Abstract
Objective:
We examined the effect of relapses—before and after progression onset—on the rate of postprogression disability accrual in a progressive multiple sclerosis (MS) cohort.
Methods:
We studied patients with primary progressive MS (n = 322) and bout-onset progressive MS (BOPMS) including single-attack progressive MS (n = 112) and secondary progressive MS (n = 421). The effect of relapses on time to Expanded Disability Status Scale (EDSS) score of 6 was studied using multivariate Cox regression analysis (sex, age at progression, and immunomodulation modeled as covariates). Kaplan-Meier analysis was performed using EDSS 6 as endpoint.
Results:
Preprogression relapses (hazard ratio [HR]: 1.63; 95% confidence interval [CI]: 1.34–1.98), postprogression relapses (HR: 1.37; 95% CI: 1.11–1.70), female sex (HR: 1.19; 95% CI: 1.00–1.43), and progression onset after age 50 years (HR: 1.47; 95% CI: 1.21–1.78) were associated with shorter time to EDSS 6. Postprogression relapses occurred in 29.5% of secondary progressive MS, 10.7% of single-attack progressive MS, and 3.1% of primary progressive MS. Most occurred within 5 years (91.6%) after progressive disease onset and/or before age 55 (95.2%). Immunomodulation after onset of progressive disease course (HR: 0.64; 95% CI: 0.52–0.78) seemingly lengthened time to EDSS 6 (for BOPMS with ongoing relapses) when analyzed as a dichotomous variable, but not as a time-dependent variable.
Conclusions:
Pre- and postprogression relapses accelerate time to severe disability in progressive MS. Continuing immunomodulation for 5 years after the onset of progressive disease or until 55 years of age may be reasonable to consider in patients with BOPMS who have ongoing relapses.
Multiple sclerosis (MS) is characterized by 2 clinical phenomena—relapses and progression. Clinical phenotype is classified based on the presence and timing of these 2 features.1 Most patients will enter a progressive phase of disease.2 Progression may be clinically manifest from onset (primary) or after a relapsing-remitting phase (secondary). Progressive disease course refers to insidious accumulation of neurologic deficits independent of relapses. Disability progression can occur from stepwise accumulation of disability from relapses, progressive disease course, or both. Progressive disease course is the dominant factor affecting disability progression. Few patients reach severe persistent ambulatory disability before onset of a progressive course in MS.2
Earlier studies suggested primary progressive MS (PPMS) portended a worse prognosis than relapsing-remitting MS (RRMS) or secondary progressive MS (SPMS).3–5 These studies, including our own, considered time to disability milestones from onset of MS rather than from onset of the progressive phase of disease.6 The absence of preceding relapses led many neurologists to argue that the pathologic processes in PPMS are fundamentally different and should not be studied together with RRMS. However, development of a progressive disease course and rate of postprogression disability accumulation seems to be age-dependent, and does not correlate with the rate of preprogression disability accumulation.2,4,7–11
Although age at progressive disease onset and pace of disability accumulation are similar across MS subtypes (e.g., PPMS vs SPMS), there is a wide range and distribution. This suggests that the presence or absence of other clinical parameters underlies the differences in long-term disability accrual between patients with progressive MS. In this study, we explored clinical parameters that influence disability accumulation and specifically whether clinical relapses before, or subsequent to progressive disease course onset affect this variability.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic institutional review board. Written informed consent to access medical records for research was obtained from all patients included.
Study populations.
All patients were evaluated at Mayo Clinic and fulfilled McDonald12,13 diagnostic criteria for MS. We studied a population-based (n = 101, age 18 years and older) and a clinic-based (n = 754, age 18 years and older) progressive MS cohort. The population-based cohort was established in 199214 and reascertained in 200215 and 2010.2 This cohort captured patients across the disease spectrum with more than 20 years of follow-up. The clinic-based cohort was all inclusive between 2003 and 2007, and all patients had documented progressive MS of greater than 1 year, as recently reported.2 This cohort captured a population enriched for PPMS and single-attack progressive MS (SAPMS), in addition to the more common SPMS. Demographic and clinical data were extracted from the medical records and validated as previously reported.2 The populations were similar in demographic and disease characteristics2 and all progressive MS patients were combined for this study.
Progression and disability.
We defined progressive disease course as insidious and irreversible worsening brain, brainstem-cerebellar, and spinal cord syndromes most frequently characterized by weakness, ataxia, or bladder dysfunction of ≥1 year.2 Age at progressive MS onset was established from the most detailed available record and documented examination nearest in time to onset of progressive disease course.2
We assigned Kurtzke Expanded Disability Status Scale (EDSS)16 scores based on the comprehensive neurologic examination sheet documented in the medical record at our center only, beginning with the first evaluation.
Classification of progressive disease course.
PPMS refers to progressive MS with no previous relapses; SAPMS refers to progressive MS developing after a single relapse9; and SPMS refers to progressive MS developing after RRMS. When appropriate, SAPMS and SPMS were combined as bout-onset progressive MS (BOPMS). If there were ongoing relapses after progressive MS onset, these were considered as part of an overlap between a resolving relapsing phase of MS and the early progressive phase of MS rather than a separate diagnostic category of progressive-relapsing MS.
