Abstract
Objective:
To determine the safety and tolerability of 3 doses of intranasal oxytocin (Syntocinon; Novartis, Bern, Switzerland) administered to patients with frontotemporal dementia (FTD).
Methods:
We conducted a randomized, parallel-group, double-blind, placebo-controlled study using a dose-escalation design to test 3 clinically feasible doses of intranasal oxytocin (24, 48, or 72 IU) administered twice daily for 1 week to 23 patients with behavioral variant FTD or semantic dementia (clinicaltrials.gov registration number NCT01386333). Primary outcome measures were safety and tolerability at each dose. Secondary measures explored efficacy across the combined oxytocin vs placebo groups and examined potential dose-related effects.
Results:
All 3 doses of intranasal oxytocin were safe and well tolerated.
Conclusions:
A multicenter trial is warranted to determine the therapeutic efficacy of long-term intranasal oxytocin for behavioral symptoms in FTD.
Classification of evidence:
This study provides Class I evidence that for patients with FTD, intranasal oxytocin is not significantly associated with adverse events or significant changes in the overall neuropsychiatric inventory.
Loss of empathy is a hallmark symptom of the most common subtype of frontotemporal dementia (FTD), behavioral variant FTD (bvFTD).1,2 Presently, there are no treatments for the emotional blunting, lack of empathy, and social behavioral decline in FTD. Without treatments targeting these difficulties, physicians are unable to manage the symptoms most destructive and emotionally challenging to caregivers.3,4
Research suggests that the neuropeptide oxytocin is an important mediator of social behavior, potentially enhancing empathy and prosocial behaviors.5 Oxytocin administration to healthy adults or patients with autism improves emotional expression processing,6,7 empathy,8 and cooperative behavior.9 A single dose of intranasal oxytocin vs placebo was associated with a transient improvement in social and neuropsychiatric behaviors in patients with FTD.10 Thus, upregulation of oxytocin-mediated mechanisms of empathy and prosocial behavior may be a potential treatment approach in FTD.
The optimal dosage and outcome measures for a clinical trial of oxytocin in FTD are unknown. Animal studies report increases in some forms of aggression after oxytocin administration, leading to concerns regarding potential adverse effects of extended dosing in humans.11 Other potential dose-limiting toxicities include uterine contractions and hyponatremia. The objectives of this study were (1) to determine the optimum dosage of intranasal oxytocin based on safety, feasibility, and tolerability in patients with FTD; (2) to preliminarily evaluate the efficacy of repeated intranasal oxytocin dosing for improving empathic behaviors and neuropsychiatric symptoms in FTD; and (3) to identify which outcome measures are most sensitive to the effects of oxytocin in patients with FTD.
METHODS
This was a randomized, parallel-group, double-blind, placebo-controlled trial of 3 doses of intranasal oxytocin (24, 48, and 72 IU) in patients with FTD based on a modified dose-escalation design.12,13 Medication was administered twice daily for 1 week with telephone assessments after 1 week washout. The study was completed at the Cognitive Neurology and Alzheimer Research Unit at St. Joseph's Hospitals, London, Canada. Study visits occurred between June 2011 and October 2013. Primary outcome measures were safety and tolerability of each dose of oxytocin. Secondary measures explored the efficacy of oxytocin on ameliorating the behavioral symptoms and emotion deficits hallmark in FTD.
Participants.
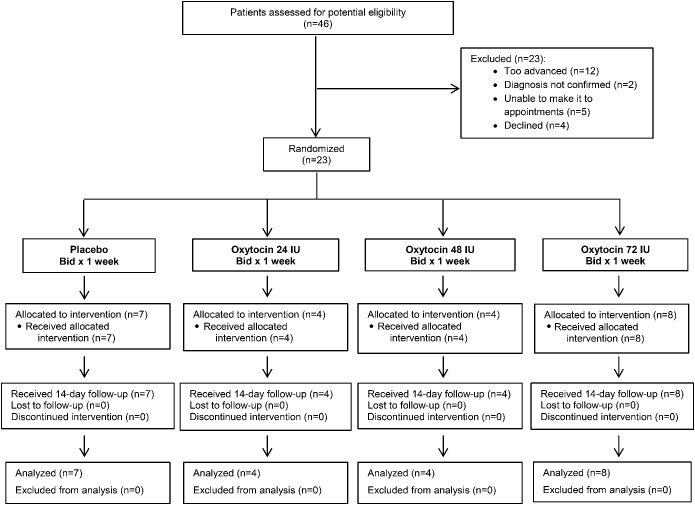
Forty-six patients known to our clinic or referred for the study were reviewed for potential eligibility (figure 1). For inclusion, participants had to meet the revised international consensus criteria for probable bvFTD14 or Neary criteria for semantic dementia with concomitant behavioral features, and demonstrate significant deficits in empathy as reported by caregiver responses on the Frontal Behavioral Inventory (FBI).15 Exclusion criteria included history of stroke, tumor, or brain lesion (see appendix e-1 on the Neurology® Web site at Neurology.org for detailed inclusion/exclusion criteria). While no specific cutoff scores to limit disease severity were used, on chart review, 12 patients (figure 1) were found to be too advanced to participate, based on their inability to follow basic instructions in prior clinical assessments.
