Abstract
Objective:
To discover, by using metabolomics, novel candidate biomarkers for stroke recurrence (SR) with a higher prediction power than present ones.
Methods:
Metabolomic analysis was performed by liquid chromatography coupled to mass spectrometry in plasma samples from an initial cohort of 131 TIA patients recruited <24 hours after the onset of symptoms. Pattern analysis and metabolomic profiling, performed by multivariate statistics, disclosed specific SR and large-artery atherosclerosis (LAA) biomarkers. The use of these methods in an independent cohort (162 subjects) confirmed the results obtained in the first cohort.
Results:
Metabolomics analyses could predict SR using pattern recognition methods. Low concentrations of a specific lysophosphatidylcholine (LysoPC[16:0]) were significantly associated with SR. Moreover, LysoPC(20:4) also arose as a potential SR biomarker, increasing the prediction power of age, blood pressure, clinical features, duration of symptoms, and diabetes scale (ABCD2) and LAA. Individuals who present early (<3 months) recurrence have a specific metabolomic pattern, differing from non-SR and late SR subjects. Finally, a potential LAA biomarker, LysoPC(22:6), was also described.
Conclusions:
The use of metabolomics in SR biomarker research improves the predictive power of conventional predictors such as ABCD2 and LAA. Moreover, pattern recognition methods allow us to discriminate not only SR patients but also early and late SR cases.
Stroke is the leading cause of acquired neurologic incapacity.1 In almost 20% of cases, stroke is preceded by TIA, providing a great opportunity for prevention.2 Thus, the risk of stroke is particularly high during the few days after the onset of TIA symptoms. However, TIA patients are a heterogeneous group in terms of symptoms, risk factors, underlying pathology, and early prognosis.3 The presence of large-artery atherosclerosis (LAA) has been the main established predictor of subsequent stroke4–6 so far, but a definitive prognostic tool for stroke recurrence (SR) is not defined.
Recently, in stroke research, there has been significant interest in biomarker development for predicting SR. Candidate approaches have rendered few biomarkers to be useful in the prognosis of TIA patients such as C-reactive protein,7 copeptin,8 and lipoprotein-associated phospholipase A (Lp-PLA),9 but the validity of the proposed biomarkers is debated.10 Regarding biomarker discovery for etiology classification, only the activity of Lp-PLA2 has been shown effective for the detection of LAA,9,11 and very recently pro–brain natriuretic peptide levels have been associated with the diagnosis of atrial fibrillation.12
New techniques such as metabolomics provide the opportunity to identify new biomarkers.13 This has been applied in stroke research14,15 but no description of SR after early recovery such as that present in TIA patients appeared.
The aim of the present study was to perform a metabolomic analysis of plasma from a cohort of TIA patients in order to find (1) new candidate biomarkers associated with SR, (2) temporal patterns of recurrence, and (3) the potential impact of LAA in the plasma metabolomic profile. Results were then validated in an independent cohort, thus ensuring robustness of the resulting candidate biomarker.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethics committee of the Arnau de Vilanova University Hospital. Patient consent was obtained from all the donors.
Subjects.
We prospectively recruited consecutive patients with transient neurologic deficit who were attended by a neurologist in the emergency department during the first 24 hours after the onset of symptoms (REGITELL registry).16 These patients were aleatorized into 2 cohorts (e-Methods on the Neurology® Web site at Neurology.org). TIA was defined as acute onset of focal cerebral or monocular symptoms lasting <24 hours attributable to brain ischemia. The number of TIA patients attended in our hospital during the study period (January 2008 to January 2012) determined the sample size. Risk factor profile; clinical characteristics; age, blood pressure, clinical features, duration of symptoms, and diabetes score (ABCD2)17; ultrasound; and neuroimaging protocols were recorded as described in e-Methods.
Follow-up and clinical endpoints.
Endpoint event was SR. A stroke physician performed clinical visits during the follow-up at 7 days, 3 months, and every 6 months. Imaging data were required to confirm brain ischemia in all SR patients considered. Recurrence of a TIA was not considered an endpoint.
Metabolomic analysis.
For nontargeted metabolomics analysis, metabolites were extracted from plasma samples and analyzed using liquid chromatography coupled to mass spectrometry as described in e-Methods.
Statistical analysis.
Statistics calculations were performed using SPSS software for Macintosh, version 20 (SPSS, Chicago, IL), R software, or the Stata 11 statistics package (StataCorp, College Station, TX). Further information for tests used appears in e-Methods.
RESULTS
Clinical characteristics of the study population.
