelitis optica (NMO). (G–I) Multiple sclerosis (MS). (A) Glial fibrillary acid protein (GFAP) staining is decreased in a spinal cord NMO lesion (GFAP, scale bar = 200 µm). (B) GFAP-positive astrocytes (arrows) present within the lesion are small, with few short and blunted processes, i.e., dystrophic astrocytes (GFAP, scale bar = 100 µm). (C) Besides dystrophic astrocytes (arrows and insets), the lesion also shows the presence of macrophages containing GFAP-positive degradation products (white arrows) (GFAP, scale bar = 50 µm, scale bar in insets = 16.5 µm). (D) Low magnification of this brain NMO lesion also shows a decreased density of astrocytes (GFAP, scale bar = 500 µm). (E) Higher magnification reveals dystrophic astrocytes (arrows) and GFAP-positive astrocytic profiles (arrowheads) (GFAP, scale bar = 100 µm). (F) Besides dystrophic astrocytes (arrows) and GFAP-positive astrocytic profiles (arrowheads), the lesion also shows macrophages containing GFAP-positive degradation products (white arrows) (GFAP, scale bar = 50 µm). (G) Dystrophic astrocytes (arrows) are also present in an active demyelinating MS lesion (GFAP, scale bar = 33 µm). (H, I) Another MS biopsy reveals Creutzfeldt-Peters cells (arrows) and reactive astrocytes with prominent processes, more typical of demyelination in MS (arrowheads) (H, hematoxylin & eosin, scale bar = 50 µm; I, GFAP, scale bar = 100 µm).
All 18 biopsies with AQP4 loss lacked classical Creutzfeldt-Peters cells, but occasional mitotic astrocytes were noted. Creutzfeldt-Peters cells were present in 1 of the 2 biopsies with increased AQP4 immunoreactivity (figure 3, H and I, arrows).
Myelin pathology.
Nineteen biopsies (18 with AQP4 loss; 1 with AQP4 increase) exhibited tissue and myelin vacuolation within the active demyelinating regions or the adjacent PPWM consistent with edema (figure 4, A–C and G), while 1 biopsy with increased AQP4 immunoreactivity did not.
Figure 4. Myelin vacuolation and granulocytic inflammatory infiltrates in neuromyelitis optica and multiple sclerosis.
(A–F) Neuromyelitis optica (NMO). (G–I) Multiple sclerosis (MS). (A) White matter exhibits tissue vacuolation (hematoxylin & eosin [H&E], scale bar = 100 µm). (B, C) Myelin is vacuolated at the lesion edge (B; arrow) (LFB/PAS, scale bar = 12.5 µm) as well as at the lesion margin (C; Luxol fast blue [LFB]/periodic acid–Schiff [PAS], scale bar = 33 µm). (D) The lesion is infiltrated by eosinophils (arrows) (H&E, scale bar = 25 µm). (E) Neutrophils (arrows; higher magnification showed in inset) (H&E, scale bar = 50 µm; inset scale bar = 17 µm) and (F) eosinophils (arrow; inset shows higher magnification) and blood vessels with thickened, hyalinized walls are common findings within NMO lesions (H&E, scale bar = 25 µm; inset scale bar = 12.5 µm). (G) Tissue vacuolation (H&E, scale bar = 100 µm) and (H, I) eosinophilic inflammatory infiltrates (H) in the periplaque white matter (H&E, scale bar = 100 µm) and (I) in lesion (H&E, scale bar = 50 µm) can also be found in MS; inset in G shows tissue vacuolation in an actively demyelinating lesion (H&E, scale bar = 67 µm).
Three biopsies with AQP4 loss contained cortex besides white matter, but no cortical demyelinating lesions were observed.
Vascular pathology.
Nonspecific vascular thickening and hyalinization was noted in numerous small blood vessels in 17 of 20 biopsies (16 biopsies with AQP4 loss; 1 with increased AQP4 IR) (figure 4, E and F).
Inflammatory infiltrate.
All biopsies showed perivascular or parenchymal inflammatory infiltrates. Macrophages and lymphocytes were present in all 20 biopsies (figure 1, C–E and I–K). Granulocytes were present in 19 biopsies (17 with AQP4 loss; 2 with increased AQP4). Two biopsies contained only eosinophils, 3 biopsies only neutrophils, and 14 biopsies contained both eosinophils and neutrophils (figure 4, D–F, H and I). The 2 biopsies with increased AQP4 contained both eosinophils and neutrophils.