Figure 1. Trial profile.
Bid = twice a day.
Procedures.
Screening visit.
At the first visit, neurologic and psychometric assessments were completed to confirm the diagnosis of probable FTD, including symptoms of emotional blunting/empathy deficits (figure e-1). Caregivers completed patient baseline behavioral and severity ratings on the Neuropsychiatric Inventory (NPI),16 Interpersonal Reactivity Index (IRI),17 FBI,15 Frontotemporal Lobar Degeneration–modified Clinical Dementia Rating,18 and Frontotemporal Dementia Rating Scale19 (see appendix e-1 supplemental methods for scale descriptions). The NPI was designated a priori as the main efficacy measure based on prior findings.10 The IRI includes an “empathic concern” scale, which we predicted would capture increases in empathy after oxytocin treatment. Patients completed an exploratory baseline performance measure of empathy, the Multifaceted Empathy Task.20 Vital signs and serum sodium levels were measured before the first dose of oxytocin. Premenopausal women completed urine pregnancy tests on the screening visit and prior to the first dose of medication.
Randomization, masking, and dose escalation.
This was a double-blind, parallel-group study. Because of the frequent occurrence of behavioral problems in FTD, which could confound attribution of adverse behaviors, we included a small placebo group (figure e-2). The intranasal oxytocin was manufactured by Novartis Switzerland (Syntocinon) and purchased from International Apotheke (Bern, Switzerland). The study drug and placebo (Salinex saline spray) were repackaged by our institutional research pharmacy (appendix e-1 supplemental methods). The dosages selected (24, 48, and 72 IU twice daily) are based on Fibonacci sequences recommended for dose escalation12,13 and supported by (1) prior published studies of intranasal oxytocin on social cognition and neuropsychiatric symptoms, which indicate beneficial effects without significant safety concerns at these doses; and (2) the volume that can be absorbed intranasally. Optimal intranasal dose volumes are considered to be less than 0.5 mL per nostril; thus, for larger volumes (48 and 72 IU), doses were divided over 10-minute intervals to maximize absorption (i.e., 3 sprays per nostril every 10 minutes). The number of intranasal sprays was matched in the oxytocin and placebo groups. Based on the estimated duration of action of oxytocin in the CNS between 2 and 5 hours,21,22 twice-daily dosing (8 am/2 pm) was used to augment CNS levels of oxytocin during daytime hours. Caregivers administered the sprays and compliance was measured through logs recording administration times and measurement of remaining volumes on day 7.
Four cohorts were randomized to oxytocin or placebo (figure e-2). All randomization was conducted by the research pharmacy. The randomization ratio for the initial 3 cohorts was 4 treatment to 1 placebo. After identification of the highest dose tolerated, a final cohort of 8 patients was randomized in a 1:1 ratio to oxytocin or placebo at the maximum tolerated dose (72 IU) so that a total of 8 participants received the maximum dose.
On day 7, study drug was administered in the morning by the study physician. Twenty minutes after the administration of the nasal solution, safety assessments were completed, followed by patient- and caregiver-completed behavioral measures and physician-completed Clinician's Global Impression of Change (figure e-1). Caregivers were instructed to report on the average behaviors over the 7-day treatment period. Adverse events were assessed each visit through standardized medical symptom-based questionnaires, and heart rate, blood pressure, and serum sodium levels were monitored at baseline and posttreatment on day 7. A follow-up telephone assessment was conducted with caregivers on day 14, after 7 days of medication washout, to assess for any unanticipated effects of medication discontinuation.
Methods/primary research question: Is intranasal oxytocin safe and well tolerated when administered twice daily to patients with bvFTD?
Standard protocol approvals, registrations, and patient consents.
