Abstract
Objective:
The purpose of this study was to determine the prevalence of bicuspid aortic valves (BAVs) and thoracic ascending aortic aneurysms (TAAs) in a retrospective cohort of patients treated for intracranial aneurysms (IAs).
Methods:
Patients treated for IA at our institution between 2002 and 2011 were identified and their clinical records reviewed. Those without an echocardiogram of sufficient quality to assess the aortic valve were excluded. The prevalence of BAVs and TAAs in this remaining cohort was determined based on echocardiography reports, medical records, and cross-sectional chest imaging.
Results:
Of 1,047 patients, 317 had adequate echocardiography for assessment of BAV. Of these, 82 also had cross-sectional chest imaging. Of the 317 patients, 2 had BAV and 15 had TAA. The prevalence of BAVs (0.6%, 95% confidence interval 0.2%–2.2%) was similar to population prevalence estimates for this condition; however, the prevalence of TAAs (4.7%, 95% confidence interval 2.9%–7.6%) was larger than expected in a normal age- and sex-matched population.
Conclusions:
Our data demonstrate an association between IA and TAA, but not independently for BAV.
The prevalence of intracranial aneurysms (IAs) in the general population is approximately 2% to 3%.1 Recently, among 61 patients with bicuspid aortic valves (BAVs), 6 (9.8%) were found to harbor IAs.2 This raises the possibility that patients with BAV should be screened for IA. However, this would have important and potentially costly consequences for the care of patients with BAV, who comprise approximately 1% to 2% of the general population.3
Among the 6 patients with BAV, 5 also harbored a thoracic aortic aneurysm (TAA), raising the possibility that the association between BAV and IA was mediated by their common association with TAA. BAV is known to be associated with a significantly higher rate of aortic complications, including TAA, which increases in prevalence after diagnosis of BAV to greater than 20%.4 Similarly, IA has been found to associate with aortic conditions, including coarctation of the aorta5 and TAA.6 Familial clustering and genetic linkage of aortic aneurysms and IAs have also been reported.7–9 The purpose of this study was to test the hypothesis that the reported association between BAVs and IAs might instead be related to their common association with TAAs, by measuring the prevalence of TAAs and BAVs retrospectively in a large cohort of patients harboring a known IA.
METHODS
Standard protocol approvals, registrations, and patient consents.
Data collection was approved by the Washington University School of Medicine institutional review board. Informed consent was waived because of the retrospective nature of this study.
Patients.
We reviewed records for 1,047 patients consecutively treated for ruptured or unruptured nonmycotic IA at our institution between 2002 and 2011. Among these, we identified 317 patients with an echocardiogram, transthoracic (TTE) or transesophageal (TEE), in our hospital medical records that was sufficiently adequate to evaluate for BAV by report. TEE is more sensitive than TTE for identification of BAV, but by a relatively small amount (TEE 87% vs TTE 78%).10 Echocardiography was performed for a variety of reasons in these patients, typically not in relation to their IA; no specific reason such as heart failure or syncope was more frequently seen than expected. Presence of BAV or TAA was determined by the echocardiogram report. When BAV or TAA was suspected, available echocardiogram images were reviewed for accuracy.
Among the 317 patients, we identified 82 patients who had chest CT in our medical records obtained for any reason; as for echocardiography, indications varied widely and no specific trend was identified. One of the authors reviewed these CTs and measured the largest diameter of the thoracic ascending aorta using multiplanar reconstruction without knowledge of the presence of TAA based on echocardiography.11,12 In these patients, TAA was defined according to established age- and sex-matched nomograms.11 Thus, TAA presence in the total 317 patient cohort was defined as (1) TAA on echocardiogram report, (2) history of repaired TAA, or (3) TAA based on chest CT measurements. In cases when an unrepaired TAA was identified by either echocardiography or chest CT, but absent on the other, chest CT was preferentially used to define the presence of TAA given its ability to more completely evaluate the aorta.13 History of hypertension or smoking was also assessed among these 82 individuals without knowledge of TAA status.
Confidence intervals (CIs) and comparisons between groups for subarachnoid hemorrhage, hypertension, smoking status, sex, and age were assessed by 2-sided proportion CIs, χ2 test, and Student t test, as implemented in the R statistical package (http://www.R-project.org).
RESULTS
Patient characteristics and prevalence of BAVs and TAAs are shown in table 1 and figure 1. Prevalence of BAVs in the total cohort was 0.6% (95% CI 0.2%–2.2%). Prevalence of TAAs in the total cohort was 4.7% (95% CI 2.9%–7.6%). The 2 patients with BAV also had TAA, and one of the 2 had stented coarctation of the aorta. Another patient with TAA did not have BAV but also had stented coarctation of the aorta. Subarachnoid hemorrhage occurred in one of the 2 patients with BAV. Among patients with TAA, subarachnoid hemorrhage occurred in 6 patients (40% vs 55%, 2-tailed t test, p > 0.20).
