Abstract
Objective:
We employed functional MRI (fMRI) to assess whether (1) patients with disorders of consciousness (DOC) retain the ability to willfully engage in top-down processing and (2) what neurophysiologic factors distinguish patients who can demonstrate this ability from patients who cannot.
Methods:
Sixteen volunteers, 8 patients in vegetative state (VS), 16 minimally conscious patients (MCS), and 4 exit from MCS (eMCS) patients were enrolled in a prospective cross-sectional fMRI study. Participants performed a target detection task in which they counted the number of times a (changing) target word was presented amidst a set of distractors.
Results:
Three of 8 patients diagnosed as being in a VS exhibited significant activations in response to the task, thereby demonstrating a state of consciousness. Differential activations across tasks were also observed in 6 MCS patients and 1 eMCS patient. A psycho–physiologic interaction analysis revealed that the main factor distinguishing patients who responded to the task from those who did not was a greater connectivity between the anterior section of thalamus and prefrontal cortex.
Conclusions:
In our sample of patients, the dissociation between overt behavior observable in clinical assessments and residual cognitive faculties is prevalent among DOC patients (37%). A substantial number of patients, including some diagnosed with VS, can demonstrate willful engagement in top-down cognition. While neuroimaging data are not the same as observable behavior, this suggests that the mental status of some VS patients exceeds what can be appreciated clinically. Furthermore, thalamo-frontal circuits might be crucial to sustaining top-down functions.
Assessing the amount of residual cognitive function that can be maintained after severe brain injury is a major challenge. The issue is particularly relevant in the context of patients with disorders of consciousness (DOC) such as vegetative state (VS)1,2 and minimally conscious state (MCS).3 Indeed, assessment of residual cognitive processing in this cohort is well known to be problematic,4–7 and raises several complex scientific, clinical, and ethical issues.8–10
In recent years, an increasing number of studies have demonstrated the potential of neuroimaging approaches for assessing residual cognition, as well as the presence of consciousness, in patients who appear nonresponsive in standard clinical testing (see reference 11 for a comprehensive review). Yet very little is known about why some patients respond to neuroimaging protocols while others do not. We employ a previously validated functional MRI (fMRI) paradigm12 to explore top-down cognition in a group of 28 DOC patients. In particular, we address 3 main questions. First, to what extent, in our available sample, do DOC patients retain the ability to maintain information through time and voluntarily engage in top-down processing? Second, what proportion of patients who appear nonresponsive (and thus VS) during clinical testing can demonstrate the presence of consciousness via neuroimaging? Third, what neurophysiologic differences, in terms of large-scale cortico-thalamo-cortical connectivity, distinguish patients who respond to the neuroimaging task from patients who do not?
METHODS
Patient population.
A convenience sample of 28 patients participated in the study. Patients were recruited, over a time span of 2.5 years, from specialized long-term care-taking centers. Inclusion criteria for being invited to our center were a DOC diagnosis, consent of the legal representative, and suitability to undergo transportation from their care-taking facility to our center. The only exclusion criterion was unsuitability for entering the MRI environment (e.g., any type of non-MRI-safe implant) or any medical condition making it unsafe for the patient to participate (a decision that rested with clinical personnel blinded to the specific aims of this study). After having been admitted to our center, each patient underwent clinical testing (reported in tables 1 and 2). Patients remained in our facility for a period of 5 days (including arrival and departure days) for clinical and neuroimaging testing. The selection of patients who were invited to our center was not performed in a statistical manner, thus circumscribing the inferential scope of our observational study. The sample included 8 patients with a VS diagnosis, 16 MCS, and 4 exit from MCS (eMCS), as classified by the Coma Recovery Scale–Revised.13 Demographic, injury, and clinical data are presented in tables 1 and 2. Following the taxonomy introduced by Bruno et al.,14 minimally conscious patients were further divided into MCS− (i.e., patients demonstrating low-level behavioral responses such as visual pursuit, localization of noxious stimulation, or contingent behavior such as appropriate smiling or crying to emotional stimuli; n = 4) and MCS+ (i.e., patients demonstrating high-level behavioral responses such as command following, intelligible verbalizations, or nonfunctional communication; n = 12).
Table 1.