Complement immunohistochemistry was performed on 8 AQP4 loss biopsies with sufficient tissue available for staining. All demonstrated deposition of complement activation products in a perivascular rim or rosette pattern.
Figure 5A summarizes the frequency of pathologic features among the biopsy cohort, segregated according to whether AQP4 immunoreactivity is lost or increased in active demyelinating lesions.
Figure 5. Histopathologic, clinical, and serologic features of the cohort.
(A) Histopathologic features and clinical and serologic outcomes of the cohort segregated based on whether the biopsy showed aquaporin-4 (AQP4) loss or increase in regions of active demyelination. (B) Clinical features of the cohort show the type and frequency of exacerbations (i.e., unilateral or bilateral optic neuritis [ON], transverse myelitis [TM], intractable nausea/vomiting [N/V], or brain syndrome) relative to the timing of either spinal cord or brain biopsy of the neuromyelitis optica (NMO)/NMO spectrum disorders (NMOSD) cohort. Patient 1 was known to have multiple relapses of optic neuritis but the exact timing of these attacks was unknown and therefore represented with dashed lines. GFAP = glial fibrillary acid protein; LETM = longitudinally extensive transverse myelitis; PPWM = periplaque white matter; RRMS = relapsing-remitting multiple sclerosis. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Clinical and serologic features of the biopsy cohort.
Figure 5A summarizes clinical and serologic outcomes in the AQP4 loss or AQP4 increase cohorts. Among the AQP4 loss cohort (17 patients; 14 female/3 male), the average age at biopsy was 50.5 years (range 9–83) and the median time from disease onset to first biopsy was 3 months (range 1 week–15 years). Presenting symptoms were brain onset in 7 (including intractable nausea and vomiting in 2), spinal cord onset in 8, and optic nerve onset in 2. A single case had 2 biopsies separated by a 26-year interval. This patient initially presented with an ataxic syndrome and cerebellar lesion biopsied at age 9, and then had an episode of longitudinally extensive TM (LETM) and was biopsied at the local institution at age 35 (case 2 in figure 5B). Both biopsies revealed loss of AQP4 immunoreactivity related to blood vessels and astrocyte surface membranes.
AQP4-IgG was positive in all 14 cases pathologically characterized by AQP4 loss with serum available (figure 5A). Serologic testing was done after spinal cord or brain biopsy in all, the interval postbiopsy ranging from weeks to >26 years. Diagnosis at last follow-up in the AQP4 loss cohort was NMO (6 patients [1 diagnosed at autopsy]), NMOSD (9), relapsing LETM (1), and LETM (1). Figure 5B illustrates the clinical course relative to biopsy in the AQP4 loss cohort.
Both biopsies with increased AQP4 immunoreactivity were from women (age 34 and 44 years at biopsy); the median time from disease onset to biopsy was 1 month and 6 months, respectively. Both had brain presentations. AQP4-IgG testing was negative in both, and diagnosis at last follow-up was MS according to McDonald criteria.
DISCUSSION
Our study highlights the potential diagnostic utility of AQP4 immunohistochemistry to help identify active demyelinating surgical biopsies suggestive of NMO/NMOSD. Archival autopsy neuropathologic studies have indicated AQP4 loss as a pathologic hallmark of active NMO lesions.3,19 However, several additional histopathologic hallmarks have been suggested to be more typical of NMO/NMOSD than MS. These include (1) myelin and tissue vacuolation23; (2) granulocytic inflammatory infiltrates19; (3) dystrophic astrocytes18; and (4) macrophages containing GFAP-positive debris.20 Previous case reports and small studies on NMO biopsies have reported the usefulness of detecting the loss of AQP4 immunoreactivity, the presence of dystrophic astrocytes, and the presence of vascular wall thickening for the diagnosis of biopsied NMO lesions.18,21,26,27 However, in the majority of these studies, the diagnosis of NMO/NMOSD was known before the biopsy interpretation or the fulfilment of NMO/NMOSD diagnostic criteria or seropositivity for AQP4-IgG led to biopsy reassessment. Our study is the first to assess the potential diagnostic utility of AQP4 immunohistochemistry for NMO/NMOSD in a prospective manner. The identification of 20 surgical biopsies from 19 patients exhibiting active demyelination with a combination of the above MS-unusual histopathologic features prompted us to perform AQP4 immunohistochemistry on a research basis using a commercially available antibody. We found AQP4 was lost or decreased in active demyelinating lesions in 18 biopsies (17 patients). This led us to test serum retrospectively (available from 14 cases), and review of clinical and imaging information confirmed the diagnosis of NMO/NMOSD in 15 patients. Two additional AQP4 loss patients without available serum had a diagnosis of relapsing LETM or LETM, respectively, at last follow-up. In contrast, astrocytic and perivascular AQP4 immunoreactivity in active demyelinating lesions in surgical biopsies from 2 patients was increased relative to baseline normal AQP4 immunoreactivity.4 Both were negative for serum AQP4-IgG, and follow-up confirmed a diagnosis of relapsing-remitting MS. Both of these MS cases demonstrated several histopathologic features that have also been described in NMO (i.e., myelin vacuolation, granulocytes, dystrophic astrocytes), underscoring the utility of AQP4 immunohistochemistry to help discriminate between NMO and MS on biopsy.