Table 1.
Patient characteristics in the total cohort and patients with TAA
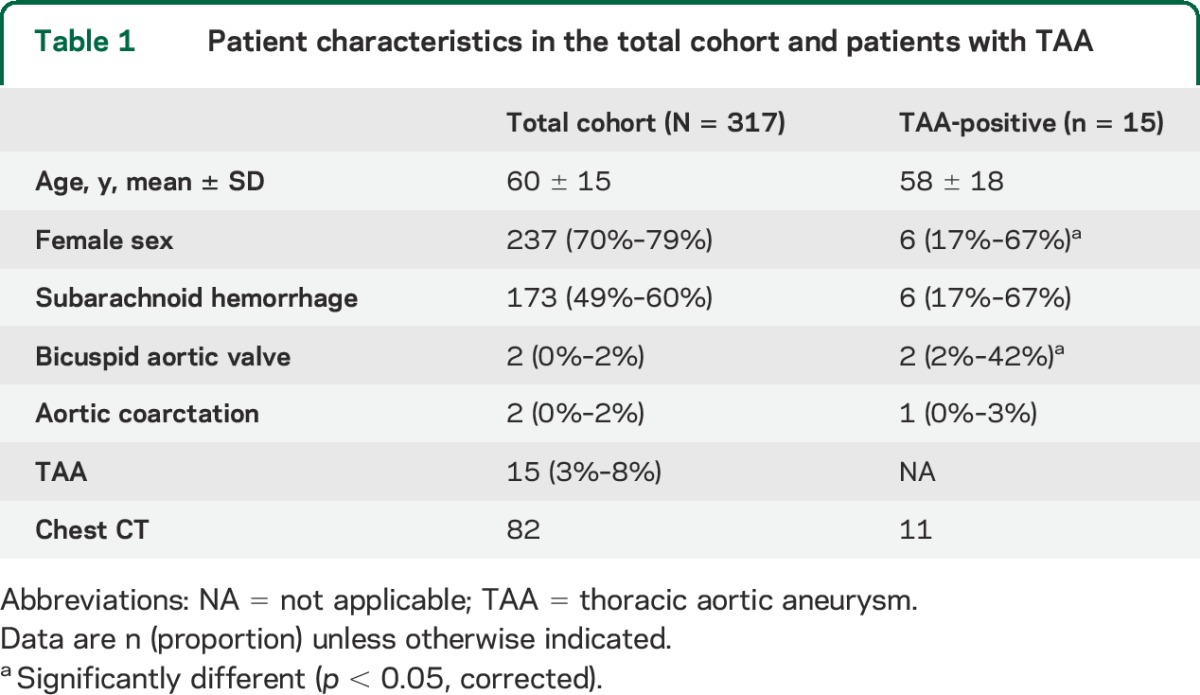
Figure 1. Flowchart of case evaluation and prevalence ascertainment.
BAV = bicuspid aortic valve; IA = intracranial aneurysm; TAA = thoracic aortic aneurysm.
Among the 82 patients with chest CT, 11 (13.4%, 95% CI 7%–23%) had TAA based on age- and sex-matched nomograms, more than expected (p < 0.03) (figure 2); the use of body-surface-area–based nomograms for these patients did not disqualify any as having TAA.12 Males were more likely to have a TAA (p < 0.01). Patients with TAA were also more likely to have hypertension (11/11 vs 49/71, p = 0.03), but not smoking history (p = 0.40). No significant difference in subarachnoid hemorrhage or age was seen between those with and without TAA.
Figure 2. Increased prevalence of TAA as measured by chest CT in 82 patients with IA.