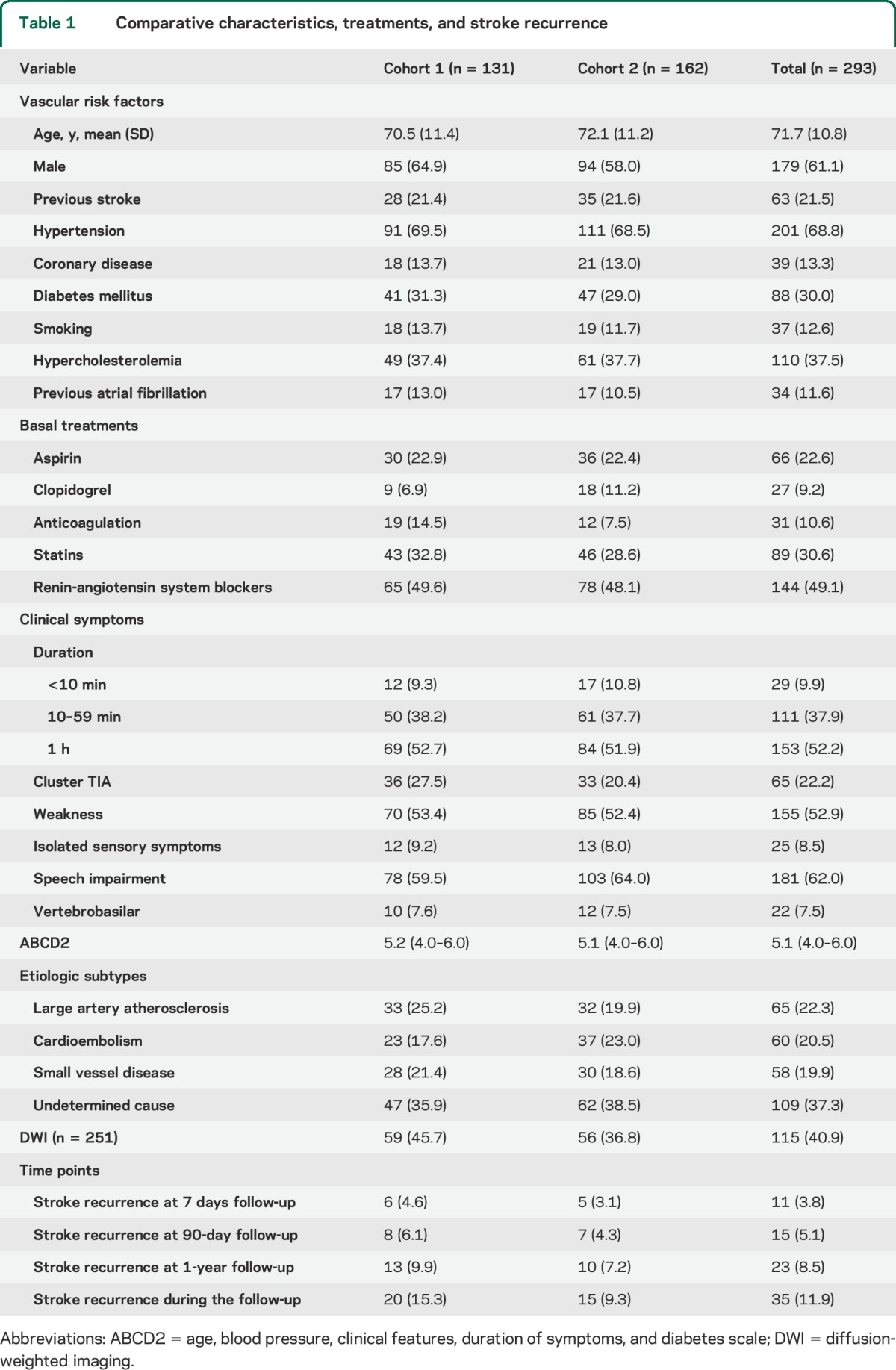
Table 1 shows baseline characteristics of both cohorts, comprising 293 patients. As confounders for the metabolomic analyses, we excluded significant differences in characteristics for vascular risk factors, clinical symptoms, ABCD2 score, etiologic subtypes, or discharge treatments between the 2 cohorts. Previous anticoagulation tended to be more frequent among those patients from the first cohort. The cohorts did not differ significantly in terms of SR.
Table 1.