The study was approved by the human ethics review board at Western University, London, Canada, and by Health Canada. The trial is registered at clinicaltrials.gov, registration number NCT01386333. Written informed consent was obtained from all patients and their caregivers.
Statistical analysis.
The t tests were performed on descriptive data to determine any group differences at baseline (age, age at onset), and χ2 analysis for sex. Because of the small sample size, brief duration of treatment, and multiple secondary outcome measures of interest, formal statistical testing is not reported for the secondary outcome measures.
RESULTS
Twenty-three patients with FTD exhibiting prominent behavioral symptoms and emotional blunting met trial eligibility criteria and were enrolled (n = 20 bvFTD; with 2 of 20 also with features of progressive nonfluent aphasia and 3 with semantic dementia with prominent behavioral features) (table e-1 and figure e-3). For the 3 patients with semantic dementia, randomization resulted in one assigned to the placebo treatment, one to 24 IU oxytocin twice daily, and one to 72 IU oxytocin twice daily. All 23 participants were able to complete the study and were included in the analysis. According to the logs and caregiver reports, all patients received each scheduled dose of the study drug. This was confirmed with measurements of the volume of remaining medication.
Baseline demographics and screening material.
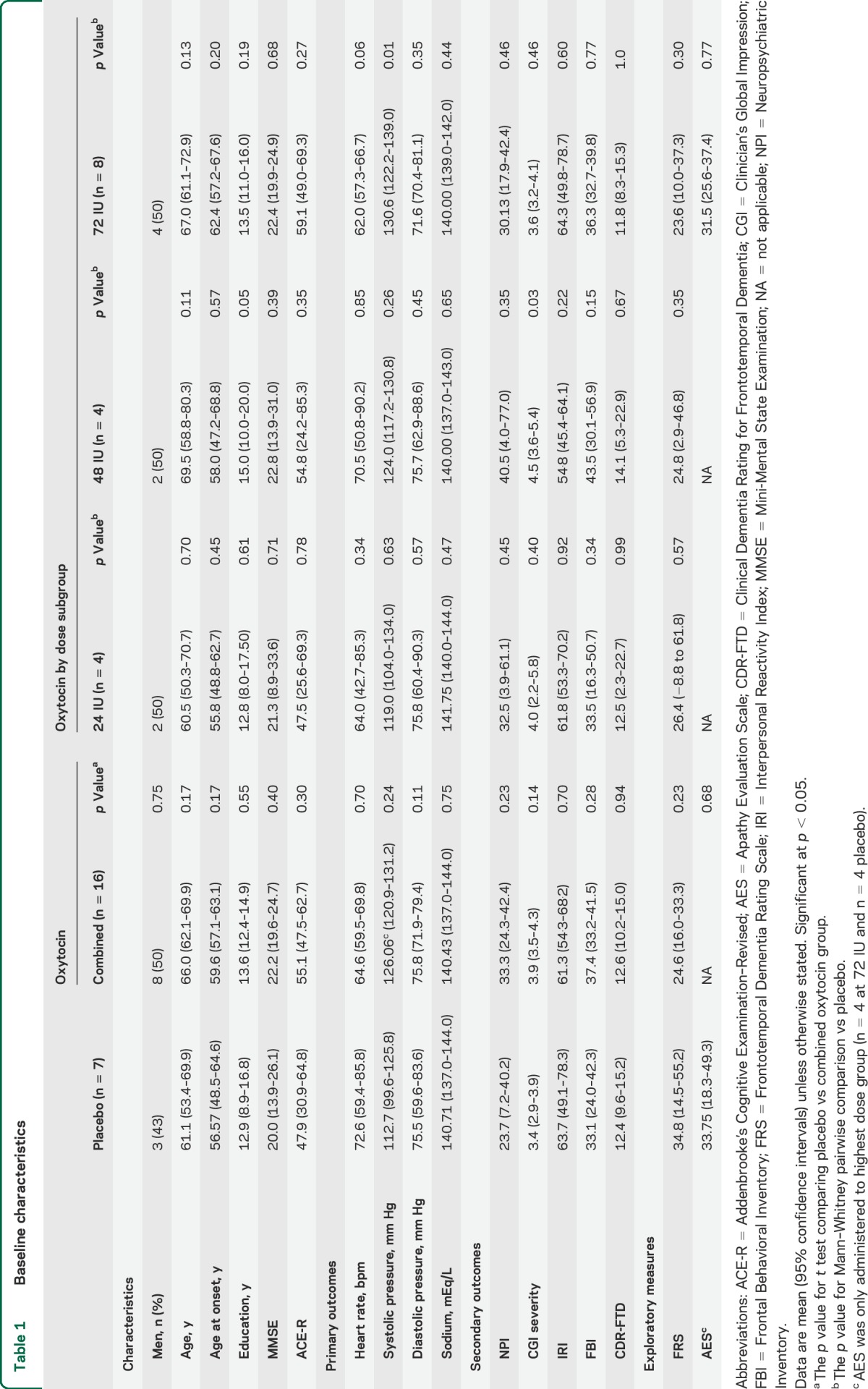
Demographic information and neuropsychological testing are presented in table 1. There were no significant differences in baseline demographics, cognitive test scores, or disease severity between the combined oxytocin and placebo treatment groups.
