Abstract
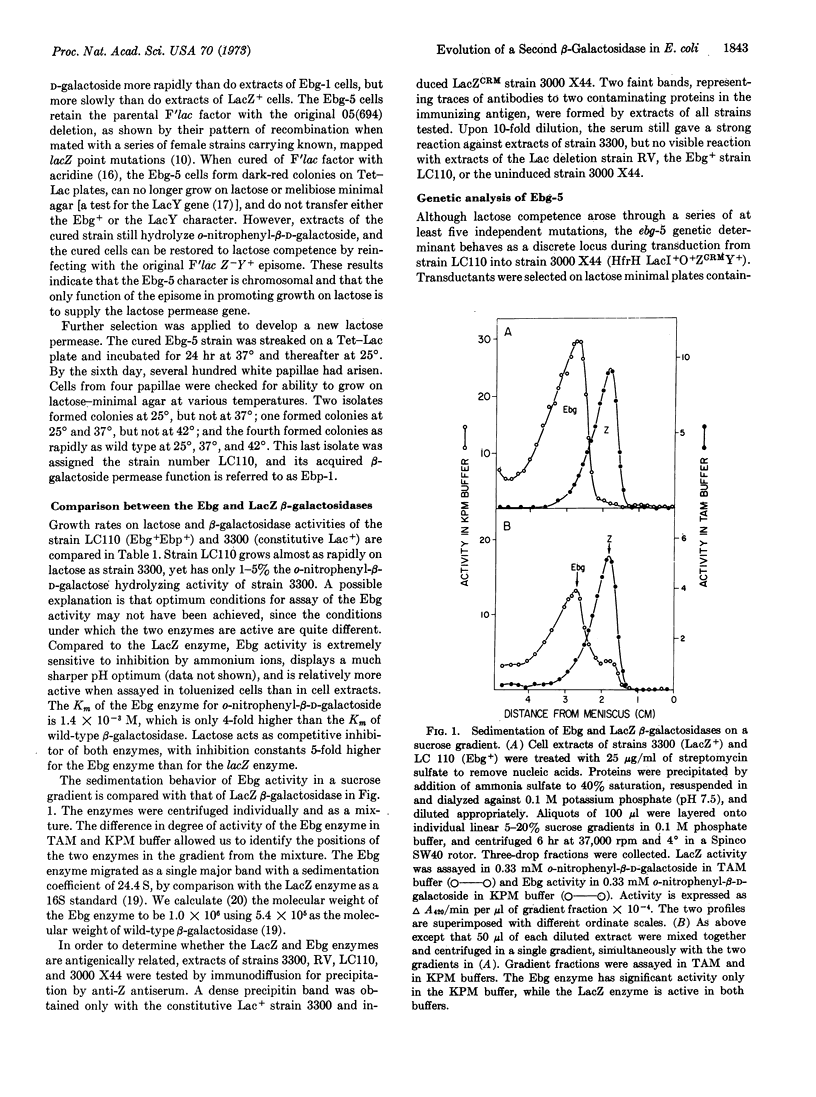
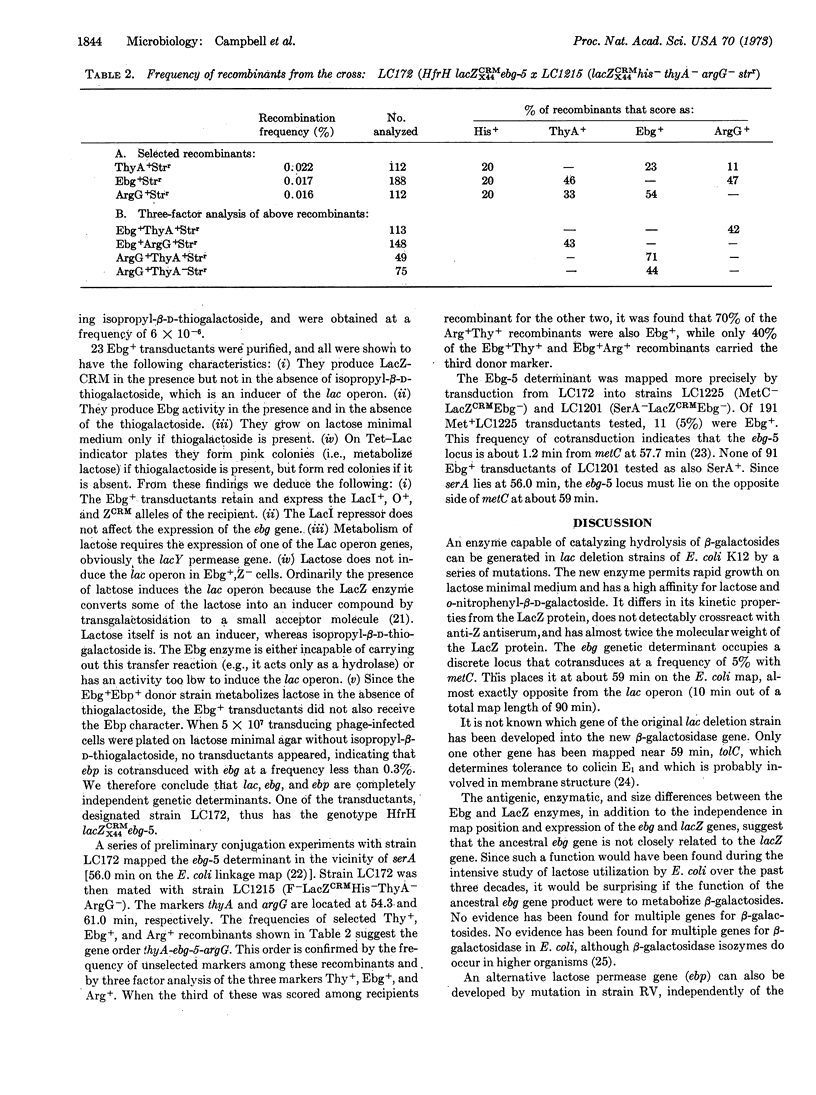
Mutants of E. coli K12 with deletions of the β-galactosidase gene (lacZ) can reacquire the ability to hydrolyze β-galactosides during prolonged intense selection for growth on lactose. Full lactose competence is restored through a sequence of at least five mutations. Cell extracts of these derived strains hydrolyze o-nitrophenyl-β-D-galactoside, the standard substrate for assay of β-galactosidase. The enzyme responsible for this activity differs in its immunological, kinetic, and sedimentation characteristics from the lacZ β-galactosidase of wild-type E. coli. Its genetic determinant, designated ebg-5, maps at 59 min on the E. coli chromosome, whereas the lac operon maps at 10 min. We suggest that a gene not involved in lactose utilization has been progressively changed into a form capable of specifying a β-galactosidase and that this process is similar to that whereby genes with new functions are evolved by natural selection.
Keywords: lac operon, gene mapping, enzyme characterization, lactose permease, enzyme evolution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., JOLIT M. Isolement par sélection de mutants d'Escherichia coli synthétisant spontanément l'amylomaltase et la beta-galactosidase. Ann Inst Pasteur (Paris) 1953 May;84(5):937–945. [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. The lac repressor-operator interaction. VII. A repressor with unique binding properties: the X86 repressor. J Mol Biol. 1972 Dec 14;72(1):139–152. doi: 10.1016/0022-2836(72)90075-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langridge J., Campbell J. H. Classification and intragenic position of mutations in the beta-galactosidase gene of Escherichia coli. Mol Gen Genet. 1969;103(4):339–347. doi: 10.1007/BF00383484. [DOI] [PubMed] [Google Scholar]
- Langridge J. Genetic evidence for the disposition of the substrate binding site of beta-galactosidase. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1260–1267. doi: 10.1073/pnas.60.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Newton A. Effect of nonsense mutations on translation of the lactose operon of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1966;31:181–187. doi: 10.1101/sqb.1966.031.01.026. [DOI] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S. Gene unstabilization induced by heat and by nitrous acid. J Bacteriol. 1961 Jan;81:111–117. doi: 10.1128/jb.81.1.111-117.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