Comparative characteristics, treatments, and stroke recurrence
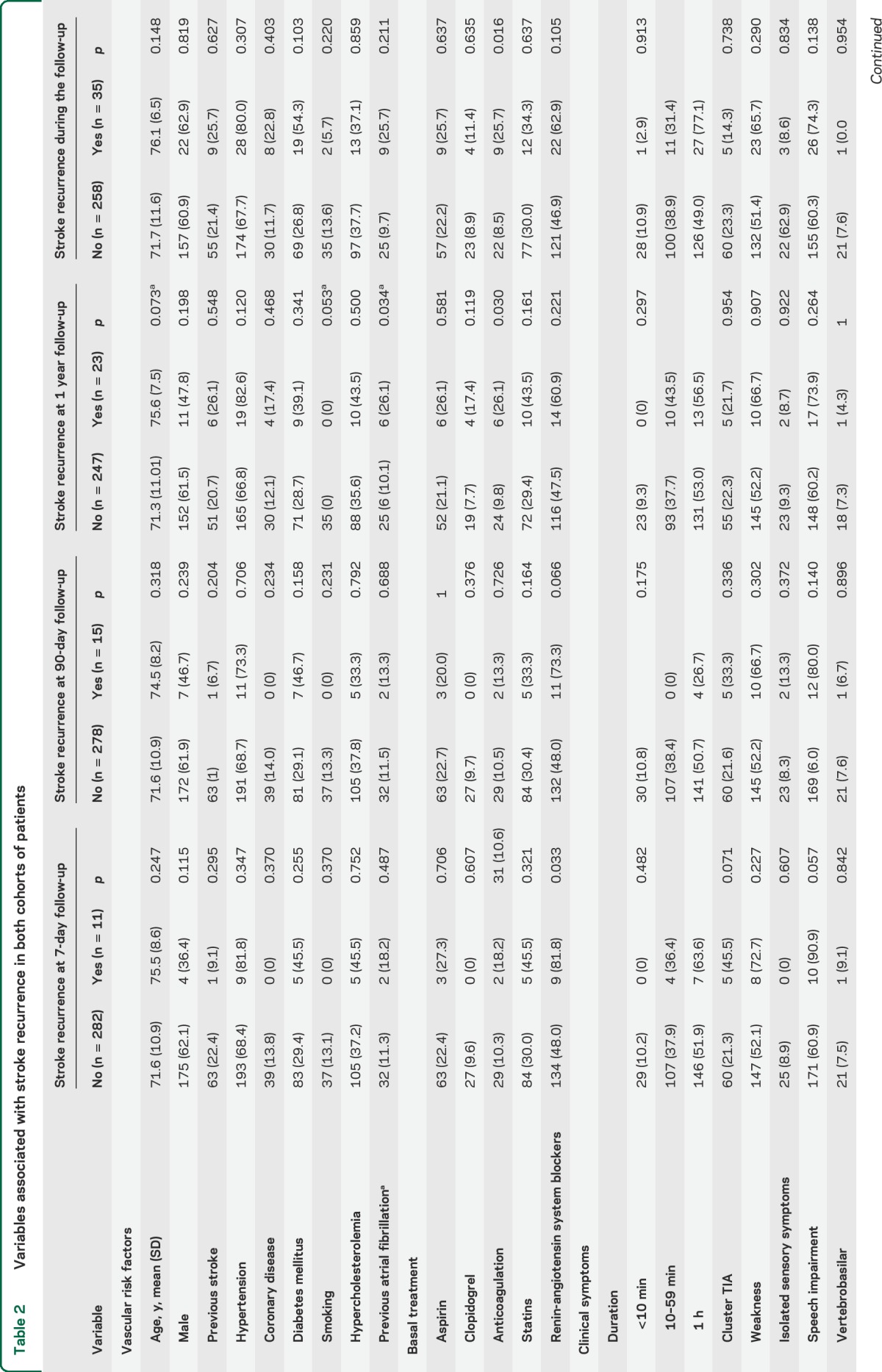
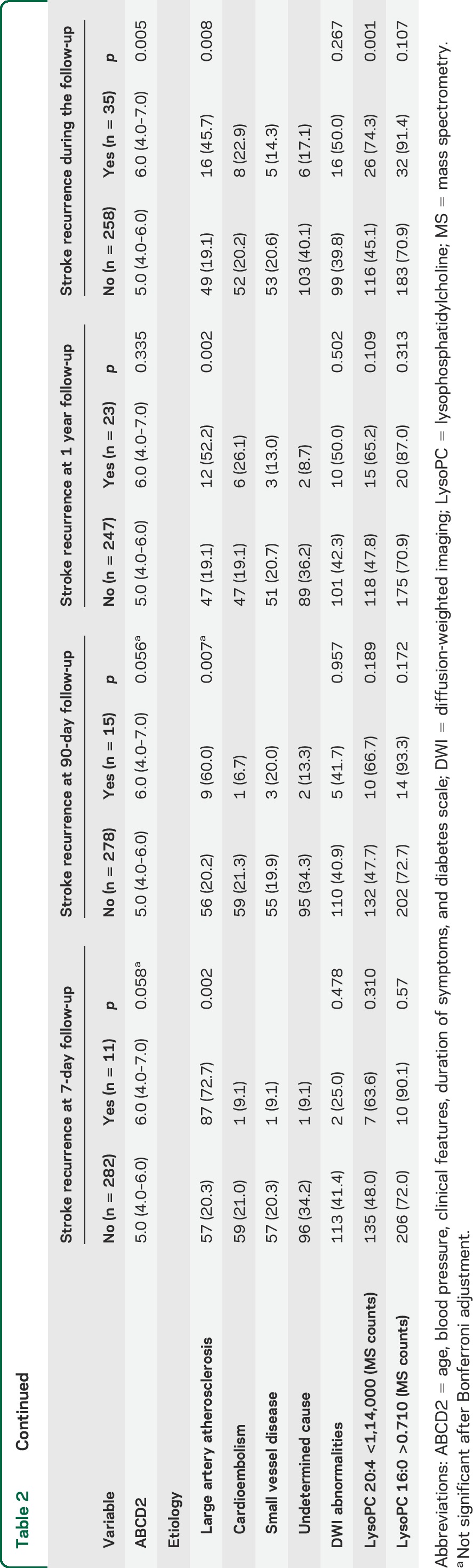
A total of 35 patients had SR. Two patients died due to recurrent ischemia. In the univariate analysis (table 2), only LAA and ABCD2 score were associated with SR. Clopidogrel and anticoagulation at discharge were associated with SR.
Table 2.
Variables associated with stroke recurrence in both cohorts of patients
Subsequent stroke risk exhibits a specific metabolomic profile.