Study variables and outcomes.
Variables studied included sex, age at progressive MS onset, occurrence of relapse(s) before progressive MS onset, occurrence of relapse(s) after progressive MS onset, treatment with (≥3 months' duration) immunomodulatory therapy before progressive MS onset, and treatment with (≥3 months' duration) immunomodulatory therapy after progressive MS onset. To evaluate the potential confounding effect of immunomodulation on relapse-related disability accumulation, either before or after progressive MS onset, we included immunomodulation in the multivariate analyses. Because this study compares progressive phases of BOPMS to PPMS, symptomatic disease duration before progressive MS onset was not included as a covariate, as by definition this is asymptomatic in PPMS. While EDSS at the time of BOPMS onset was available for the population-based cohort, it was not complete for the clinic-based cohort, many of whom were already in the progressive phase at the time of referral, typical of such real-world clinical practice. We compared the EDSS and functional system scores at onset of progressive MS between PPMS and BOPMS (including SAPMS and SPMS) as well as between population-based (all patients) and clinic-based (when this was available) cohorts to help guide interpretation of results. However, because of lack of data points in some patients, EDSS at progressive MS onset was not included as a covariate in the combined analyses. Outcome measures included hazard ratios (HRs) for study variables and time from progressive MS onset to EDSS 6.
Data analyses.
SAS software (9.3.1; SAS Institute, Cary, NC) was used for all data analyses. Demographic and clinical features were studied using multivariate Cox regression analysis. Kaplan-Meier analysis was used to generate survival curves from progressive MS onset to EDSS 6, stratified for factors with significant HRs. Patients were censored at the time of last assessment whenever the endpoint had not been reached. Age-scaled measures were analyzed as continuous variables or binned into 5-year groups.
Two of the study variables (relapses after progressive MS onset and immunomodulatory therapy after progressive MS onset) occurred during the observation period for survival analysis (progressive MS onset to EDSS 6). Generation of survival curves for these variables relied on the following a priori assumptions and adjustments: (1) patients were assumed to fall within the relapse groupings at the time of progressive MS onset; (2) because patients with PPMS who initiated immunomodulatory therapy did so at variable times after progressive MS onset, survival curves for postprogression immunomodulatory therapy included only patients with BOPMS; and (3) assessment of treatment effect was based on a dichotomized variable of whether the patient was ever treated after onset of progressive MS.
The primary Cox regression analysis also relied on the assumptions outlined above, with relapses and immunomodulatory therapy modeled as binary (or ternary) variables. In addition, we performed a second Cox regression analysis to model postprogression relapses and immunomodulatory therapy as time-dependent variables. In this model, all patients fell within the no postprogression relapse group at the time of progressive MS onset and switched groups only when a postprogression relapse occurred. For postprogression immunomodulatory therapy, patients were allowed to switch back and forth between treated vs untreated groups throughout the observation period.
RESULTS
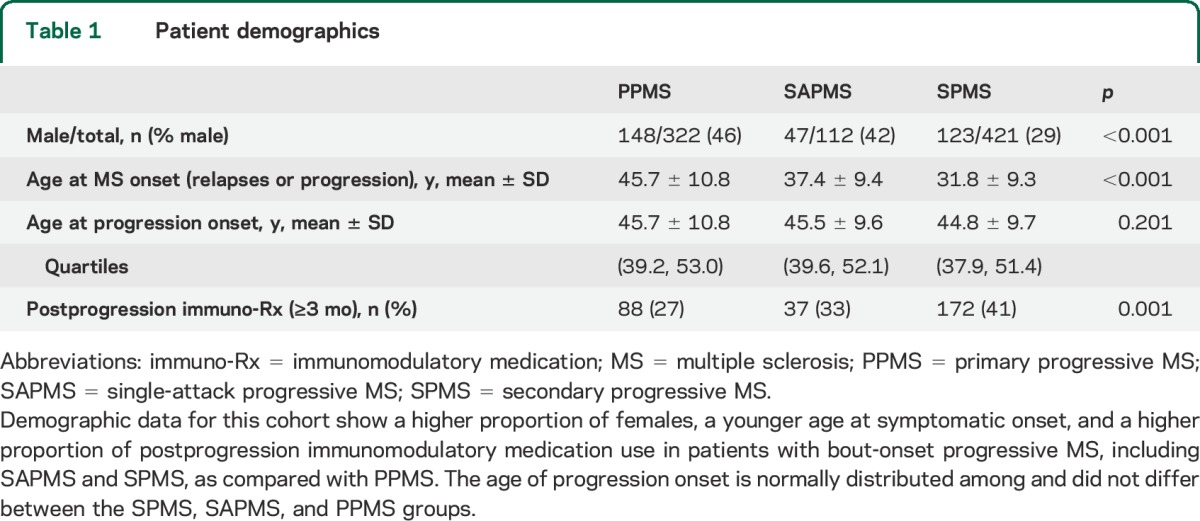
The proportion of females and age at symptomatic onset were consistent with known demographics of MS phenotypes (table 1). A higher proportion of patients with BOPMS compared with PPMS were treated with immunomodulatory medications after progressive MS onset. Age at progressive MS onset was similar among PPMS, SAPMS, and SPMS (table 1).2
Table 1.