The accurate classification of demyelinating activity within NMO lesions is crucial for interpreting AQP4 immunohistochemistry. Early NMO lesions can demonstrate AQP4 loss in the absence of obvious demyelination; more advanced lesions are typically demyelinated.3,4 Demyelinating lesions can be classified by the presence or absence of myelin degradation products within macrophages. Macrophages in acute active lesions contain myelin degradation products that stain with LFB and are immunoreactive for both minor myelin proteins (MOG, MAG, CNPase) and major myelin products (PLP, MBP), whereas macrophages in inactive lesions do not contain either LFB-positive or myelin immunoreactive intracytoplasmic debris.22 While some investigators reported that AQP4 loss is seen not only in NMO/NMOSD, but also in MS and Balo concentric sclerosis,28 we and others have shown in archival autopsy material that AQP4 is lost in NMO lesions irrespective of the demyelinating activity stage, whereas AQP4 in MS lesions is increased in active but lost in inactive lesions.3,4 Similarly, in the present surgical biopsy cohort, all 17 cases ultimately diagnosed as NMO, NMOSD, or LETM demonstrated active demyelination with AQP4 loss whereas the 2 MS cases with active demyelination had increased AQP4 immunoreactivity. Moreover, NMO cases with Balo-like lesions have been previously reported, and further studies are required to assess the significance and value of AQP4 immunohistochemistry in Balo concentric sclerosis.29 Unlike active lesions, AQP4 immunoreactivity was increased in the PPWM adjacent to active demyelinated lesions in both NMO and MS. Therefore, AQP4 immunohistochemistry of PPWM cannot reliably differentiate these 2 conditions.
Active demyelination associated with myelin and tissue vacuolation, granulocytic inflammatory infiltrates, dystrophic astrocytes, macrophages containing GFAP-positive debris, complement activation, blood vessel thickening, or absence of Creutzfeldt-Peters cells in surgical biopsies suggest NMO/NMOSD and should prompt AQP4 immunohistochemistry. These neuropathologic features are consistent with antibody-mediated primary destruction of astrocytes in NMO and secondary demyelination.3,4,18–20 The astrocytic profiles containing AQP4 immunoreactive foci we describe in NMO but not MS lesions likely represent remnants of astrocytic processes, and are consistent with both complement-mediated destruction of astrocytes and AQP4 internalization by astrocytes following binding of AQP4-IgG to AQP4.23 While they may represent a useful histopathologic feature that may help in differentiating NMO from MS, their absence should not dismiss the possibility of NMO considering that a minority of NMO cases showed the presence of such astrocyte profiles. Although common in NMO lesions and rare in MS, granulocytic inflammatory infiltrates are not entirely specific and can occur in other destructive demyelinating etiologies including fulminant MS or acute disseminated encephalomyelitis.
Myelin vacuolation in active demyelinating lesions and normal-appearing white matter as seen in all NMO/NMOSD biopsies is consistent with in vitro demonstrations that binding of AQP4-IgG to AQP4 disrupts water homeostasis via AQP4 internalization or direct blockade of water flux.23 Myelin vacuolation is the pathologic hallmark of fluid accumulation within myelin and is considered a sensitive marker of injury to the oligodendrocyte–myelin sheath complex. It is also encountered preceding or accompanying primary, secondary, or toxin-induced demyelination, and is not specific to NMO.23,30–32
Creutzfeldt-Peters cells, which are reactive astrocytes with fragmented nuclear inclusions (micronuclei) identifiable by light microscopy,33 were absent in NMO/NMOSD biopsies. These cells are a frequent but not universal pathologic finding in active MS lesions.33 Micronuclei originate from chromosomes that do not attach properly to the spindle apparatus during mitosis and fail to be included in the daughter nuclei, but are enclosed by the nuclear membrane.34 Micronuclei are a marker of clastogen- or aneugen-induced chromosomal instability and cells containing micronuclei are commonly found in cancer.35 Micronuclei are also induced by inflammation and oxidative stress in preneoplastic or non-neoplastic conditions.25,36 The presence of Creutzfeldt-Peters cells in MS but not NMO suggests that different inflammatory or oxidative environments may exist in MS and NMO. Alternatively, Creutzfeldt-Peters cells in MS may reflect astrocyte proliferation, whereas their absence in NMO may reflect astrocyte death or compromise.