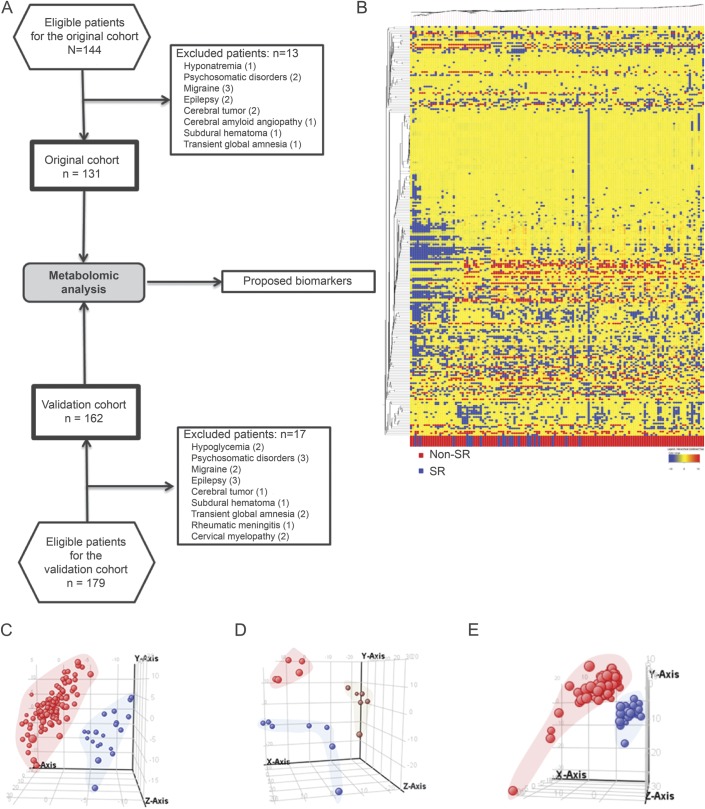
Untargeted metabolomic analysis in cohort 1 indicated plasma biomarker potentially indicating future SR. Molecular features detected (present in at least 50% of samples in the same group) are represented in the heat map (figure 1B). TIA patients with SR had a specific metabolomic signature, as shown by partial least squares discriminant analysis (PLS-DA) (figure 1C), reaching an accuracy ranging from 66% (in positive ionization, specificity = 0.63, sensitivity = 0.95) to 73% (in negative ionization, specificity = 0.68, sensitivity = 1). The individual importance of molecules in the model is presented in the supplemental data (dataset 1).
Figure 1. Study design and metabolomics profile of TIA patients.
(A) Flow diagram of experiment design. Metabolomic profile of stroke recurrence (SR) and large-artery atherosclerosis (LAA) in TIA patients in cohort 1. (B) Heat map representation of hierarchical clustering of molecular features found in each sample. Each line of this graphic represents an accurate mass ordered by retention time, colored by its abundance intensity and baselining to median/mean across the samples. The scale from −10 blue (low abundance) to +10 red (high abundance) represents this normalized abundance in arbitrary units. Tridimensional partial least squares discriminant analysis (PLS-DA) graphs demonstrate that SR ([C] blue spots represent SR plasma samples; red ones represent non-SR samples) and TIA temporal patterns recurrence ([D] early recurrence [<90 days] is represented in blue spots, medium [>90 days and <1 any] in red, and late [>1 year] in brown) determine a plasma metabolome. (E) Tridimensional PLS-DA graphs show differences between patients with LAA. Blue spots represent LAA and red ones non-LAA plasma samples.
Univariate statistics revealed that 94 ions differentiated SR from non-SR patients (p < 0.05) (dataset 2, supplemental data). By using orthogonal approaches (exact mass, isotope distribution, tandem mass spectrometry [MS/MS], and retention time database), 6 molecules were identified: 1-monopalmitin, dodecanoic acid, meso-erythritol, threonate, and lysophosphatidylcholine (LysoPC[16:0]) downregulated and myristoyl-ethanolamine upregulated (p < 0.05) in SR patients. After pathway analysis, these molecules clustered into free fatty acid metabolism, energy metabolism, and solute carrier (SLC)–mediated transmembrane transport pathways (dataset 3, supplemental data).
Temporal patterns of stroke recurrence define specific metabolomic profiles.
The results (figure 1D) show that PLS-DA of metabolomic profiles has a high accuracy in differentiating early SR from later SR groups (dataset 1). A total of 325 ions differentiated the groups by analysis of variance (p < 0.05, dataset 2). These molecules (14 metabolites identified among all the differential ions), indicating early recurrence, clustered into different metabolic pathways with 13 common between recurrence and temporal patterns of recurrence, including fatty acid metabolism (biosynthesis, transport, and receptors) and regulation of insulin secretion (table e-1, dataset 3).
Patients with LAA have a specific metabolomic pattern.
We focused on LAA etiology because of its importance as a SR predictor.4–6 We evaluated metabolomic profiles of patients with symptomatic carotid or intracranial stenosis of at least 50%. The results (figure 1E) revealed that metabolomic profiles are able to offer a high accuracy predicting presence of LAA (PLS-DA accuracy 91%–98%, positive: specificity = 0.79, sensitivity = 1; negative: specificity = 0.86, sensitivity = 1; dataset 1). A total of 73 metabolites were significantly different (Student t test) between LAA and non-LAA (dataset 2), with the resulting molecules identified after MS/MS analyses being androsterone, stearic acid, ascorbic acid, and LysoPC(22:6), all upregulated in patients with LAA (p < 0.05).
Results validation using an independent cohort.