Patient demographics
Multivariate analysis revealed that preprogression relapses (HR: 1.63; 95% confidence interval [CI]: 1.34–1.98), postprogression relapses (HR: 1.37; 95% CI: 1.11–1.70), female sex (HR: 1.19; 95% CI: 1.00–1.43), and progressive disease onset after age 50 years (HR: 1.47; 95% CI: 1.21–1.78) were negatively associated with time from onset of progressive disease course to EDSS 6 (table 2). The age cutoff of 50 years was determined by a best-fit analysis (data not shown).
Table 2.
Cox regression analyses of time to EDSS 6 after onset of a progressive disease course
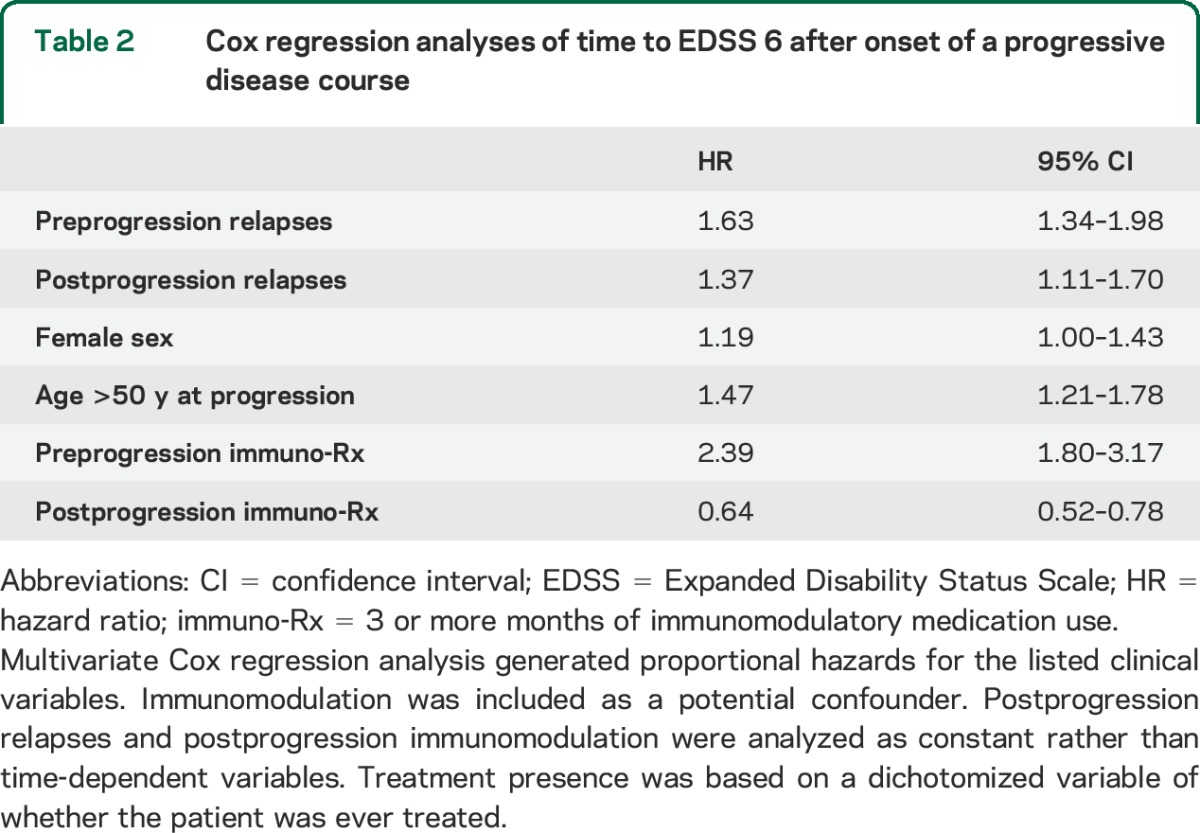
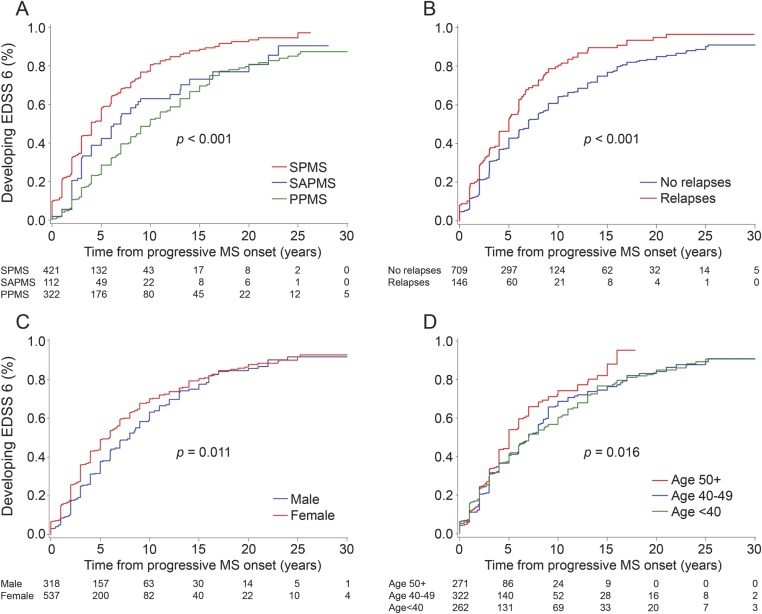
Figure 1 shows Kaplan-Meier analyses for significant factors from the multivariate analysis. Time to EDSS 6 from progressive MS onset was longest for PPMS (50% in 10 years), intermediate for SAPMS (50% in 7 years), and shortest for SPMS (50% in 4 years) (p < 0.001). Ongoing relapses after progressive MS onset shortened time to EDSS 6 by approximately 2 years (p < 0.001). Stratifying postprogression relapses further, survival curves for 1 vs 2 or more relapses were superimposed (data not shown). Postprogression relapses were more frequent in SPMS (29.5%) than SAPMS (10.7%) than PPMS (3.1%), and most happened within 5 years (91.6%) after onset of progressive disease and/or before age 55 (95.2%).
Figure 1. Clinical features affecting postprogression disability accumulation.
Survival curves from progressive MS onset to EDSS 6 are shown. Both pre- and postprogression relapses increase the pace of postprogression disability accumulation (A, B). Women accumulate disability mildly faster during the early progressive disease phase (C). Progressive MS onset at 50 years or older mildly increases the pace of postprogression disability accumulation (D). EDSS = Expanded Disability Status Scale; MS = multiple sclerosis; PPMS = primary progressive MS; SAPMS = single-attack progressive MS; SPMS = secondary progressive MS.
Patients with PPMS typically had low EDSS score (1.5–2) at the time of first clinical assessment. For patients with BOPMS followed longitudinally (predominantly the population-based cohort), mean EDSS score at progressive disease onset was 2.2 (±0.4) for SAPMS and was 3.4 (±1.1) for SPMS. Mean pyramidal functional system scores (main functional system affecting EDSS 6) at progressive disease onset were similar among PPMS (1.9 ± 0.4), SAPMS (2.0 ± 0.6), and SPMS (2.6 ± 0.8). EDSS at onset of progressive MS was similar between the clinic-based cohort (when available) and population-based cohort (data not shown).