Table 1.
Baseline characteristics
Primary outcome measures: Safety and tolerability and adverse events.
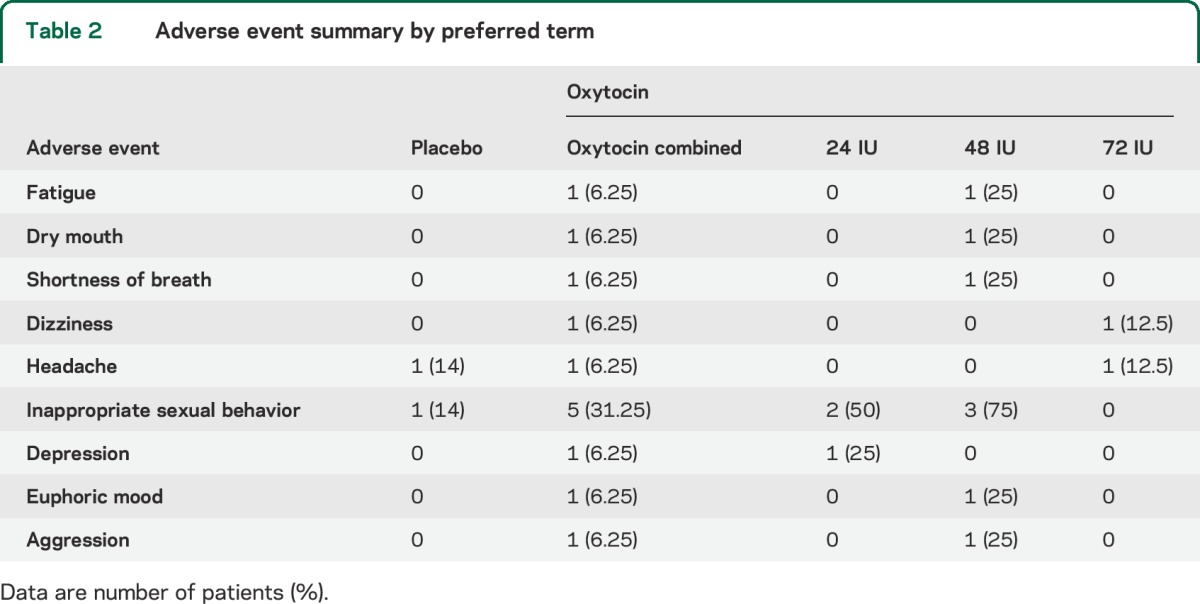
There were no serious adverse events during the course of the study (table 2). After a caregiver report of increased hypersexual behaviors, the hypersexuality item on the FBI was reviewed for all participants, and was increased in 31% of patients receiving oxytocin, compared with 14% of the placebo group (χ21 = 0.73, p = 0.4).
Table 2.
Adverse event summary by preferred term
Safety measures.
Comparison of heart rate, systolic and diastolic blood pressure, and serum sodium levels at baseline (pretreatment) and day 7 revealed no clinically relevant differences between treatment groups (table e-2).
Secondary outcome measures: Efficacy.