Patient demographic data, injury information, and total CRS-R score
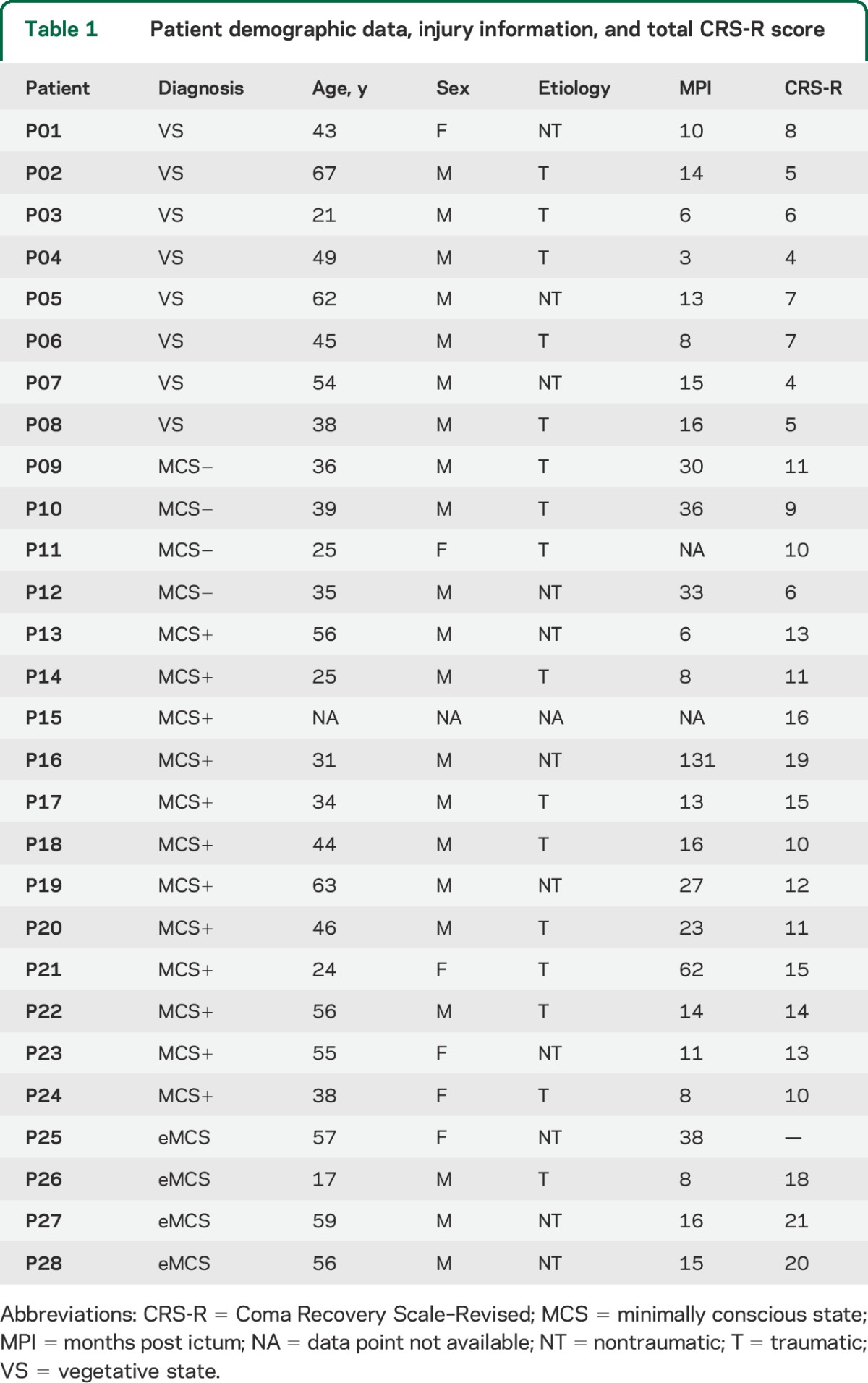
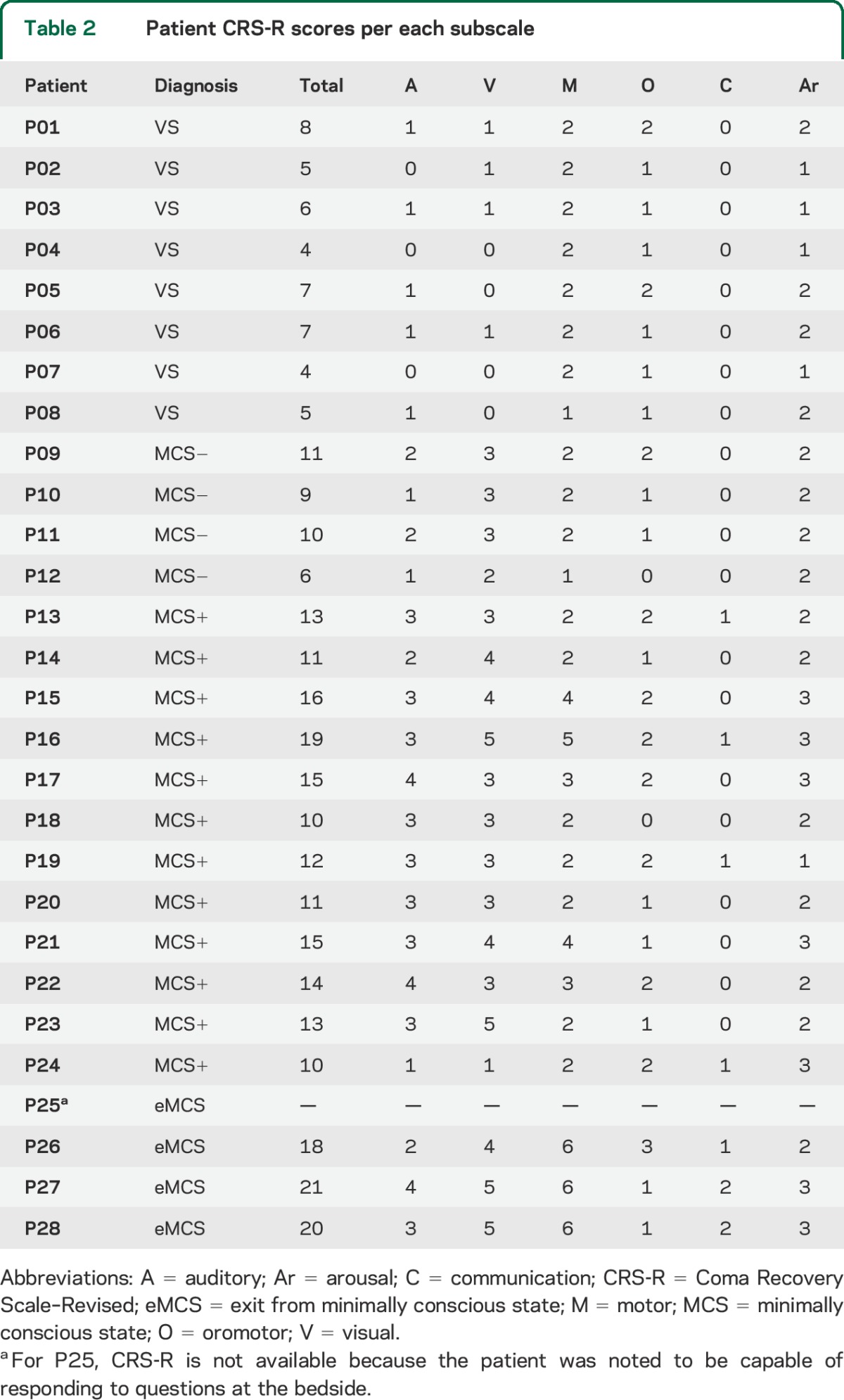
Table 2.
Patient CRS-R scores per each subscale
Healthy volunteers.
Sixteen (previously described12) healthy volunteers underwent the same procedure.
Standard protocol approval, registrations, and patient consent.
For each patient, we collected written assent from his or her legal representative. Healthy volunteers provided written consent personally. The described procedures were approved by the Cambridge Local Research Ethics Committee.
Experimental design.
Each participant took part in one functional and one structural scan (as part of a larger battery of fMRI tasks). The functional scan consisted of performing 5 target detection blocks and 5 passive listening blocks (see Task), which repeated in an ABAB fashion, with passive listening always being performed first. The start of each block was signaled by a 4-second aural cue that indicated the nature of the block, and revealed, for counting blocks, the target word. The cue was then followed by a sequence of non–intrinsically salient words, presented aurally at 1 Hz.12
Task.
Participants thus performed 2 alternating tasks.12 While in both tasks participants were exposed to identical levels of stimulation (i.e., 26 words—see Stimuli), during the “passive listening” baseline task, they were asked to just listen to the sequence of words, and during “target detection” task (or “counting” task, interchangeably), they were asked to count the number of times a given target word (randomly selected in each of the 5 target detection blocks) was repeated.12 For participants to know which task to perform, 2 aural cues were used. In both