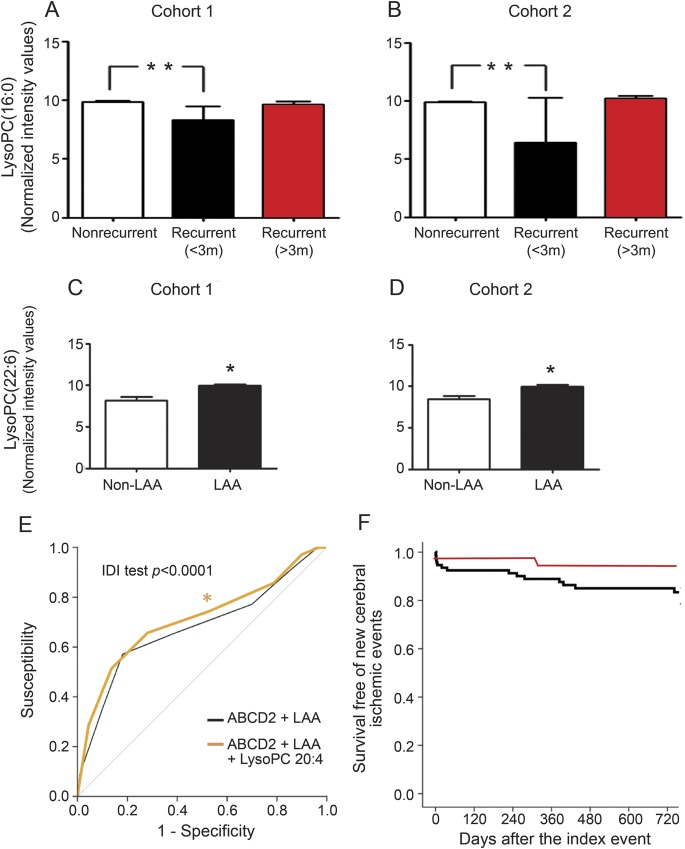
The multivariate analysis of an independent cohort (cohort 2) confirmed (1) metabolomic signature of SR; (2) metabolomics-based differentiation of SR temporal patterns; and (3) differences between LAA and non-LAA (figure e-1, A–C). Common pathways affected by temporal patterns of recurrence between the 2 independent cohorts included biosynthesis of unsaturated fatty acids and SLC-transport-related pathways (dataset 3). In both cohorts, LysoPC(16:0) was significantly decreased in early SR patients (figure 2, A and B), whereas LysoPC(22:6) was upregulated in LAA patients (figure 2, C and D).
Figure 2. Plasma lysophosphatidilcholines as TIA recurrence biomarkers.
(A) Lysophosphatidylcholine (LysoPC) (16:0) arose as a potential blood biomarker of stroke recurrence ([A] cohort 1; [B] cohort 2). **Indicates significant differences (p < 0.0001) by analysis of variance test with Tukey multiple comparison test. LysoPC(22:6) as a blood biomarker of large-artery atherosclerosis (LAA) ([C] cohort 1; [D] cohort 2). *Indicates significant differences by Student t test (at least p < 0.05). (E) The inclusion of LysoPC (20:4) levels to age, blood pressure, clinical features, duration of symptoms, and diabetes scale (ABCD2) and LAA score to receiver operating characteristic curve increase the predictive power of stroke recurrence (areas: ABCD2 = 0.646, p = 0.0.05; ABCD2 + LAA = 0.678, p = 0.001; ABCD2 + LAA + LysoPC(20:4) = 0.711, p < 0.001; integrated discrimination improvement (IDI) test for comparison of prediction models: p < 0.0001). (F) Kaplan-Meier estimates of the proportion of patients remaining free from any cerebral ischemic event. Red line indicates LysoPC(20:4) >1,14,000 MS counts; black line indicates LysoPC(20:4) <1,14,000 MS counts. MS = mass spectrometry.
Predictive potential of SR biomarkers described.