Among the 3 AQP4 loss NMO/NMOSD biopsies with available cortex, none had cortical demyelination. This is consistent with previous studies that reported cortical demyelinating lesions as a characteristic of MS but not NMO.37,38
A cerebral presentation occurred in 7 of 17 NMO/NMOSD patients, and prompted brain biopsy in 6. Brain involvement, as initial event,13 or at relapse,12,14 is now recognized and included among the diagnostic criteria for NMO/NMOSD.10,39 Despite increasing awareness in the clinical community, the diagnosis of NMO/NMOSD is still overlooked, especially when brain involvement precedes episodes of TM or ON. Furthermore, supraspinal and spinal lesions are similar pathologically,4,26 providing an additional means of diagnosing NMO/NMOSD with initial brain presentations when brain biopsy tissue is available.
Many patients in this report underwent diagnostic biopsy to exclude neoplasm.21,27 Furthermore, AQP4-IgG testing was performed after biopsy in all patients, and in several cases there was a long interval between symptom onset, biopsy, AQP4-IgG serologic testing, and eventual NMO/NMOSD diagnosis, stressing the importance of considering NMO in the differential diagnosis of a tumefactive brain or spinal cord lesion. AQP4-IgG testing may avert biopsy and avoid the use of potentially aggravating therapies if these patients are erroneously treated for MS.40 Whether AQP4-IgG seronegative NMO has a similar pathology to seropositive NMO is unknown, and will require the careful analysis of such cases, should they become available.
Although future studies on a larger cohort are needed to validate AQP4 immunohistochemistry as a specific marker for the diagnosis of NMO on neurosurgical biopsy, our findings underscore the importance of recognizing several unusual findings in a biopsy of active demyelinating disease, including the presence of dystrophic astrocytes, myelin vacuolation, granulocytes, and vascular hyalinization, which should prompt the neuropathologist to perform immunohistochemistry for AQP4, as well as recommend AQP4-IgG serologic testing to exclude a diagnosis of NMO/NMOSD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Patricia Ziemer, Mayo Clinic, for technical assistance.
GLOSSARY
- AQP4
aquaporin-4
- GFAP
glial fibrillary acid protein
- IgG
immunoglobulin G
- LETM
longitudinally extensive transverse myelitis
- LFB
Luxol fast blue
- MAG
myelin-associated glycoprotein
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorders
- ON
optic neuritis
- PLP
proteolipid protein
- PPWM
periplaque white matter
- TM
transverse myelitis
Footnotes
Editorial, page 110
AUTHOR CONTRIBUTIONS
B.F.G.P., Y.G., M.E.J., J.E.P., C.G., I.M., W.B., and C.F.L. collected, analyzed, and interpreted the pathologic data. Y.G., M.E.J., B.S., J.C., C.B., B.C., S.C., S.J.P., B.W., D.M.W., and C.F.L. collected, analyzed, and interpreted the clinical data. S.J.P. and V.A.L. collected, analyzed, and interpreted the serologic data. C.F.L. conceived this study. B.F.G.P., Y.G., J.E.P., and C.F.L. drafted and B.F.P., J.E.P., Y.G., V.A.L., S.J.P., B.W., D.W., C.G., B.C., I.M., and C.F.L. edited the manuscript. All authors read and approved the final manuscript.
STUDY FUNDING
Supported by grants RO1-NS049577-01-A2 from the NIH (Dr. Lucchinetti), NMSS RG 3185-B-3 from the National Multiple Sclerosis Society (Dr. Lucchinetti), and the Guthy Jackson Charitable Foundation (Dr. Lucchinetti).