tasks a 250-msec tone alerted participants to the beginning of a new block. The sound was followed by the instruction “Listen all” to identify listening blocks, and the instruction “Count [target word]” to identify a counting block (and to reveal the target word). Both cues lasted a total of 4 seconds.12 Detailed instructions were given to participants before starting the session.
Stimuli.
The full stimulus set included 120 neutral (i.e., not intrinsically salient) monosyllabic words, half describing living items (e.g., horse, tree) and half describing nonliving items (e.g., table, stone), recorded in a neutral (i.e., nonemotional) female voice.15 From the set, 50 were randomly chosen and allocated, in subgroups of 5, to each of the 10 blocks (5 baseline, 5 target detection). Within each block, words were randomly selected to repeat 7, 6, 5, or 4 times (with 2 words repeating 4 times), resulting in 26 word presentations per block. The 26 words were then allocated, in (pseudo-) random fashion, within each block, that is, with the only constrain that no word be presented multiple times back-to-back. In the 5 counting blocks, the target word appeared twice as the 7-repetition word, twice as the 6-repetition word, and once as the 5-repetition word. The order in which counting blocks featured the 7-, 6-, or 5-repetition target was randomly determined.12 Importantly, each of the randomizations described above was unique to each participant, ensuring no systematic difference across conditions. Overall, our design ensures that perceptual stimulation is matched across baseline and target detection blocks, in terms of number of words and repetition frequencies, while prompting for different mental sets.12,16,17
fMRI data acquisition.