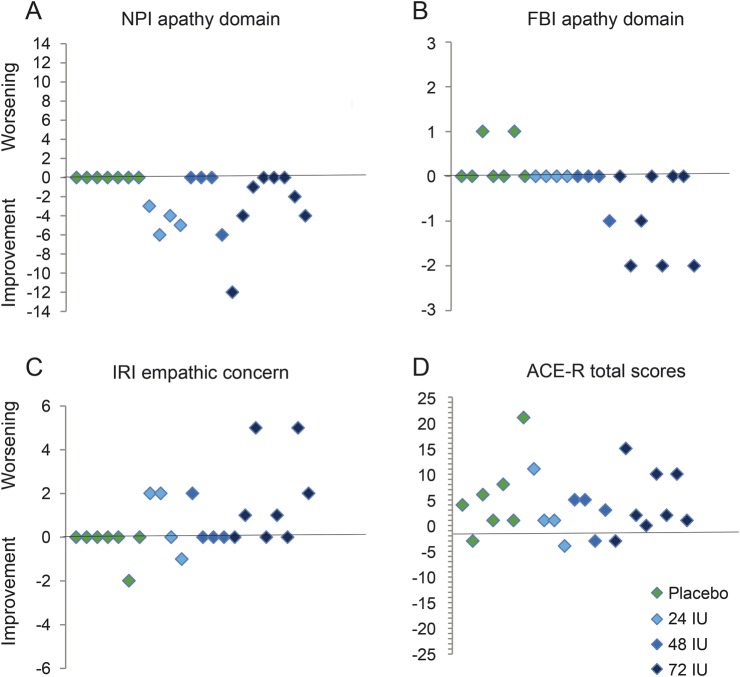
Mean differences and 95% confidence intervals for the secondary efficacy measures are reported in table 3. Possible trends of improvement were observed for the oxytocin-treated group on the measures hypothesized to be sensitive to the effects of oxytocin including the NPI apathy and FBI apathy domains and the IRI empathic concern scale (figure 2), with the Addenbrooke's Cognitive Examination–Revised included as a nonsocial cognitive measure for comparison.
Table 3.
Mean differences in change from baseline to day 7 of treatment
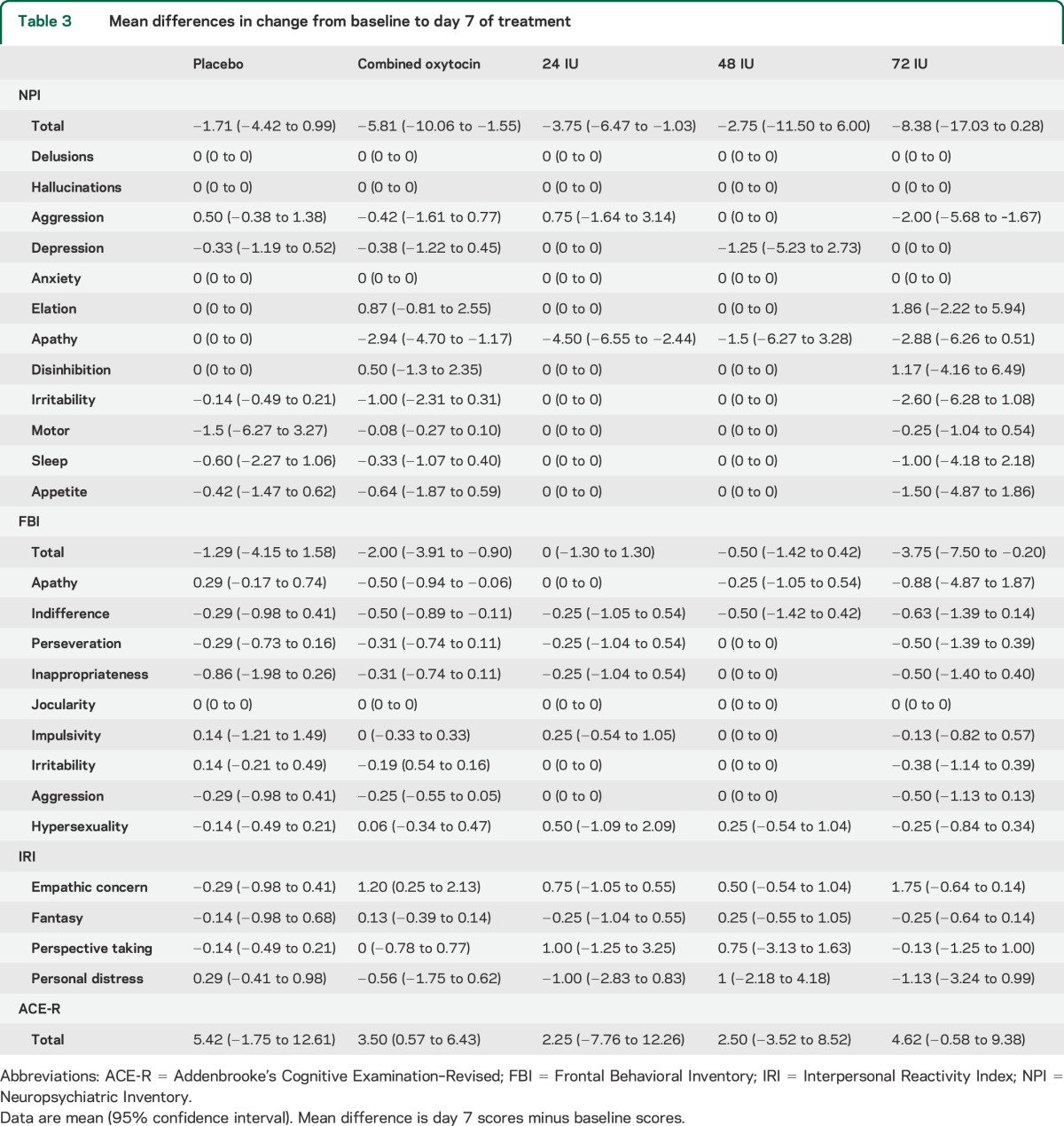
Figure 2. Clinical efficacy measures.