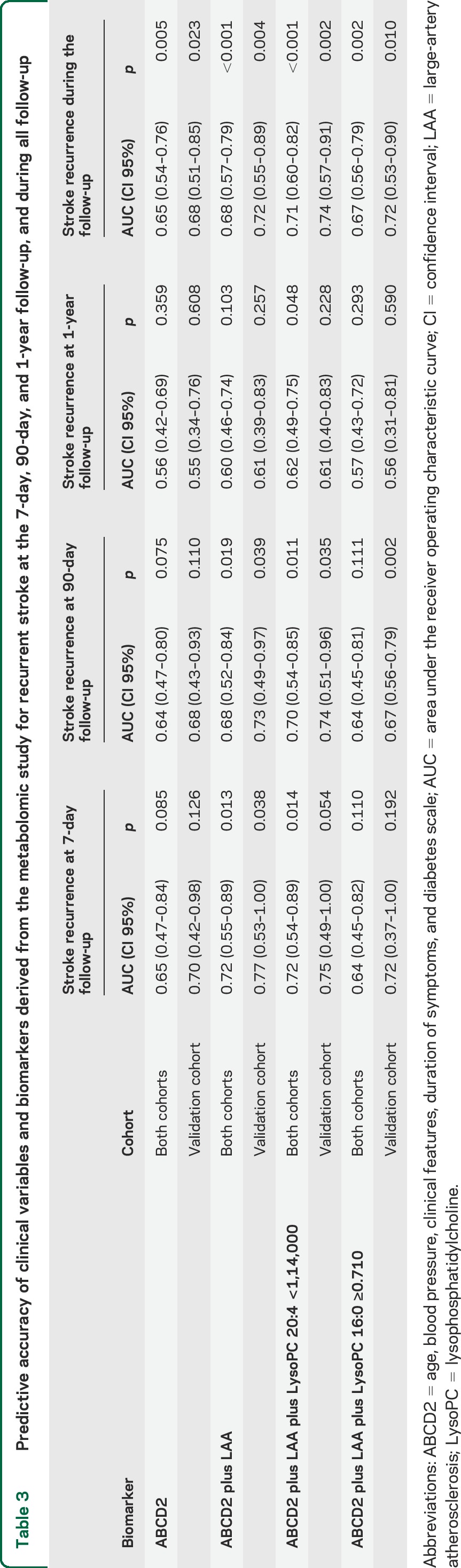
In order to define the capacity of these potential biomarkers, receiver operating characteristic (ROC) curves were performed using metabolites present in at least 70% of the samples in the same group (SR and non-SR), pooling both cohorts, due to low number of SR. The results (figure 2E, table 3, and table e-2) support the role of plasma LysoPC(20:4) as a potential biomarker, increasing significantly the predictive power of ABCD2 or LAA score from 64%–67% to 71%, as assessed by net reclassification improvement (NRI) or integrated discrimination improvement (IDI) tests (see supplemental data). The Hosmer-Lemeshow test for calibration of the risk of prediction models shows that while the ABCD2 model was well-calibrated (χ2 7.05, p = 0.069), the ABCD2 + LAA model was not (χ2 13.79, p = 0.003). The ABCD2 + LAA + LysoPC(20:4) model showed the highest calibration (χ2 3.58, p = 0.309), whereas the ABCD2 + LAA + LysoPC(16:0) model showed the lowest calibration values (χ2 17.33, p = 6e-04).
Table 3.
Predictive accuracy of clinical variables and biomarkers derived from the metabolomic study for recurrent stroke at the 7-day, 90-day, and 1-year follow-up, and during all follow-up
When only early SR was considered, an unidentified metabolite (figure e-1D) arose as a potential biomarker also increasing the predictive power of ABCD2 or LAA score (from 62%–67% to 71%).
DISCUSSION
The results demonstrate the feasibility and utility of the metabolomic approach to reveal potential biomarkers for SR after TIA, and for its most frequent independent predictor, LAA. We identified candidate biomarkers for both SR prediction and LAA detection, both belonging to LysoPC family. These biomarkers arose after analysis in 2 independent cohorts (n = 131; n = 162), reinforcing potential study generalizability.
We initially identified that a small set of metabolites are different between SR and no SR. Considering only this small set of metabolites, we reach an accuracy of >60% in segregating these 2 populations, suggesting that a combination of relatively low number of metabolites is, at least, as good as previous clinical, imaging, or etiologic variables in predicting SR. Future work devoted to identify these and new metabolites will help to increase the accuracy of the test and ultimately develop a more scalable tool for patients to assess their risk of SR.
Low plasma levels of LysoPC(16:0) arose as a potential predictor of recurrence, especially in patients with early SR. This compound is one of the products of Lp-PLA2 whose levels have been shown to be increased in adverse prognosis after stroke.11,18 Moreover, high levels of Lp-PLA2 and LysoPC(16:0) are associated with symptomatic carotid plaques.19 LysoPC(16:0) by itself increases on brain after stroke20,21 where it mediates phagocyte recruiting22 that can contribute to ischemic brain injury23 and also produces neuroinflammatory effects when applied on CNS.24 All these results could pinpoint a beneficial effect of reduced LysoPC(16:0) levels that argue against our claim that it marks an early SR risk, but although high levels of plaque LysoPC(16:0) are correlated with high plasma and plaque levels of Lp-PLA2, plasma LysoPC(16:0) levels are not.25 It has been demonstrated that plasma LysoPC(16:0) can inhibit Lp-PLA2 activity both in vitro and in vivo,26 thereby suppressing their neuroinflammative properties.27 Apart from suppressing Lp-PLA2 activity, plasmatic LysoPC(16:0) is neuroprotective on brain ischemia models,28 mainly through activation of TREK-1 potassium channels.29 Finally, it is known that LysoPC(16:0) is a potent inducer of superoxide ion production in endothelial cells30 and controlled levels of superoxide serve as angiogenic factors in ischemic angiogenesis.31 Due to all these neuroprotective properties, decreased plasma levels of LysoPC(16:0) could contribute to an early SR risk. Nonetheless, when we evaluated potential risk models accounting LysoPC(16:0), it did not add a significant value over the clinical parameters explained by ABCD2 and LAA presence. This fits with the fact that ABCD2 and LAA are closely related to pathophysiologic events previously invoked in the homeostasis of LysoPC16:0 (enumerated above).