T1-weighted magnetization-prepared rapid gradient echo images (repetition time [TR] = 2.250 msec, echo time [TE] = 2.99 msec, flip angle [FA] = 9°, field of view = 256 × 240 × 160 mm, 1 mm3 isovoxel resolution) were acquired on a 3T Siemens (Munich, Germany) Tim Trio scanner at the Wolfson Brain Imaging Centre at Addenbrookes Hospital, Cambridge, UK. T2*-sensitive images were acquired using an echoplanar sequence (TR = 2,000 msec, TE = 30 msec, FA = 78°, 32 descending slices, 3 × 3 × 3.75 mm2 resolution).12
Data analysis.
fMRI data analysis.
Analyses were performed using FSL.18 Single-subject data underwent standard preprocessing steps including brain extraction, motion correction, spatial (8 mm full width at half maximum) and temporal (with high-pass filters using a gaussian-weighted least-squares straight line fitting, with sigma = 30.0 seconds) smoothing, grand-mean intensity scaling, and coregistration to the T1-weighted image (using 7 degrees of freedom). Data were analyzed with a general linear model approach (which includes prewhitening correction). Given the ABAB block design, only the onset of the counting task was modeled and included in the generalized linear model (GLM) regression, as well as each cue period onset. In addition, we also included 18 regressors of noninterest (6 motion correction parameters and their first and second derivative) to parcel out the effect of motion. Single-subject statistical parametric maps were thresholded using cluster correction of Z > 2.3 and a (corrected) significance threshold of p = 0.05.19 For display purposes, individual results were coregistered to the standard Montreal Neurological Institute (MNI) template space (using a 12 degrees of freedom transformation).
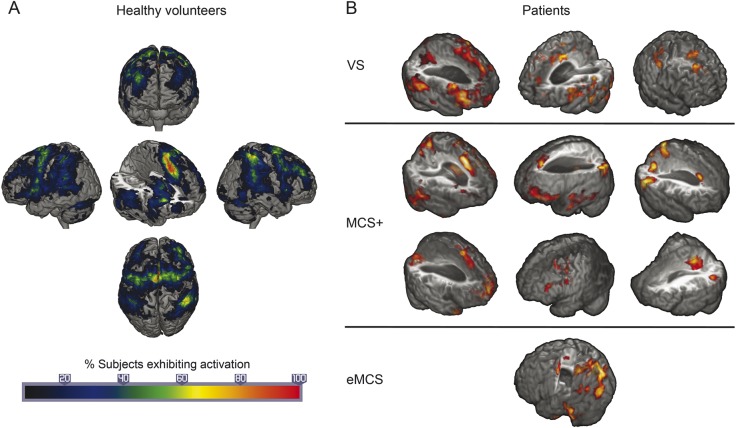
Healthy volunteers were analyzed with similar methods. However, the analysis detailed here departs from what we have previously presented12 in 2 respects. First, as mentioned above, we included as covariates of noninterest, in the single-subject GLM, not only 6 motion parameters but also their first and second derivatives. Second, instead of carrying out a standard mixed-effects group analysis, we aggregate individual results by calculating the percentage of individuals exhibiting activation, at each voxel, for the counting minus baseline task contrast. The group result is thus no longer a map of voxels exhibiting large effect sizes, as compared to the within- and between-subjects variance, but rather a map of the frequency with which a given voxel was found to be active in single subject analysis within our sample of healthy volunteers. In other words, rather than comparing the single-subject patient data to the central tendency of the effect across a group of healthy volunteers, we compare them to a map depicting the probability with which each voxel is found active across each single-subject analysis of healthy volunteers (which indexes both central tendency—as high probability areas—as well as variability—as low probability areas; see figure 1A). For these reasons, this procedure provides for a more appropriate benchmark as compared to the conventional mixed-effects group analysis.
Figure 1. Activation results.
(A) Percent of healthy volunteers exhibiting activation in each region of the brain. (B) Individual activation results for vegetative state (VS), minimally conscious state (MCS+), and exit from minimally conscious state (eMCS) patients.
Psycho–physiologic interaction analysis.
