We identified several families in Antioquia, Colombia, with early-onset Alzheimer disease (AD) due to the mendelian autosomal dominant inheritance of a PSEN1 E280A gene mutation. Extended family members were interviewed and parish baptism certificates in Antioquian municipalities examined.1 The size of these extended families (including carriers and noncarriers) approaches 5,000 individuals. Full genomes in carriers proved a single founder.2 To support an AD prevention clinical trial, we established a registry in 2010 of all family members over age 8 years.3 Since then we genotyped 3,407 family members and identified 823 (24%) carriers of the PSEN1 E280A mutation. The Comite de Bioetica de la Sede de Investigacion Universitaria, SIU Universidad de Antioquia, approved this study. All participants provided written informed consent. Despite the size of this exceptionally large family and frequent consanguinity, homozygosity at this gene locus had not been reported. The apparent absence of homozygous PSEN1 mutations led to the speculation that E280A homozygosity could be lethal. Generally, homozygous dominant mutations are more severely affected than heterozygotes in both humans and model systems.4 However, human cases in which dominant point mutations are homozygous are rare.
We identified 6 individuals with homozygous PSEN1 E280A gene mutation (g.50024A>C) (figure). In all cases where ascertainable, the parents were mutation carriers. For determination of cognitive status, we utilized the behavior rating scale developed by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) and a composite cognitive test consistent with National Institute on Aging criteria for mild cognitive impairment and dementia.5 To preserve subject anonymity, we present key data in aggregate form. Two of the 6 subjects (age range, 44–46 years) had dementia for 1 to 7 years before the time of ascertainment (range of dementia onset 37–45 years). The age range of the remaining adults with dementia was 27–38 years; there was one child, aged 11 years, with mild mental retardation. Five of the 6 were female. We adjusted scoring on cognitive status tests for those individuals with minimal or no schooling by setting the cutoff at 1–1.5 SD below the population mean. Education and CERAD cutoffs were based on data for the Antioquia cohort. Most individuals with the homozygous mutation live in rural areas and had little or no schooling.
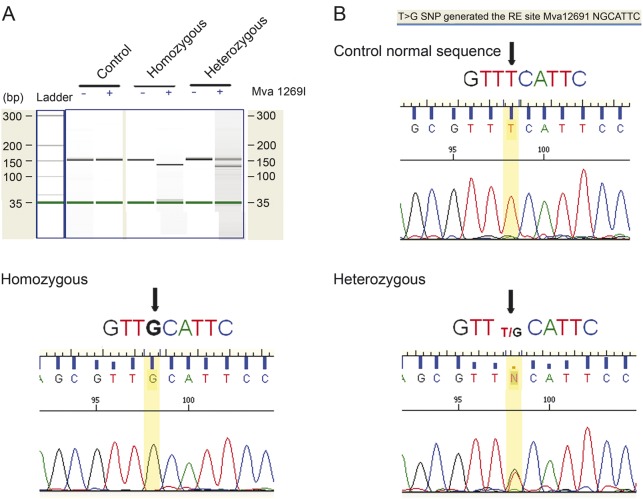
Figure. Genotype analysis.
(A) PSEN1 was amplified using the following primers—forward AACAGCTCAGGAGAGGAATG3, reverse TGAACAGAGTAG—around the mutation site. We used 40 ng of PCR product for restriction digestion with 0.5 units of Mva1269I (Fermentas, Vilnius, Lithuania) for 16 hours at 37°C, and cleaned digestion products using the Qiagen clean up reaction kit. We ran the products on a Bioanalyzer DNA chip (Agilent, Santa Clara, CA). (B) In one patient, we confirmed the mutation by sequencing 20 ng of PCR product (sent to UCLA genotyping and sequencing core). The reported sequences were analyzed using Sequence scanner v1.0 (Applied Biosystems, Foster City, CA).
Discussion.
There are no previous reports of individuals with homozygous mutations among familial AD patients. We demonstrate that individuals with the homozygous PSEN1 E280A can be viable; however, we have not excluded the possibility that other protective mutations may be present. Nor do these data apply to the viability of individuals with other AD mutations. Some subjects have children with a 100% likelihood of getting the disease. The small sample does not allow statistical conclusions as to whether homozygosity accelerates the age at AD onset. However, the E280A kindred at large have a very narrow range for mean age at onset, with very few individuals aged more than 1 SD different at onset.6 Among 449 mutation carriers with symptoms, the mean age for MCI onset was 45 years and for AD was 50 years. Among the homozygotes, 2 individuals had AD dementia, which presented 5 and 13 years before the mean age at onset for the entire kindred. Data for other individuals were not informative for dementia due to younger age at the time of this study. PSEN1 mutations affect both epsilon cleavage and subsequent carboxypeptidase-like processing of Aβ, resulting in longer Aβ peptides of 42, 43, or more amino acids and reduced peptide of 40 amino acids in length.7 Therefore, the composition of the Aβ peptides in these individuals would be of interest. Nevertheless, even with homozygosity, disease onset did not occur until individuals were in their 40s. Notch, Syndecan, and N-cadherin are also γ-secretase substrates, and therefore we might expect a phenotype related to these non-APP substrates if homozygosity resulted in complete loss of function. However, the homozygous mutation was not associated with any obvious phenotype related to these substrates. Several individuals in this sample who were unschooled, but did not have dementia, scored at the lower boundary of normal on cognitive assessments. We do not know if this was due to their socioeconomic status or mild baseline cognitive deficits independent of amyloid deposition. The fact that 5 of the 6 patients are female is notable (probability of 5 females out of 6 independent births = 0.09375), but insufficient to draw conclusions regarding increased lethality among males or consequent deviation from Hardy-Weinberg equilibrium. E280A homozygotes are viable and the phenotypic consequence of homozygosity may be moderately accelerated age at onset relative to heterozygotes.