The potential implication of LysoPC metabolism in SR was further reinforced by the finding of LysoPC(20:4) as a potential biomarker of SR based on ROC curves of aggregated cohorts. Interestingly, after the use of NRI, IDI, and Hosmer-Lemeshow test, the statistics indicated that LysoPC(20:4) significantly improves prediction capacities of combined ABCD2 and LAA scores. These data are in line with other LysoPC species containing fatty acids of 20C length, decreased in plasma in experimental models of ischemia.32 This molecule exhibits anti-inflammatory potential in preclinical models,33 reinforcing the role of inflammation in ischemic recurrence or tolerance. Interestingly, as with other biomarkers,8,11 LysoPCs exhibit the potential to increase the prognostic accuracy of clinical scores or LAA and could help management decisions, a hypothesis to be validated in further studies.
Finally, we identified LysoPC(22:6) as a potential LAA biomarker. As far as we know, no previous studies have focused on the role of LysoPC(22:6) on carotid plaque formation or stability but increased levels in symptomatic LAA patients could point to a role in this processes. N-3 polyunsaturated fatty acids, such as docosahexaenoic acid (22:6), are precursors of molecules implicated in the resolving phase of inflammation. Therefore, the higher levels of LysoPC(22:6) in LAA could be an indicator of reactive response to inflammation associated with atheroma plaque development.
As limitations of the present work, we recognize the significant heterogeneity and small sample size for SR among the studied population. The metabolome, even in healthy phenotypically similar patients, exhibits a considerable variance across the time.34–36 Furthermore, the samples were taken in the emergency room, without controlling for previous food intake. Nevertheless, the results provided were strengthened by the use of 2 independent cohorts. Further, we are not able to identify an important percentage of metabolites present in samples, due to the major bottleneck in metabolomics: the lack of comprehensive metabolite databases.37 However, the use of databases repositories with accurate mass and retention time in reproducible chromatographic systems will ease the future identification of these metabolites.
In the future, metabolomics could be useful to detect new biomarkers related to other interesting phenomena related to transient ischemia, the ischemic tolerance. Nonlacunar ischemic stroke patients with recent previous episodes of TIA have a favorable outcome, suggesting a neuroprotective effect of TIA by inducing a phenomenon of precondition ischemia.38
Supplementary Material
ACKNOWLEDGMENT
The authors thank the plasma donors for their support and permission.
GLOSSARY
- ABCD2
age, blood pressure, clinical features, duration of symptoms, and diabetes scale
- IDI
integrated discrimination improvement
- LAA
large-artery atherosclerosis
- Lp-PLA
lipoprotein-associated phospholipase A
- NRI
net reclassification improvement
- LysoPC
lysophosphatidylcholine
- MS/MS
tandem mass spectrometry
- PLS-DA
partial least squares discriminant analysis
- ROC
receiver operating characteristic
- SLC
solute carrier
- SR
stroke recurrence
Footnotes
Editorial, page 17
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Mariona Jové: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript for intellectual content. Gerard Mauri-Capdevila: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript for intellectual content. Idalmis Suárez: analysis of the data, revising the manuscript for intellectual content. Serafi Cambray: design and conceptualization of the study, interpretation of the data, revising the manuscript for intellectual content. Jordi Sanahuja: analysis and interpretation of the data, revising the manuscript for intellectual content. Alejandro Quílez: analysis and interpretation of the data, revising the manuscript for intellectual content. Joan Farré: analysis and interpretation of the data, revising the manuscript for intellectual content. Ikram Benabdelhak: analysis and interpretation of the data, revising the manuscript for intellectual content. Reinald Pamplona: design and conceptualization of the study, revising the manuscript for intellectual content. Manuel Portero-Otín: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript for intellectual content. Francisco Purroy: design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript for intellectual content.
STUDY FUNDING
Supported by the Autonomous Government of Catalunya (2009SGR-735), the Spanish Ministry of Health (FIS 11-02033), and the Marató of TV3 Foundation (95/C/2011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Samples were obtained with the support of IRBLleida biobank and RETICS BIOBANCOS (RD09/0076/00059).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Heuschmann PU, Di Carlo A, Bejot Y, et al. Incidence of stroke in Europe at the beginning of the 21st century. Stroke 2009;40:1557–1563. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology 2005;64:817–820. [DOI] [PubMed] [Google Scholar]
- 3.Merwick A, Kelly PJ. Transient ischaemic attack clinics and management of transient ischaemic attacks. Curr Opin Neurol 2011;24:50–58. [DOI] [PubMed] [Google Scholar]
- 4.Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Alvarez-Sabín J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 2007;38:3225–3229. [DOI] [PubMed] [Google Scholar]
- 5.Ois A, Gomis M, Rodríguez-Campello A, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke 2008;39:1717–1721. [DOI] [PubMed] [Google Scholar]
- 6.Purroy F, Jiménez Caballero PE, Gorospe A, et al. Prediction of early stroke recurrence in transient ischemic attack patients from the PROMAPA study: a comparison of prognostic risk scores. Cerebrovasc Dis 2012;33:182–189. [DOI] [PubMed] [Google Scholar]