Finally, to assess the question of why some patients responded to the task while others did not, we employ a psycho–physiologic interaction approach (PPI).20 This analysis is referred to as a PPI because it captures the interaction between a psychological variable (i.e., the onset and offset of a cognitive task) and a physiologic variable (i.e., the blood oxygenation level–dependent signal within a region of interest [ROI]). Following the recent proposal that specific thalamo-cortical connections might be crucial to the maintenance of large-scale neural circuits underlying willful behavior,21–23 we use as a seed ROI the section of thalamus connecting to prefrontal cortex (as defined by the Harvard-Oxford atlas,24 which is consistent with the anatomical location of sections of the mediodorsal and ventral anterior nuclei, and the anterior complex), and assess its full brain connectivity. This analysis shows the changes in connectivity patterns between thalamus and any brain region that occur during the counting task as compared to the passive listening task. Finally, by comparing the results of the PPI analysis across patients who respond to the task and patients who do not, we can reveal whether differences in the engagement of thalamic circuits during the 2 tasks is associated with the patients' ability to perform the task. For each patient, the PPI regressor was calculated by computing the interaction between the task regressor and the time course associated with the seed mask. All patients for whom excessive motion was noted (i.e., motion >3 mm; subjects marked with “m” in table 3) were excluded from the analysis. Comparison of the PPI regressors between patients who did and patients who did not respond to the task was performed using a fixed-effects 2-group design (because of the low number of subjects in each group) and evaluated against a criterion of p < 0.05 cluster-corrected (determined through 3dClustSim to require a minimum of 387 contiguous voxels with individual significance of p < 0.02).
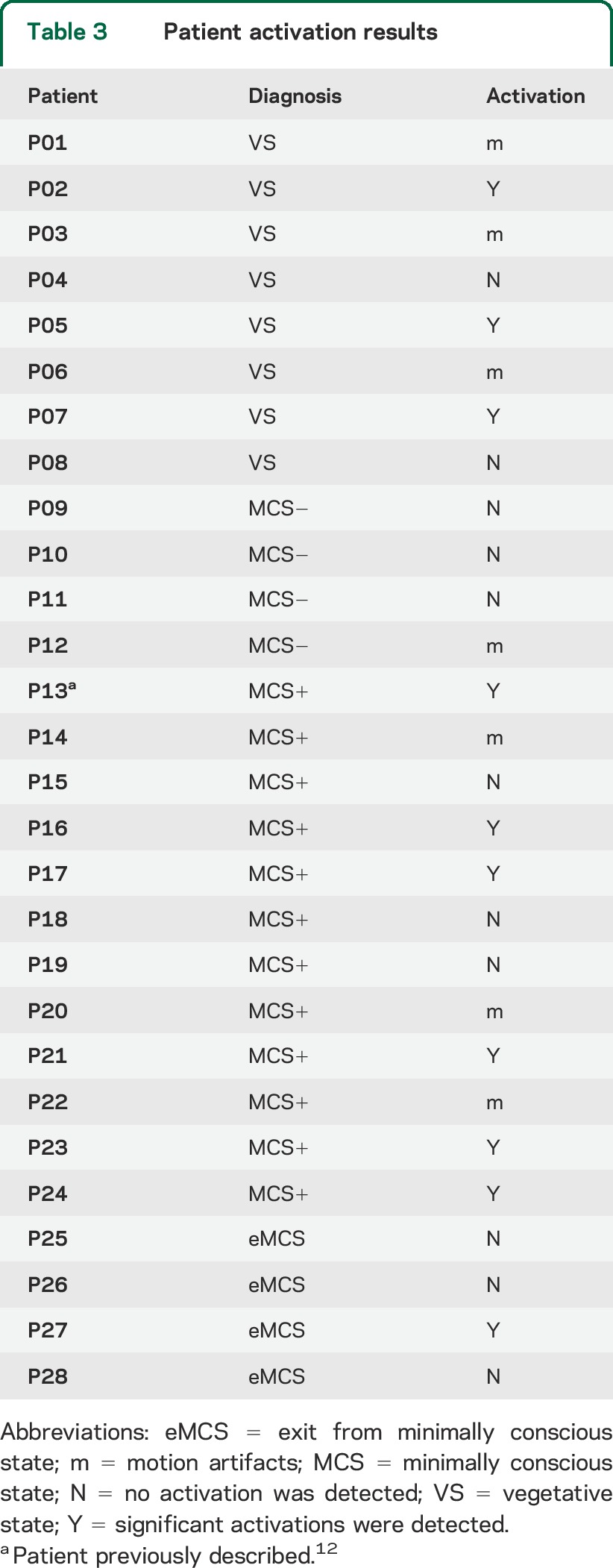
Table 3.
Patient activation results
RESULTS
Results for all patient groups are reported in table 3 and figure 1B. Significant activations for the count minus listen contrast were uncovered in a total of 10 out of 28 patients. More specifically, 3 of 8 VS patients exhibited differential activations in the 2 tasks, thereby revealing a mental state of (at least minimal) consciousness. In the remaining patient groups, significant activity was detected in 6 of 12 MCS+ patients and 1 of the 4 eMCS patients. Interestingly, no MCS− patient exhibited significant activity. With respect to patients exhibiting negative results, we distinguish in table 3 those for whom no result was observed (marked with “N”) from patients for whom excessive movement was detected (>3 mm) precluding meaningful analyses (3 VS, 1 MCS−, and 3 MCS + patients, marked with “m”).