Scatter plots depicting individual patients' change in scores from baseline to day 7 of treatment according to treatment group in the combined oxytocin vs placebo groups for (A) NPI apathy domain, (B) FBI apathy domain, (C) IRI empathic concern scale, and (D) ACE-R. ACE-R = Addenbrooke's Cognitive Examination–Revised; FBI = Frontal Behavioral Inventory; IRI = Interpersonal Reactivity Index; NPI = Neuropsychiatric Inventory.
Classification of evidence.
This interventional study provides Class I evidence that intranasal oxytocin at 24, 48, and 72 IU twice daily is safe and well tolerated in patients with FTD.
DISCUSSION
All 3 doses of intranasal oxytocin administered twice daily for 1 week were safe and well tolerated in patients with FTD. We identified convergent changes in subscales of the NPI, FBI, and IRI in oxytocin compared with placebo, suggesting that intranasal oxytocin may improve a subset of behavioral symptoms in FTD, namely, levels of apathy and expressions of empathy, resulting in improved patient–caregiver interactions (based on informant interviews). While there were no reported instances of increased aggression, one-third of patients receiving oxytocin had reported increases in hypersexual behaviors (vs 14% in placebo group). These increases were mild, but raise concerns that this effect could become a limiting side effect for some patients receiving oxytocin. While the small sample size and other factors precluded statistical analysis of the secondary efficacy measures, examination of performance suggests that intranasal oxytocin may improve a subset of behavioral symptoms in FTD, namely, levels of apathy and expressions of empathy. As anticipated, no trends of effects of intranasal oxytocin were observed on nonsocial aspects of cognition. Taken together, these results suggest a favorable benefit/risk ratio for chronic oxytocin treatment over 1 week in FTD. Because approximately 70% to 80% of caregivers of patients with FTD rate loss of empathy and apathy as particularly burdensome,5 our findings strongly suggest that longer-term efficacy studies of intranasal oxytocin for treating social and behavioral deficits in FTD are feasible and should be pursued.
Although preliminary and requiring confirmation by a larger study powered for efficacy, the results indicate that effects of oxytocin on patients' outward empathic behaviors may best be captured by caregiver interview tools that include measures of apathy and empathy (the NPI, FBI, and IRI).
While we found all oxytocin doses to be safe and well tolerated, the efficacy data indicate the maximum feasible dose used (72 IU) may be most promising. We found a significant, dose-related improvement on the FBI apathy subscale, although the same dose response was not observed on the NPI. Inspection of the interaction between Clinician's Global Impression severity index by dose group suggests that the failed response in the 48 IU group may be confounded by greater disease severity in that cohort relative to the placebo group (U = 3, p = 0.03) or the 72 IU group (U = 5, p = 0.04) (table 1). While there was a correlation between the FBI and NPI apathy scores (t = 0.32, p < 0.05 1-tailed, Kendall tau test), slight differences in results on these 2 apathy measures may be attributable to the differences in the interview prompts they contain; the FBI item focuses more on social interactions (“Has the patient lost interest in friends or activities”) compared with the NPI (“Has the patient lost interest in the world around him/her?”). Supporting this hypothesis, a correlation was observed between the IRI empathic concern and the FBI apathy scores (t = 0.46, p < 0.05 2-tailed), but not the NPI apathy and IRI empathic concern scores.