DISCLOSURE
B. Popescu served as a speaker for Teva Innovation Canada, received honorarium for publishing in Continuum: Lifelong Learning in Neurology, and receives research support from the Canada Research Chairs program (principal investigator). Y. Guo and M. Jentoft report no disclosures relevant to the manuscript. J. Parisi serves on scientific advisory boards for the FDA and the DoD Defence Health Board; receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003); and receives research support from the NIH (NS32352-13; coinvestigator). V. Lennon is a named inventor on a patent relating to AQP4 as a target of pathogenic autoantibodies in NMO and related disorders and on a pending patent related to AQP4 applications to cancer; has received greater than the federal threshold for significant interest from licensing of this technology; receives no royalties from the sale of Mayo Medical Laboratories' service serological tests; however, Mayo Collaborative Services, Inc., receives revenue for conducting these tests; is named inventor on 2 patent applications filed by the Mayo Foundation for Medical Education and Research relating to functional assays for detecting NMO/AQP4 antibody; and receives research support from the NIH (NS65829; coinvestigator). S. Pittock is a named inventor on patents (12/678,350 filed 2010 and 12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and the NIH (NS065829). He has provided consultation to Alexion Pharmaceutical, MedImmune LLC, and Chugai Pharma, but has received no personal fees or compensation for these consulting activities. All compensation for consulting activities is paid directly to Mayo Clinic. B. Weinshenker serves on data safety monitoring boards for Novartis, Biogen Idec, and Mitsubishi. He serves on an adjudication panel for MedImmune Pharmaceuticals. He has received payment for consultation from Elan Pharmaceuticals, GlaxoSmithKline Pharmaceuticals, Ono Pharmaceuticals, CHORD Therapeutics, and Chugai Pharmaceuticals. He serves on the editorial boards of Neurology®, the Canadian Journal of Neurological Sciences, and the Turkish Journal of Neurology. He has received research support from the Guthy-Jackson Charitable Foundation. He receives license royalties from RSR Ltd. for a patent regarding AQP4-associated antibodies for diagnosis of neuromyelitis optica. D. Wingerchuk has received research support from Alexion, TerumoBCT, and the Guthy Jackson Charitable Foundation, receives financial compensation for participation on a relapse adjudication panel for MedImmune, and has served as a consultant to Alexion, MedImmune, and Chugai Pharmaceuticals. C. Giannini reports no disclosures relevant to the manuscript. I. Metz received grants from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” [KKNMS], Pattern MS/NMO) and speaking honoraria and travel expenses from Biogen Idec, Bayer Healthcare, Serono, and TEVA. W. Brück receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Genzyme, and Novartis. He is member of scientific advisory boards of Teva Pharmaceutical Industries Ltd., Biogen Idec, Novartis, and Genzyme/Sanofi. Dr. Brück serves on speaker's bureaus for Bayer Vital, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries Ltd., Genzyme/Sanofi, and Novartis. Dr. Brück serves on editorial boards of Acta Neuropathologica, Neuropathology and Applied Neurobiology, and Therapeutic Advances in Neurological Disorders. E. Shuster received compensation for a PRIME educational event. J. Carter serves on a Data Safety Monitoring Committee for a MS clinical trial sponsored by Merck-Serono, has a consulting contract with Med-IQ to develop MS CME materials, and receives research support to Mayo Clinic from Genzyme, Actelion, Elan Pharmaceuticals, MedImmune, and Roche. C. Boyd and S. Clardy report no disclosures relevant to the manuscript. B. Cohen has received personal compensation for consultation services from Accorda, Biogen-Idec, EMD-Serono, Genentech, Genzyme, Questcor, and Teva Neuroscience. He receives research funding through Northwestern University from Biogen-Idec, Novartis, and Hoffman La Roche. C. Lucchinetti may accrue revenue for a patent re: Aquaporin-4-associated antibodies for diagnosis of neuromyelitis optica; receives royalties from the publication of Blue Books of Neurology: MS 3 (Saunders Elsevier, 2010); has received personal compensation for consultation services from Biogen-Idec; and receives research support from the NIH (NS49577-R01; principal investigator), the Guthy Jackson Charitable Foundation (principal investigator), and Koltan Pharmaceutical (principal investigator). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 2007;130:1224–1234. [DOI] [PubMed] [Google Scholar]
- 4.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007;130:1194–1205. [DOI] [PubMed] [Google Scholar]
- 5.Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol 2010;68:757–761. [DOI] [PubMed] [Google Scholar]