When assessing the results with respect to patient etiology, it is remarkable that 6 of the 10 responders had a nontraumatic brain injury (table 1). Of these, 2 VS and 1 MCS+ had anoxic brain injuries (P05, P07, and P13, respectively), while the remaining 2 MCS+ and 1 eMCS patients (P16, P23, and P27, respectively) had an intracerebral hemorrhage.
As depicted in figure 2, the PPI analysis revealed that patients who responded to the task exhibited a greater increase in thalamo-frontal connectivity during the counting task as compared to patients who did not respond to the task. Specifically, greater thalamo-cortical connectivity was observed in the left frontal pole (peaking at MNI coordinates −30, 50, 16) spanning the superior and medial frontal gyri.
Figure 2. Psycho–physiologic interaction approach analysis.
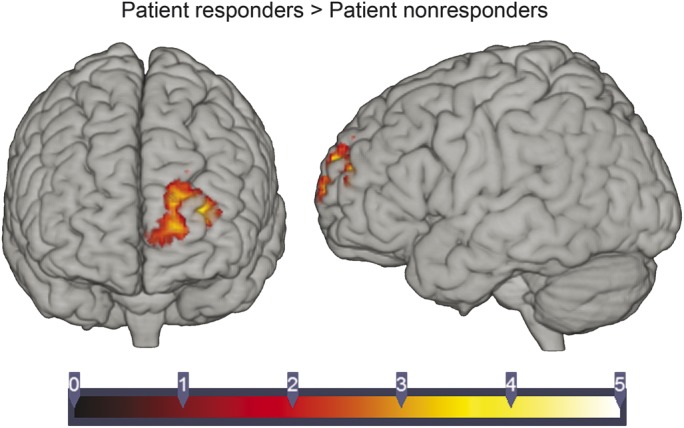
Depiction of the psycho–physiologic interaction approach (PPI) analysis results comparing thalamo-cortical connectivity in patients who responded to the task vs patients who did not (see text for activation peak coordinates).
DISCUSSION
The current study presents 3 main findings. First, we show that, in our convenience sample, a substantial number of patients with disorders of consciousness due to severe brain injury can retain high-level cognitive functions. Although our sample of VS patients is relatively small (n = 8), it is remarkable that the incidence of residual high-level cognitive processing identified in this study (37%) is even larger than what has been reported in previous neuroimaging investigations.25,26 At a minimum, our data suggest that the patients who did respond to the task retain the ability to comprehend language to an extent sufficient to understand the instructions and the request to adopt different mental sets throughout the experimental session. In addition, the presence of differential activation during the active “counting” blocks, as compared to the passive “listening” blocks, suggests that responding patients were able to engage in and disengage from different mental sets, as prompted by each cue. Whether patients were indeed counting the target stimuli, as instructed, cannot be determined on the basis of neuroimaging data. Nonetheless, it is clear that they were complying with some aspect of the instructions and differentially processing the stimuli under the 2 conditions as a result of willful top-down cognitive processing. Indeed, our experimental design, in which patients undergo exactly the same level of stimulation under different mental sets prompted by 1-second cues, implies that any differential activation between the 2 tasks cannot be ascribed to features of the stimuli (since they are matched) and can only be a consequence of the patient voluntarily processing stimuli in a different way.17,27 Crucially, unlike experiments making use of intrinsically salient materials (e.g., the patient's own name),28,29 our stimuli did not possess any intrinsic salience and were randomly allocated (uniquely for each participant) to each condition, preventing any interpretation of our effects as resulting from systematic differences in linguistic features across stimuli appearing in the 2 conditions. Thus, any difference in processing must be the result of some stimuli becoming salient through a voluntary top-down process. We note that different patients match to a different degree the patterns seen in healthy volunteers. In most cases (i.e., all 3 VS, 4 of the 6 MCS+ patients, and the one eMCS patient exhibiting activations) activations fall within regions seen in volunteers (figure 1A), supporting the view that they are engaging in some aspect of the task. In the 2 remaining MCS+ patients (in the rightmost column in figure 1B), systematic and nonartifactual (e.g., motion-driven) activity is found in the posterior temporal and medial parietal regions. Considering the matching of our conditions discussed above (and in more detail elsewhere12), which follows previously described criteria,17 the multiple levels of stimuli randomization, and the knowledge that simple cues such as those employed here do not lead, in the absence of top-down activity, to protracted activations (see reference 30 for a data-based investigation of this point, and references 12, 16, and 17 for discussion), we follow previous literature31 in interpreting these systematic activations as marking top-down activity.