There are few data regarding the effects of repeated dosing of oxytocin on behavior and cognition, with suggestions of possible habituation in other populations.8 In the present study, behavioral ratings were not collected after the first dose, and thus habituation to repeated dosing cannot be determined. However, day 7 caregiver reports indicate that at least some clinically apparent effects may be maintained over 1 week. Future clinical trials focused on efficacy may address this question with additional interim assessments. However, even if habituation occurs, the potential may remain for the use of intranasal oxytocin on an as-needed basis.
How oxytocin may increase empathy and improve corresponding social behaviors in patients with FTD has yet to be determined. The integrity of oxytocin-producing neurons in the hypothalamic nuclei is preserved in patients with TDP-43 (TAR DNA-binding protein 43) frontotemporal lobar degeneration,23 suggesting that disease pathology at the sites of afferent projections including the amygdala, orbitofrontal cortex, and insula may disrupt oxytocinergic systems, and that increased oxytocin receptor–dependent signaling to these regions may partially overcome deficits due to neuronal loss or dysfunction. In support of this hypothesis, intranasal oxytocin administration to mice increases levels of oxytocin in the amygdala and hippocampus after 30 minutes.24 This and related work has led to the hypothesis that oxytocin may mediate prosocial behaviors by increasing positive social stimulus processing, reducing anxiety related to threat cues, and improving the capacity to attend to social cues.25 Studies examining the neural response to oxytocin administration in FTD are under way to test this hypothesis.
Limitations of the study were that escalation to higher doses of oxytocin was not feasible because of the oxytocin formulation commercially available, the small sample size, and the relatively short duration of follow-up. However, the lack of limiting safety issues suggests that follow-up clinical trials should consider inclusion of higher maximum doses should they become available.
Effect sizes from the present study used to estimate the sample size required for a definitive study of the efficacy of intranasal oxytocin in FTD indicate that approximately 22 patients per group would be required (appendix e-1). An efficacy trial would be further strengthened by powering for possible differential sex and oxytocin receptor genotype effects that might influence the response to exogenous oxytocin administration, because the distribution of oxytocin receptors is known to vary by sex, and variations in the oxytocin receptor gene have been associated with differential baseline empathic traits.
The current study indicates that repeated doses of intranasal oxytocin are safe and well tolerated at doses up to 72 IU twice daily in patients with FTD. The results suggest that a multicenter trial is warranted to determine the therapeutic efficacy of intranasal oxytocin for the difficult and currently untreatable loss of empathy and related social behavior changes in FTD.
Supplementary Material
GLOSSARY
- bvFTD
behavioral variant frontotemporal dementia
- FBI
Frontal Behavioral Inventory
- FTD
frontotemporal dementia
- IRI
Interpersonal Reactivity Index
- NPI
Neuropsychiatric Inventory
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
E.C.F. obtained funding, designed and supervised the study, and wrote the report. A.B. participated in study conception and design, reviewed and made substantive comments on the report. A.K. participated in patient enrollment and made substantive comments on the report. M.C.T., J.W., M.B., and S.P. participated in patient recruitment and evaluation, and review and revision of the final report. I.D. and K.R. participated in study design and review and revision of the final report. S.J., J.M., and L.D.O. assisted with study conduct, data collection, cleaning, and analysis, and review and revision of the final report. M.B. assisted with statistical analysis and made substantive comments on the report.
STUDY FUNDING
The study was funded entirely by a peer-reviewed grant from the Canadian Consortium of Cognitive Clinical Research (C5R) and by the Hazel Soper Foundation (E.F.). Novartis and the International Apotheke had no role in the funding, study design, data collection, analysis, interpretation, or manuscript preparation.