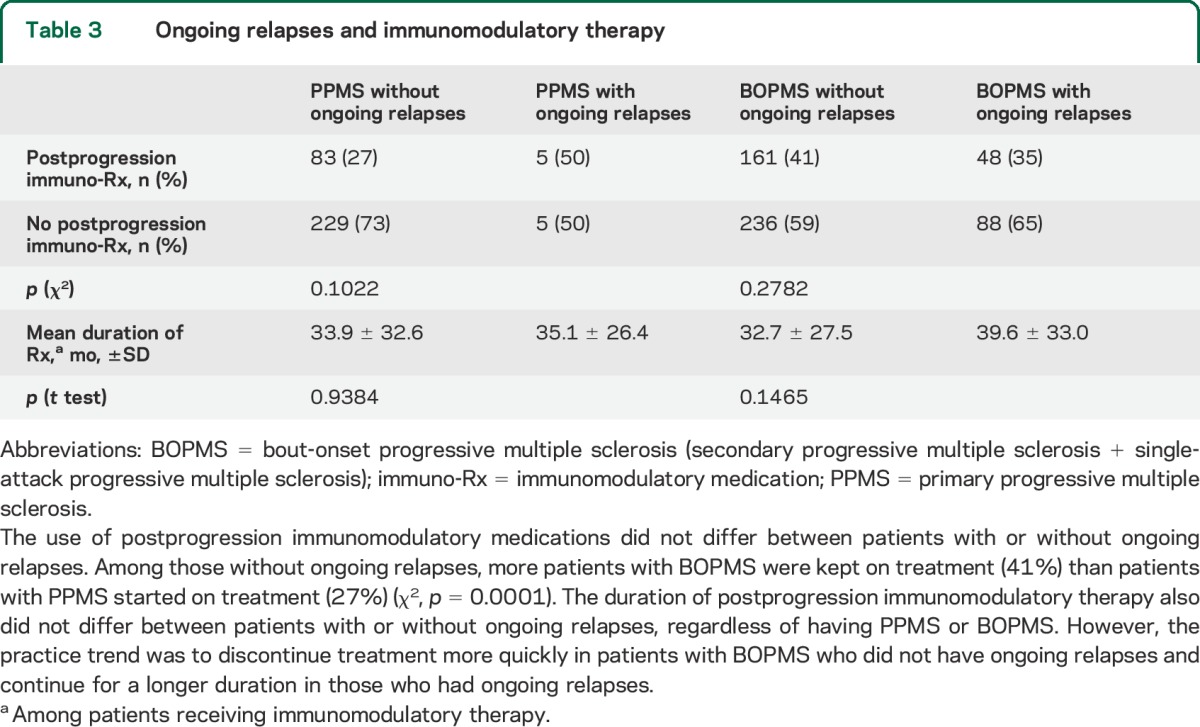
Immunomodulation before progressive MS onset (HR: 2.39; 95% CI: 1.80–3.17) correlated with increased risk of attainment of postprogression EDSS 6, whereas immunomodulation after progressive disease onset (HR: 0.64; 95% CI: 0.52–0.78) correlated with decreased risk (table 2). An independent protective effect of immunomodulatory therapy, assessed by the regression model to account for interactions, is marginally evident among patients with BOPMS who had ongoing relapses (p = 0.049) and as a trend among those who did not (p = 0.067). Illustration of this effect is shown using survival analysis with dichotomous variables (figure 2). The frequency of immunomodulation after progressive disease onset was similar between patients with or without ongoing relapses (table 3). Among those without ongoing relapses, more patients with BOPMS were kept on treatment (41%) than patients with PPMS started on treatment (27%) (χ2, p = 0.0001). The duration of postprogression immunomodulatory therapy did not differ between patients with or without ongoing relapses, regardless of having PPMS or BOPMS. The nonsignificant trend was to discontinue treatment in BOPMS patients with ongoing relapses after a longer interval from progression than in those without ongoing relapses (table 3). Mean EDSS (±SD) at progressive disease onset was 3.4 ± 1.0 for patients who continued or started treatment after progression onset, and was 3.2 ± 1.5 for those who were not treated.
Figure 2. Effect of immunomodulation on postprogression disability accumulation in BOPMS.
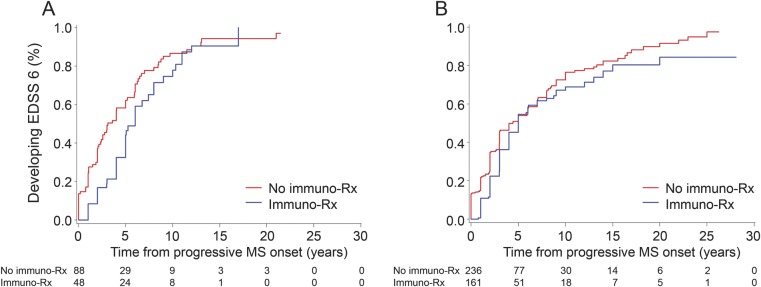
Survival curves from progressive MS onset to EDSS 6 are shown. Postprogression immunomodulatory medication use seemingly slows the pace of postprogression disability accumulation among patients with BOPMS who have ongoing relapses (A), but not clearly so among those who do not (B). For this analysis, patients were assumed to fall within the relapse groupings at the time of progressive MS onset; patients with primary progressive MS were excluded because of variability of when immunomodulatory therapy was initiated and the small number