Our second main finding relates to the well-documented problem of MCS patients being mistakenly diagnosed as VS (estimated to be approximately 40%)4–7 and the ability of neuroimaging to uncover willful “brain behavior” in a subset of patients unable to produce unambiguous and recognizable willful motor behavior.16 Indeed, in our sample, 37% of patients who appeared unresponsive in bedside clinical evaluations could nonetheless produce willful brain responses during the neuroimaging assessment. The power of neuroimaging in this context has now been demonstrated in multiple instances.26,32,33 Furthermore, in a simple adaptation of our design,12 it has been recently shown that a nonresponsive patient could answer simple questions by selectively deploying top-down attention.34 However, it is important to note that the dissociation between what is observable at the bedside and what is observable with neuroimaging can go both ways,31 stressing the fact that behavioral and neuroimaging assessments are best seen as complementary tools.10 Indeed, while 3 of the 8 patients who appeared unresponsive at the bedside could demonstrate a state of consciousness via neuroimaging-based testing, 12 of the 19 patients who could demonstrate consciousness in standard clinical assessments failed to show any detectable activation in our task, a finding that further highlights the complexity of interpreting negative results. While this result might be expected in patients characterized by minimal and fluctuating signs of consciousness (i.e., MCS patients), it is important to stress that we also observed negative results in 3 eMCS, further stressing the complex relationship between the ability to demonstrate willful behavior at the bedside and in advanced neuroimaging protocols.31
Third, the PPI analysis shows that, in this sample, the difference between patients who did or did not respond to the task relates directly to the strength of activation in cortico-thalamo-cortical circuits uniting the anterior segments of thalamus and prefrontal cortex. These specific regions are believed to underlie willful behavior35 and to be implicated in the recovery from disorders of consciousness.11,23,36 Indeed, thalamic integrity has been related to the severity of a patient's disorder of consciousness in both the acute22 and chronic37 settings. Furthermore, changes in thalamo-cortical connectivity have also been shown to underlie the dynamic reconfiguration of brain networks during loss of consciousness by anesthetic agent.38 While we stress that because of the low sample the results should not be generalized beyond our cohort, these findings are consistent with the idea that functional or structural impairment (which we cannot distinguish in our dataset) within a cortico-striatopallidal-thalamo-cortical mesocircuit is characteristic of severe DOC.23,35
In this work, we have shown that several levels of cognitive processing, including the ability to comprehend verbal information, maintain information through time, and adopt voluntary mindsets—a set of high-level cognitive processes thought to be crucial for consciousness39—can be maintained in patients with DOC. This finding thus informs the important discussion of which cognitive processes might be retained after severe brain injury. Our findings are particularly relevant to the subset of patients diagnosed as being in a VS prior to their neuroimaging examination, further confirming the misdiagnosis rates that have been reported in retrospective studies,4,5 evaluation of different clinical protocols,6,7 as well as other neuroimaging assessments.25,26 We stress, however, that generalizable estimation of the incidence with which residual high-level cognition is present in patients with a VS diagnosis will require a larger sample and a more controlled experimental setting. Finally, we have also shown that the level of patient responsiveness in this task directly correlates with quantitative differences in thalamo-frontal connectivity, a finding that confirms the idea that DOC might be best interpreted as a “disconnection syndrome”11 whereby, because of an impairment in specific cortico-thalamo-cortical circuits, even a functioning cortex might not necessarily give rise to a state of consciousness.23,40
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients' families and the staff at the Royal Hospital for Neuro-disability (London, UK), Leamington Spa Rehabilitation Hospital (Warwick, UK), and the Gardens and Jacobs Neurological Center (Heartfordshire, UK).
GLOSSARY
- DOC
disorders of consciousness
- eMCS
exit from minimally conscious state
- FA
flip angle
- fMRI
functional MRI
- GLM
generalized linear model
- MCS
minimally conscious state
- MNI
Montreal Neurological Institute
- PPI
psycho–physiologic interaction approach
- ROI
region of interest
- TE
echo time
- TR
repetition time
- VS
vegetative state
Footnotes
Editorial, page 114
AUTHOR CONTRIBUTIONS
Martin M. Monti: designed the study, collected neuroimaging data, performed data analysis, interpreted the results, and drafted the manuscript. Matthew Rosenberg: performed data analysis and revised the manuscript. Paola Finoia: collected and interpreted behavioral data. Evelyn Kamau: collected and interpreted behavioral data and revised the manuscript. John D. Pickard: revised the manuscript. Adrian M. Owen: designed the study and revised the manuscript.