- 6.Magana SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 2009;72:712–717. [DOI] [PubMed] [Google Scholar]
- 7.Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 2005;65:1479–1482. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Miyazawa I, Misu T, et al. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: a herald of acute exacerbations. J Neurol Neurosurg Psychiatry 2008;79:1075–1078. [DOI] [PubMed] [Google Scholar]
- 9.Weinshenker BG, Wingerchuk DM. Neuromyelitis optica: clinical syndrome and the NMO-IgG autoantibody marker. Curr Top Microbiol Immunol 2008;318:343–356. [DOI] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107–1114. [DOI] [PubMed] [Google Scholar]
- 12.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–396. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler 2011;17:1107–1112. [DOI] [PubMed] [Google Scholar]
- 14.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968. [DOI] [PubMed] [Google Scholar]
- 15.Viegas S, Weir A, Esiri M, et al. Symptomatic, radiological and pathological involvement of the hypothalamus in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2009;80:679–682. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Misu T, Fujihara K, et al. Occurrence of acute large and edematous callosal lesions in neuromyelitis optica. Mult Scler 2009;15:695–700. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 2007;130:1235–1243. [DOI] [PubMed] [Google Scholar]
- 18.Bruck W, Popescu B, Lucchinetti CF, et al. Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate. Ann Neurol 2012;72:385–394. [DOI] [PubMed] [Google Scholar]
- 19.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misu T, Hoftberger R, Fujihara K, et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol 2013;125:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato DK, Misu T, Rocha CF, et al. Aquaporin-4 antibody-positive myelitis initially biopsied for suspected spinal cord tumors: diagnostic considerations. Mult Scler 2014;20:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruck W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995;38:788–796. [DOI] [PubMed] [Google Scholar]
- 23.Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci USA 2012;109:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vass K, Lassmann H, Wekerle H, Wisniewski HM. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol 1986;70:149–160. [DOI] [PubMed] [Google Scholar]
- 25.Andreassi MG, Barale R, Iozzo P, Picano E. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis 2011;26:77–83. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Metz I, Berthele A, et al. Supraspinal demyelinating lesions in neuromyelitis optica display a typical astrocyte pathology. Neuropathol Appl Neurobiol 2010;36:685–687. [DOI] [PubMed] [Google Scholar]
- 27.Ringelstein M, Metz I, Ruprecht K, et al. Contribution of spinal cord biopsy to diagnosis of aquaporin-4 antibody positive neuromyelitis optica spectrum disorder. Mult Scler Epub 2013 Nov 5. [DOI] [PubMed]
- 28.Matsuoka T, Suzuki SO, Suenaga T, Iwaki T, Kira J. Reappraisal of aquaporin-4 astrocytopathy in Asian neuromyelitis optica and multiple sclerosis patients. Brain Pathol 2011;21:516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graber JJ, Kister I, Geyer H, Khaund M, Herbert J. Neuromyelitis optica and concentric rings of Balo in the brainstem. Arch Neurol 2009;66:274–275. [DOI] [PubMed] [Google Scholar]
- 30.Sobel RA, Moore GR. Demyelinating diseases. In: Love S, Louis DN, Ellison DW, eds. Greenfield's Neuropathology, 8th ed Oxford: Oxford University Press; 2008:1513–1608. [Google Scholar]
- 31.Lampert P, O'Brien J, Garrett R. Hexachlorophene encephalopathy. Acta Neuropathol 1973;23:326–333. [DOI] [PubMed] [Google Scholar]
- 32.Powell H, Swarner O, Gluck L, Lampert P. Hexachlorophene myelinopathy in premature infants. J Pediatr 1973;82:976–981. [DOI] [PubMed] [Google Scholar]
- 33.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol 2012;7:185–217. [DOI] [PubMed] [Google Scholar]
- 34.Norppa H, Falck GC. What do human micronuclei contain? Mutagenesis 2003;18:221–233. [DOI] [PubMed] [Google Scholar]
- 35.Rosefort C, Fauth E, Zankl H. Micronuclei induced by aneugens and clastogens in mononucleate and binucleate cells using the cytokinesis block assay. Mutagenesis 2004;19:277–284. [DOI] [PubMed] [Google Scholar]
- 36.Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res 1994;54:1929s–1933s. [PubMed] [Google Scholar]
- 37.Popescu BF, Parisi JE, Cabrera-Gomez JA, et al. Absence of cortical demyelination in neuromyelitis optica. Neurology 2010;75:2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saji E, Arakawa M, Yanagawa K, et al. Cognitive impairment and cortical degeneration in neuromyelitis optica. Ann Neurol 2013;73:65–76. [DOI] [PubMed] [Google Scholar]
- 39.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 40.Papeix C, Vidal JS, de Seze J, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.