with relapses; and assessment of treatment effect was based on a dichotomized variable of whether the patient was ever treated after onset of progressive MS. However, with the secondary multivariate analysis (immunomodulation modeled as time-dependent variable), the postprogression treatment benefit was no longer detectable. BOPMS = bout-onset progressive multiple sclerosis (secondary progressive multiple sclerosis + single-attack progressive multiple sclerosis); EDSS = Expanded Disability Status Scale; immuno-Rx = 3 or more months of immunomodulatory medication use; MS = multiple sclerosis.
Table 3.
Ongoing relapses and immunomodulatory therapy
With the secondary multivariate analysis (postprogression relapses and immunomodulation modeled as time-dependent variables), ongoing relapses still had a deleterious effect (HR: 1.34; 95% CI: 1.02–1.76). However, there was no longer a detectable protective effect from attainment of EDSS 6 for postprogression immunomodulation (HR: 1.73; 95% CI: 1.41–2.13).
DISCUSSION
As we have previously reported, the age at onset of progressive disease course did not differ among PPMS, SAPMS, and SPMS in this cohort.2 Most natural history studies comparing disability accumulation in patients with PPMS or BOPMS did not account for age dependence. Time measurements from symptomatic onset of MS to disability milestones included the preprogression relapsing phase and the progressive phase in patients with BOPMS, but only time spent in the progressive phase in patients with PPMS. The tendency for patients with BOPMS to reach disability milestones after a longer interval from onset compared to those with PPMS likely reflects that patients with BOPMS were not in a progressive phase throughout their entire disease course.
We accounted for time spent only in the progressive phase by using onset of progressive disease course as a uniform starting point to demonstrate that patients with BOPMS reach severe postprogression disability faster than patients with PPMS. We previously showed that, in this cohort, approximately 2% of patients reach EDSS 6 (need for unilateral gait aid) before onset of a progressive disease course.2 EDSS 6 represents a particularly reliable milestone for severe disability onset, determined mainly by pyramidal tract involvement, and is a robust outcome measure for assessing the effect of progressive phase in MS.