STUDY FUNDING
Supported by a James S. McDonnell Foundation “Scholar Award,” Medical Research Council (U.1055.01.002.00007.01 and U.1055.01.002.00001.01), and European Commission ICT Programme Project FP7-247919.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jennett B, Plum F. Persistent vegetative state after brain damage. RN 1972;35:ICU1–ICU4. [PubMed] [Google Scholar]
- 2.Monti MM, Laureys S, Owen AM. The vegetative state. BMJ 2010;341:c3765. [DOI] [PubMed] [Google Scholar]
- 3.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58:349–353. [DOI] [PubMed] [Google Scholar]
- 4.Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ 1996;313:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993;43:1465–1467. [DOI] [PubMed] [Google Scholar]
- 6.Schnakers C, Giacino J, Kalmar K, et al. Does the FOUR score correctly diagnose the vegetative and minimally conscious states? Ann Neurol 2006;60:744–745; author reply 745. [DOI] [PubMed] [Google Scholar]
- 7.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fins JJ, Illes J, Bernat JL, Hirsch J, Laureys S, Murphy E. Neuroimaging and disorders of consciousness: envisioning an ethical research agenda. Am J Bioeth 2008;8:3–12. [DOI] [PubMed] [Google Scholar]
- 9.Jennett B. The vegetative state. J Neurol Neurosurg Psychiatry 2002;73:355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti MM. Ethics, neuroimaging and disorders of consciousness: what is the question? AJOB Neurosci 2013;4:1–2. [Google Scholar]
- 11.Monti MM. Cognition in the vegetative state. Annu Rev Clin Psychol 2012;8:431–454. [DOI] [PubMed] [Google Scholar]
- 12.Monti MM, Coleman MR, Owen AM. Executive functions in the absence of behavior: functional imaging of the minimally conscious state. Prog Brain Res 2009;177:249–260. [DOI] [PubMed] [Google Scholar]
- 13.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale–Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 14.Bruno MA, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011;258:1373–1384. [DOI] [PubMed] [Google Scholar]
- 15.Adapa RM, Davis MH, Stamatakis EA, Absalom AR, Menon DK. Neural correlates of successful semantic processing during propofol sedation. Hum Brain Mapp 2014;35:2935–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monti MM, Coleman MR, Owen AM. Neuroimaging and the vegetative state: resolving the behavioral assessment dilemma? Ann NY Acad Sci 2009;1157:81–89. [DOI] [PubMed] [Google Scholar]
- 17.Monti MM, Owen AM. Behavior in the brain using functional neuroimaging to assess residual cognition and awareness after severe brain injury. J Psychophysiol 2010;24:76–82. [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 19.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992;12:900–918. [DOI] [PubMed] [Google Scholar]
- 20.Friston KJ. Analyzing brain images: principles and overview. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human Brain Function. Waltham, MA: Academic Press; 1997:25–41. [Google Scholar]
- 21.Laureys S, Antoine S, Boly M, et al. Brain function in the vegetative state. Acta Neurol Belg 2002;102:177–185. [PubMed] [Google Scholar]
- 22.Lutkenhoff ES, McArthur DL, Hua X, Thompson PM, Vespa PM, Monti MM. Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury. Neuroimage Clin 2013;3:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010;33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 25.Cruse D, Chennu S, Chatelle C, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2011;378:2088–2094. [DOI] [PubMed] [Google Scholar]
- 26.Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–589. [DOI] [PubMed] [Google Scholar]
- 27.Owen AM, Coleman MR. Functional neuroimaging of the vegetative state. Nat Rev Neurosci 2008;9:235–243. [DOI] [PubMed] [Google Scholar]
- 28.Perrin F, Schnakers C, Schabus M, et al. Brain response to one's own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch Neurol 2006;63:562–569. [DOI] [PubMed] [Google Scholar]
- 29.Schnakers C, Perrin F, Schabus M, et al. Voluntary brain processing in disorders of consciousness. Neurology 2008;71:1614–1620. [DOI] [PubMed] [Google Scholar]
- 30.Owen AM, Coleman MR, Boly M, et al. Response to comments on “Detecting awareness in the vegetative state.” Science 2007;315. [DOI] [PubMed] [Google Scholar]
- 31.Bardin JC, Fins JJ, Katz DI, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain 2011;134:769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chennu S, Finoia P, Kamau E, et al. Dissociable endogenous and exogenous attention in disorders of consciousness. Neuroimage Clin 2013;3:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monti MM, Pickard JD, Owen AM. Visual cognition in disorders of consciousness: from V1 to top-down attention. Hum Brain Mapp 2013;34:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naci L, Owen AM. Making every word count for nonresponsive patients. JAMA Neurol 2013;70:1235–1241. [DOI] [PubMed] [Google Scholar]
- 35.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann NY Acad Sci 2008;1129:105–118. [DOI] [PubMed] [Google Scholar]
- 36.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 2000;355:1790–1791. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Espejo D, Bekinschtein T, Monti MM, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage 2011;54:103–112. [DOI] [PubMed] [Google Scholar]
- 38.Monti MM, Lutkenhoff ES, Rubinov M, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol 2013;9:e1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001;79:1–37. [DOI] [PubMed] [Google Scholar]
- 40.Schiff ND, Ribary U, Plum F, Llinás R. Words without mind. J Cogn Neurosci 1999;11:650–656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.