- 7.Purroy F, Montaner J, Molina CA, et al. C-reactive protein predicts further ischemic events in transient ischemic attack patients. Acta Neurol Scand 2007;115:60–66. [DOI] [PubMed] [Google Scholar]
- 8.Katan M, Nigro N, Fluri F, et al. Stress hormones predict cerebrovascular re-events after transient ischemic attacks. Neurology 2011;76:563–566. [DOI] [PubMed] [Google Scholar]
- 9.Delgado P, Chacón P, Penalba A, et al. Lipoprotein-associated phospholipase A(2) activity is associated with large-artery atherosclerotic etiology and recurrent stroke in TIA patients. Cerebrovasc Dis 2012;33:150–158. [DOI] [PubMed] [Google Scholar]
- 10.Whiteley W, Tian Y, Jickling GC. Blood biomarkers in stroke: research and clinical practice. Int J Stroke 2012;7:435–439. [DOI] [PubMed] [Google Scholar]
- 11.Cucchiara BL, Messe SR, Sansing L, et al. Lipoprotein-associated phospholipase A2 and C-reactive protein for risk-stratification of patients with TIA. Stroke 2009;40:2332–2336. [DOI] [PubMed] [Google Scholar]
- 12.Purroy F, Suárez-Luis I, Mauri-Capdevila G, et al. N-terminal pro–brain natriuretic peptide level determined at different times identifies transient ischaemic attack patients with atrial fibrillation. Eur J Neurol 2014;21:679–683. [DOI] [PubMed] [Google Scholar]
- 13.Mauri-Capdevila G, Jove M, Suarez-Luis I, Portero-Otin M, Purroy F. Metabolomics in ischaemic stroke, new diagnostic and prognostic biomarkers [in Spanish]. Rev Neurol 2013;57:29–36. [PubMed] [Google Scholar]
- 14.Jung JY, Lee HS, Kang DG, et al. 1H-NMR-based metabolomics study of cerebral infarction. Stroke 2011;42:1282–1288. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z, Sun J, Liang Q, et al. A metabonomic approach applied to predict patients with cerebral infarction. Talanta 2011;84:298–304. [DOI] [PubMed] [Google Scholar]
- 16.Purroy F, Begué R, Gil MI, et al. Patterns of diffusion-weighted magnetic resonance imaging associated with etiology improve the accuracy of prognosis after transient ischaemic attack. Eur J Neurol 2011;18:121–128. [DOI] [PubMed] [Google Scholar]
- 17.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 18.Tsai TH, Chen YL, Lin HS, et al. Link between lipoprotein-associated phospholipase A2 gene expression of peripheral-blood mononuclear cells and prognostic outcome after acute ischemic stroke. J Atheroscler Thromb 2012;19:523–531. [DOI] [PubMed] [Google Scholar]
- 19.Mannheim D, Herrmann J, Versari D, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008;39:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanta SR, Choi CS, Lee JH, et al. Global changes in phospholipids identified by MALDI MS in rats with focal cerebral ischemia. J Lipid Res 2012;53:1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koizumi S, Yamamoto S, Hayasaka T, et al. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience 2010;168:219–225. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Lee YC, Kim SJ, et al. Production of lysophosphatidylcholine by cPLA2 in the brain of mice lacking PPT1 is a signal for phagocyte infiltration. Hum Mol Genet 2007;16:837–847. [DOI] [PubMed] [Google Scholar]
- 23.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010;87:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist 2006;12:245–260. [DOI] [PubMed] [Google Scholar]
- 25.Gonçalves I, Edsfeldt A, Ko NY, et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 2012;32:1505–1512. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham TJ, Yao L, Lucena A. Product inhibition of secreted phospholipase A2 may explain lysophosphatidylcholines' unexpected therapeutic properties. J Inflamm 2008;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto F, Brenner T, Dan P, Krimsky M, Yedgar S. Extracellular phospholipase A2 inhibitors suppress central nervous system inflammation. Glia 2003;44:275–282. [DOI] [PubMed] [Google Scholar]
- 28.Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab 2002;22:821–834. [DOI] [PubMed] [Google Scholar]
- 29.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J 2004;23:2684–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao SP, Riederer M, Lechleitner M, et al. Acyl chain-dependent effect of lysophosphatidylcholine on endothelium-dependent vasorelaxation. PLoS One 2013;8:e65155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bir SC, Shen X, Kavanagh TJ, Kevil CG, Pattillo CB. Control of angiogenesis dictated by picomolar superoxide levels. Free Radic Biol Med 2013;63:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Jia H, Chang X, Ding G, Zhang H, Zou ZM. The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on a tissue targeted metabonomics. Mol Biosyst 2013;9:2823–2834. [DOI] [PubMed] [Google Scholar]
- 33.Hung ND, Kim MR, Sok DE. Anti-inflammatory action of arachidonoyl lysophosphatidylcholine or 15-hydroperoxy derivative in zymosan A-induced peritonitis. Prostaglandins Other Lipid Mediat 2009;90:105–111. [DOI] [PubMed] [Google Scholar]
- 34.Krug S, Kastenmüller G, Stückler F, et al. The dynamic range of the human metabolome revealed by challenges. FASEB J 2012;26:2607–2619. [DOI] [PubMed] [Google Scholar]
- 35.Enea C, Seguin F, Petitpas-Mulliez J, et al. (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal Bioanal Chem 2010;396:1167–1176. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Kim SB, Wang B, et al. Individual variation in macronutrient regulation measured by proton magnetic resonance spectroscopy of human plasma. Am J Physiol Regul Integr Comp Physiol 2009;297:R202–R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patti GJ, Yanes O, Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol 2012;13:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arboix A, Cabeza N, García-Eroles L, et al. Relevance of transient ischemic attack to early neurological recovery after nonlacunar ischemic stroke. Cerebrovasc Dis 2004;18:304–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.