Several studies have documented more rapid disability accumulation8,17–20 (or trend toward21) from the time of progressive MS onset (or disability milestone) among patients with SPMS compared to patients with PPMS. Other studies found no difference but, importantly, none concluded that disability accrual is faster in PPMS.9,11,22–24 Variability between these studies is likely methodologic and not intrinsic to the studied population, because the same natural history cohorts report different conclusions at different times. Some of this variability may also reflect the use of earlier disability milestones that are influenced by other functional systems and not predominantly by the pyramidal system. We conclude that presence of a clinical relapsing phase before progressive MS onset accelerates postprogression disability accumulation.
The more rapid accrual of disability among patients with BOPMS likely reflects both clinical relapse-related and relapse-independent mechanisms. At the time of progressive disease onset, the mean EDSS score was under 2 points higher and the mean pyramidal functional system score was about one-half point higher in patients with SPMS than in those with PPMS; the mean EDSS and the mean pyramidal functional system scores were not different between patients with SAPMS and PPMS. Therefore, preprogression clinical relapse-related disability should only partly account for the postprogression disability burden among patients with BOPMS. However, while the number of patients with BOPMS who had available EDSS scores at the onset of progressive MS was sufficiently sized for descriptive analysis, we lacked the power for independent multivariate analysis to assess the effect of EDSS at progressive MS onset related to the other covariates. It is also possible that a relapsing course preceding progressive disease onset reflects higher subclinical activity (MRI lesion accumulation) in these patients. However, we were unable to study the effect of radiologic activity (i.e., subclinical relapses) because MRI was not systematically performed or analyzed for this study.
We report 2 additional preprogression factors that negatively affect pace of postprogression disability accumulation: a modest effect of older age at progression and female sex. Given attainment of EDSS 6 is heavily influenced by ambulation, our finding may also reflect age-related contributions to gait changes through medical comorbidities and increasing neurodegeneration. The observation that female sex has a deleterious effect on the pace of progression seemingly contradicts long-held views of prognosis in MS; namely, male sex imparts a worse prognosis. This assumption rests on a sizeable literature documenting an elevated risk in males of developing progressive disease.3–5,25,26 However, our finding reflects postprogression course only and does not include time spent in the relapsing phase. Another study also documented reversal of the sex effect on pace of progression: women attained EDSS 6 at a longer interval than men after MS onset but at a shorter interval after progressive MS onset.7
We did not observe a protective effect of preprogression immunomodulatory medication use in delaying accrual of disability after progressive MS onset. Because we studied only patients who developed progressive MS, however, any benefit of medications in preventing disability accrual among patients who do not develop progressive MS would have been missed. Together with the tendency to initiate immunomodulation in patients with more active RRMS, these observations could explain the finding of faster disability accumulation in patients who were treated before the onset of progressive MS.
Several studies have documented no influence of superimposed relapses9,22,23,26–28 in SPMS or PPMS. Our findings conflict with these conclusions, but are consistent with a recent clinical trial.29 Studying a large cohort of patients with progressive MS likely enhanced the power of our study to detect a modest negative effect of postprogression relapses on disability accumulation. This effect was independent of the presence of a relapsing disease course before the onset of progressive MS (i.e., BOPMS vs PPMS), and was similar whether one or more postprogression relapses occurred. Modeling postprogression relapses as a constant variable assumed any relapse effect was independent of timing after the onset of progressive MS. When we eliminated this assumption, the negative effect of postprogression relapses remained evident. Although most disability accrual in MS occurs by a progressive disease course,11,24,30 we conclude that superimposed relapses further accelerate postprogression disability accumulation.
We show that only a small proportion of patients with PPMS (3%) and patients with SAPMS (11%) have relapses after onset of the progressive phase of MS, whereas 30% of the patients with SPMS had relapses. Because we also show that most postprogression relapses typically occur within time (5 years) and age limits (younger than 55 years), a logical extension is that intervention to prevent postprogression relapses would be most effective in this period, especially in BOPMS.
When analyzed as a dichotomous variable, immunomodulatory therapy after progressive MS onset was associated with longer time to EDSS 6 from onset of progression. Generally, patients with more active inflammatory disease are more likely to be continued on postprogression immunomodulation. Of note, both treated and untreated patients had a similar mean EDSS score at onset of progressive MS. Therefore, patients treated during the progressive phase were not particularly more severe cases and, conversely, patients with low EDSS score did not preferentially choose to remain on treatment during the progressive phase. Moreover, there was no overrepresentation of those with ongoing relapses among those continuing postprogression immunotherapy.