Footnotes
Author contributions: K.S.K., E.R., S.R., C.H., F.L.: conception of the study, writing and editing of the manuscript. P.T., C.H., E.R., F.L., M.G., S.R., J.L., W.C., S.S.: writing and approval of protocols. S.M., C.M., L.L., H.L.: conduct and review of subject assessments. M.L.A., G.G., K.S.K.: analysis of DNA samples.
Study funding: No targeted funding reported.
Disclosure: K. Kosik serves on the board of directors and holds stock in Minerva Biotechnologies. He serves on the scientific advisory board and holds stock options in ADRx and was a paid consultant to Genentech. Dr. Kosik receives funding from NIH grant RO1MH093661, receives personal fees and research funding from the Rainwater Charitable Foundation, receives funding from the Dr. Miriam and Sheldon Adelson Medical Foundation, receives honoraria from the Society for Neuroscience for serving on the BrainFacts.org editorial board, has received funding from the Hillblom Foundation, and has received speaker fees or travel reimbursement from Learning & the Brain produced by Public Information Resources, Inc., Emory School of Medicine, Fundación Reina Sofía, and Imagine Solutions Conference. C. Muñoz receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. L. Lopez receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. M. Arcila reports no disclosures relevant to the manuscript. G. García receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. L. Madrigal receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. S. Moreno reports no disclosures relevant to the manuscript. S. Ríos Romenets receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. H. Lopez receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. M. Gutierrez receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. J. Langbaum is funded by NIH grants RF1 AG041705 and UF1 AG046150 and receives research support from Genentech, the Banner Alzheimer's Foundation, and the State of Arizona. W. Cho is a full-time employee of Genentech, a member of the Roche Group that has provided funding for the Alzheimer's Prevention Initiative (API) Colombian study, and holds stock options in Roche. S. Suliman is an employee of Genentech, a member of the Roche Group that has provided funding for the Alzheimer's Prevention Initiative (API) Colombian study. P. Tariot reports personal fees from Abbott Laboratories, AbbVie, AC Immune, Adamas, Boehringer-Ingleheim, California Pacific Medical Center, Chase Pharmaceuticals, Chiesi, Continuing Medical Education, Elan, Medavante, Merz, Otsuka, and Sanofi-Aventis; and personal fees and site compensation for research activities from Avanir, Avid, Bristol Myers Squibb, Cognoptix, GlaxoSmithKline, Janssen, Eli Lilly, Medivation, Merck and Company, and Roche. Dr. Tariot receives site compensation for research activities from AstraZeneca, Baxter Healthcare, Functional Neuromodulation, General Electric, Genentech, Pfizer, Targacept, Toyama, National Institute on Aging, and Arizona Department of Health Services. Dr. Tariot has stock options from Adamas unrelated to the submitted work and a patent pending for Biomarkers of Alzheimer's Disease. C. Ho is a full-time employee of Genentech, a member of the Roche Group that has provided funding for the Alzheimer's Prevention Initiative (API) Colombian study, and holds stock options in Roche. E. Reiman has served as a scientific advisor to AstraZeneca, CereSpir, Eisai, Eli Lilly, and GlaxoSmithKline. He and his colleagues have received research support from Genentech, Avid Radiopharmaeuticals/Eli Lilly, the Banner Alzheimer's Foundation, Nomis Foundation, an Anonymous Foundation, the state of Arizona, and the National Institute on Aging. F. Lopera receives grant and contract support from the NIA, Banner Alzheimer's Foundation, Genentech, Colciensius, and an anonymous foundation to develop the API ADAD Registry and help conduct the API ADAD Trial in Colombia. Go to Neurology.org for full disclosures.
References
- 1.Lopera F, Ardilla A, Martínez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 1997;277:793–799. [PubMed] [Google Scholar]
- 2.Lalli MA, Cox HC, Arcila ML, et al. Origin of the PSEN1 E280A mutation causing early-onset Alzheimer's disease. Alzheimers Dement 2014;10(5 suppl):S277–S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiman EM, Langbaum JB, Fleisher AS, et al. Alzheimer's prevention initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26(suppl 3):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlotogora J. Dominance and homozygosity. Am J Med Genet 1997;68:412–416. [DOI] [PubMed] [Google Scholar]
- 5.Ayutyanont N, Langbaum JB, Hendrix SB, et al. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry 2014;75:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol 2011;10:213–220. [DOI] [PubMed] [Google Scholar]
- 7.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease: talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep 2007;8:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]