DISCLOSURE
E. Finger is funded by Canadian Institutes of Health Research (CIHR) grants 286763, 288612, and 327387, by the Alzheimer Society of Canada, The Canadian Consortium for Clinical Cognitive Research, and by the Ontario Ministry of Research and Innovation, and has received research funding for clinical trials from TauRx, the Ontario Brain Institute, and the Alzheimer's Disease Neuroimaging Initiative. J. MacKinley and M. Blair report no disclosures. L. Oliver is funded by the Alzheimer Society of London and Middlesex. S. Jesso reports no disclosures. M. Tartaglia is funded by Alzheimer Society of Canada New Investigator's Grant and by CIHR 137083. M. Borrie received research support from CIHR, NIH, Alzheimer Society of Canada, Physician Services Incorporated, Ontario Brain Institute, St. Joseph Hospital Foundation, and Lawson Health Research Institute, and clinical trials funding from Pfizer, Purdue-Pharma, Neotherapeutics, HMR Pharmacia, Boehringer-Ingelheim, Sanofi-Synthélabo, Myriad Pharmaceuticals, ONO, Pharma USA, Neurochem, Elan, Elan/Wyeth, Eli Lilly, Hoffmann-La Roche, Bristol-Myers Squibb, and Baxter. He has served as a consultant for Pfizer, Janssen-Ortho, Novartis, Lundbeck, and Eli Lilly. J. Wells has received speakers honoraria from Merck, and was a past employee with stock ownership at Pfizer Inc. Her research is supported as primary site investigator or subinvestigator for clinical trials from Eisai Limited, Merck Canada Inc., TauRx Pharmaceuticals, Eli Lilly & Company, Bristol-Myers Squibb Canada, Lundbeck Canada Inc., Physician Services Inc., and the Ontario Brain Institute. I. Dziobek reports no disclosures. S. Pasternak receives funding from the CIHR operating grants MOP-82890 and MOP-10414. He has also received research funding for clinical trial from TauRx and the Alzheimer's Disease Neuroimaging Initiative. D. Mitchell is funded by CIHR grant 286763 and by NSERC. K. Rankin is funded by NIH/NIA 1R01 AG029577, P01 AG019724, and UCSF REAC. A. Kertesz reports no disclosures. A. Boxer has been a consultant for Asceneuron, Acetylon, Archer, Bristol-Myers Squibb, iPierian, received research support from Allon Therapeutics, Cortice Biosciences, Bristol-Myers Squibb, Janssen, Forest, Pfizer, Eli Lilly, and Genentech, and is funded by NIH grants R01AG038791, R01AG031278, and U54NS092089, the Tau Research Consortium, the Alzheimer's Association, and the Bluefield Project to Cure Frontotemporal Dementia. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 2.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005;18:28–36. [DOI] [PubMed] [Google Scholar]
- 3.Diehl-Schmid J, Schmidt EM, Nunnemann S, et al. Caregiver burden and needs in frontotemporal dementia. J Geriatr Psychiatry Neurol 2013;26:221–229. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol 2013;26:174–184. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008;322:900–904. [DOI] [PubMed] [Google Scholar]
- 6.Hollander E, Bartz J, Chaplin W, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry 2007;61:498–503. [DOI] [PubMed] [Google Scholar]
- 7.Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology 2010;209:225–232. [DOI] [PubMed] [Google Scholar]
- 8.Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 2010;30:4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005;435:673–676. [DOI] [PubMed] [Google Scholar]
- 10.Jesso S, Morlog D, Ross S, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 2011;134:2493–2501. [DOI] [PubMed] [Google Scholar]
- 11.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci 2005;25:6807–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storer B. Choosing a phase I design. In: John Crowley AH, Ankerst D, editors. Handbook of Statistics in Clinical Oncology. London: Chapman and Hall; 2006:59–75. [Google Scholar]
- 13.Storer BE. Design and analysis of phase I clinical trials. Biometrics 1989;45:925–937. [PubMed] [Google Scholar]
- 14.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of consensus diagnostic criteria in autopsy confirmed patients with behavioral variant frontotemporal dementia (bvFTD): first report of the international bvFTD Criteria Consortium (FTDC). Dement Geriatr Cogn Disord 2010;30. [Google Scholar]
- 15.Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997;24:29–36. [DOI] [PubMed] [Google Scholar]
- 16.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:S10–S16. [DOI] [PubMed] [Google Scholar]
- 17.Davis MH. A multidimensional approach to individual differences in empathy. In: JSAS Catalog of Selected Documents in Psychology. Washington, DC: American Psychological Association; 1980:85. [Google Scholar]
- 18.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010;74:1591–1597. [DOI] [PubMed] [Google Scholar]
- 20.Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord 2008;38:464–473. [DOI] [PubMed] [Google Scholar]
- 21.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516. [DOI] [PubMed] [Google Scholar]
- 22.Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 2013;3:3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diodati D, Cyn-Ang L, Kertesz A, Finger E. Pathologic evaluation of the supraoptic and paraventricular nuclei in dementia. Can J Neurol Sci 2012;39:213–219. [DOI] [PubMed] [Google Scholar]
- 24.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013;38:1985–1993. [DOI] [PubMed] [Google Scholar]
- 25.Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA 2010;107:9400–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.