Initiating immunomodulation after onset of progressive MS in prospective clinical trials does not affect disability accumulation independent of any effect on relapses,31–40 consistent with our findings. The protective effect of postprogression immunomodulation shown here was more apparent in BOPMS patients with ongoing relapses than those without. We were not able to accurately assess the effect of postprogression immunomodulation among patients with PPMS, in whom postprogression relapses are far less common. Furthermore, the regression model was based on assumptions regarding postprogression relapse groupings and timing of postprogression immunomodulation. Dichotomizing the treatment variable assumed any immunomodulation may have lasting effects. When we eliminated this assumption, the postprogression treatment benefit disappeared.
We show that both pre- and postprogression relapses independently accelerate time to severe disability in progressive MS. Our findings suggest that patients with SPMS and SAPMS who have ongoing relapses may benefit from immunomodulatory therapies after onset of progressive MS; however, the retrospective nature of part of our study cohort introduced methodologic challenges that preclude definite conclusions regarding the value of postprogression immunomodulation. If a continued treatment approach is taken, our findings suggest that treatment is most likely to benefit those within 5 years after onset of progressive disease or before age 55 years. The frequency of postprogression relapses reported here may help guide power calculations in prospective clinical trials focused on addressing the value of immunomodulation in patients with progressive MS who have ongoing clinical relapses. Short of such trials, we suggest individualizing treatment continuation or discontinuation decisions after progressive MS onset.
GLOSSARY
- BOPMS
bout-onset progressive multiple sclerosis
- CI
confidence interval
- EDSS
Expanded Disability Status Scale
- HR
hazard ratio
- MS
multiple sclerosis
- PPMS
primary progressive multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- SAPMS
single-attack progressive multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
AUTHOR CONTRIBUTIONS
M.M. Paz Soldán contributed to study design, extracted, analyzed and interpreted data, and wrote the manuscript. M. Novotna, N. Abou Zeid, N. Kale, and M. Tutuncu extracted data and participated with data interpretation and manuscript revision. D.J. Crusan and E.J. Atkinson completed statistical analysis. A. Siva, B.M. Keegan, I. Pirko, S.J. Pittock, C.F. Lucchinetti, B.G. Weinshenker, and M. Rodriguez evaluated patients and participated with manuscript revision. O.H. Kantarci conceived of and designed the study, evaluated patients, analyzed and interpreted data, and participated in manuscript preparation.
STUDY FUNDING
Supported by the Mayo Clinic Department of Neurology, the European Regional Development Fund (FNUSA-ICRC), and a pilot grant from the National Multiple Sclerosis Society.
DISCLOSURE
M. Paz Soldán reports no disclosures relevant to the manuscript. M. Novotna receives support from the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123), the European Social Fund and the State Budget of the Czech Republic, and the European Social Fund (Young Talent Incubator II CZ.1.07/2.3.00/20.0117). N. Abou Zeid, N. Kale, M. Tutuncu, D. Crusan, E. Atkinson, and A. Siva report no disclosures relevant to the manuscript. B. Keegan has served as a consultant to Novartis, Bionest, and Bristol-Myers Squibb and receives research support from Terumo BCT. I. Pirko receives research support from the National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS058698, R01 NS060881), NIH CTSA (UL1 TR000135), the Department of Defense (W81XWH-13-1-0098), and Novartis Pharmaceuticals (CFTY720DUSNC18T). S. Pittock is a named inventor on patents (12/678,350 filed 2010 and 12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and NINDS (NS065829); and has provided consultation to Alexion Pharmaceuticals, MedImmune, and Chugai Pharma USA but has received no personal fees or personal compensation for these consulting activities (all compensation for consulting activities paid directly to Mayo Clinic). C. Lucchinetti receives research support from NINDS (R01 NS049577 and 2P50NS038667), the Department of Defense (W81XWH-13-1-0098), the Guthy-Jackson Charitable Foundation, and Kolltan Pharmaceuticals. B. Weinshenker serves on data safety monitoring boards for Novartis, Biogen Idec, and Mitsubishi Pharmaceuticals and an adjudication panel for MedImmune Pharmaceuticals; serves on the editorial boards of Neurology®, Canadian Journal of Neurological Sciences, and the Turkish Journal of Neurology; receives license royalties (<$5,000 to date) from RSR Ltd. for marketing of kits for the detection of AQP4 antibodies as a diagnostic aid for neuromyelitis optica; has received consulting fees from Asahi Kasei Medical Company, GlaxoSmithKline, Ono Pharmaceuticals, CHORD Pharmaceuticals, and Elan Pharmaceuticals; and receives research support from the Guthy-Jackson Charitable Foundation. M. Rodriguez receives research support from NIH (R01 GM092993, R01 NS024180, R01 NS032129, R01 NS048357, and R21 NS073684), NIH CTSA (UL1 RR024150 and UL1 TR000135), the National Multiple Sclerosis Society (CA 1060A11), Novartis Pharmaceuticals (CFTY720DUSNC18T), the Minnesota Partnership Award for Biotechnology and Medical Genomics, the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123), as well as the Applebaum, Hilton, Peterson, and McNeilus Foundations. O. Kantarci receives research support from the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123), the National Multiple Sclerosis Society, and has given a scientific presentation at a meeting supported by Teva Pharmaceuticals but has neither received personal fees or personal compensation for this activity (all compensation for consulting activities paid directly to Mayo Clinic) nor has spoken about the specific medications involving this company. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911. [DOI] [PubMed] [Google Scholar]
- 2.Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler 2013;19:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarci O, Siva A, Eraksoy M, et al. Survival and predictors of disability in Turkish MS patients. Turkish Multiple Sclerosis Study Group (TUMSSG). Neurology 1998;51:765–772. [DOI] [PubMed] [Google Scholar]
- 4.Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980;103:281–300. [DOI] [PubMed] [Google Scholar]
- 5.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study: I: clinical course and disability. Brain 1989;112:133–146. [DOI] [PubMed] [Google Scholar]
- 6.Kantarci OH, Weinshenker BG. Natural history of multiple sclerosis. Neurol Clin 2005;23:17–38, v. [DOI] [PubMed] [Google Scholar]
- 7.Minderhoud JM, van der Hoeven JH, Prange AJ. Course and prognosis of chronic progressive multiple sclerosis: results of an epidemiological study. Acta Neurol Scand 1988;78:10–15. [DOI] [PubMed] [Google Scholar]
- 8.Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129:606–616. [DOI] [PubMed] [Google Scholar]
- 9.Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 2006;129:584–594. [DOI] [PubMed] [Google Scholar]
- 10.Koch M, Mostert J, Heersema D, De Keyser J. Progression in multiple sclerosis: further evidence of an age dependent process. J Neurol Sci 2007;255:35–41. [DOI] [PubMed] [Google Scholar]
- 11.Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology 2011;77:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez M, Siva A, Ward J, Stolp-Smith K, O'Brien P, Kurland L. Impairment, disability, and handicap in multiple sclerosis: a population-based study in Olmsted County, Minnesota. Neurology 1994;44:28–33. [DOI] [PubMed] [Google Scholar]
- 15.Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology 2004;62:51–59. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 17.Cottrell DA, Kremenchutzky M, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 1999;122:625–639. [DOI] [PubMed] [Google Scholar]
- 18.Kremenchutzky M, Cottrell D, Rice G, et al. The natural history of multiple sclerosis: a geographically based study. 7. Progressive-relapsing and relapsing-progressive multiple sclerosis: a re-evaluation. Brain 1999;122:1941–1950. [DOI] [PubMed] [Google Scholar]
- 19.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006;129:595–605. [DOI] [PubMed] [Google Scholar]
- 20.Tremlett H, Zhao Y, Devonshire V. Natural history comparisons of primary and secondary progressive multiple sclerosis reveals differences and similarities. J Neurol 2009;256:374–381. [DOI] [PubMed] [Google Scholar]
- 21.Debouverie M, Pittion-Vouyovitch S, Louis S, Guillemin F. Natural history of multiple sclerosis in a population-based cohort. Eur J Neurol 2008;15:916–921. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–1438. [DOI] [PubMed] [Google Scholar]
- 23.Trojano M, Liguori M, Bosco Zimatore G, et al. Age-related disability in multiple sclerosis. Ann Neurol 2002;51:475–480. [DOI] [PubMed] [Google Scholar]
- 24.Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010;133:1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 1993;116:117–134. [DOI] [PubMed] [Google Scholar]
- 26.Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci 2003;206:135–137. [DOI] [PubMed] [Google Scholar]
- 27.Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009;73:1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 2010;133:1914–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabian MT, Lublin F, Wolinsky J; The PROMISE Trial Study Group. Evaluation of progressive relapsing MS patients in the PROMISE Trial. Neurology 2011;76:A613. [Google Scholar]
- 30.Trojano M, Avolio C, Manzari C, et al. Multivariate analysis of predictive factors of multiple sclerosis course with a validated method to assess clinical events. J Neurol Neurosurg Psychiatry 1995;58:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kappos L, Weinshenker B, Pozzilli C, et al. Interferon beta-1b in secondary progressive MS: a combined analysis of the two trials. Neurology 2004;63:1779–1787. [DOI] [PubMed] [Google Scholar]
- 32.SPECTRIMS Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001;56:1496–1504. [DOI] [PubMed] [Google Scholar]
- 33.Andersen O, Elovaara I, Farkkila M, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2004;75:706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59:679–687. [DOI] [PubMed] [Google Scholar]
- 35.Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F. Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology 2001;57:1969–1975. [DOI] [PubMed] [Google Scholar]
- 36.Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology 2003;60:44–51. [DOI] [PubMed] [Google Scholar]
- 37.Montalban X, Sastre-Garriga J, Tintore M, et al. A single-center, randomized, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Mult Scler 2009;15:1195–1205. [DOI] [PubMed] [Google Scholar]
- 38.Panitch H, Miller A, Paty D, Weinshenker B. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004;63:1788–1795. [DOI] [PubMed] [Google Scholar]
- 39.Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007;61:14–24. [DOI] [PubMed] [Google Scholar]
- 40.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009;66:460–471. [DOI] [PubMed] [Google Scholar]