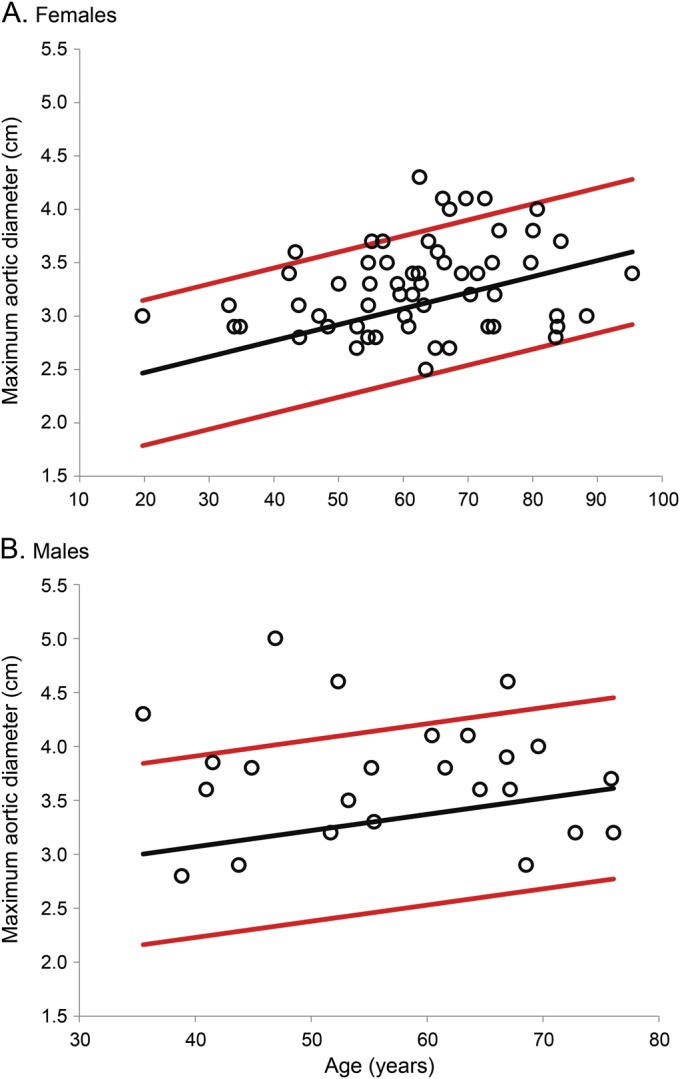
Thoracic aortic diameters (open circles) of 82 patients (A: females; B: males) are demonstrated as a function of age. Expected mean (black line) and 95% confidence interval (red lines) thoracic aortic diameters according to age are derived from previously established sex-specific nomograms.11 Several thoracic aortic diameters fall above the 95% confidence interval (n = 11, 13.3%, more than expected, p < 0.03), showing that there is an increased prevalence of TAA in this cohort of 82 patients with IA. IA = intracranial aneurysm; TAA = thoracic aortic aneurysm.
DISCUSSION
Our data demonstrate increased prevalence of TAAs but not BAVs in this retrospective cohort of patients treated for IAs. The study by Schievink et al.,2 which demonstrated an increased prevalence of IAs in patients with BAVs, may have been biased to show this association because 5 of the 6 patients with BAV and IA also had TAA; excluding patients with TAA, only one of their 24 patients with BAV had IA. Similarly, the prevalence of BAVs in our study is near that found in a normal population (1%–2%),14 and both of the patients with BAV in our study also had TAA. Another recent report also found BAV in only 1 of 56 patients (1.8%) presenting with subarachnoid hemorrhage.15
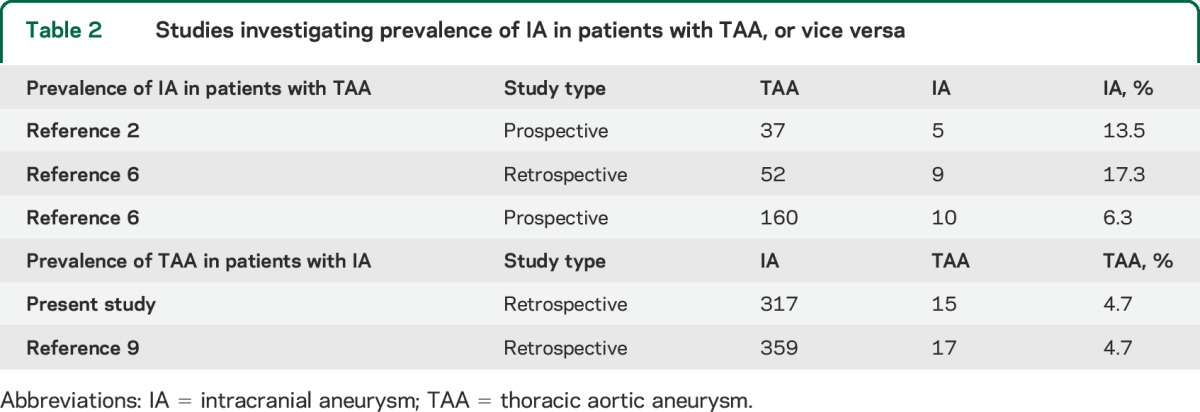
TAA, however, was associated with IA in our study. While prior estimates of TAAs in the general population have greatly varied (<1%–4.2%),11,12,16,17 these estimates are lower than the TAA prevalence in our cohort and are outside the 95% proportion CI for TAA prevalence among the 82 patients with chest CT (7%–23%). The increased prevalence of TAAs in patients with IAs is consistent with prior studies (table 2).
Table 2.
Studies investigating prevalence of IA in patients with TAA, or vice versa
The association between IA and TAA may be attributable to heritable and environmental factors. In particular, all of the patients with IA in this study identified by chest CT to have TAA also had hypertension, suggesting that this is likely a common etiologic factor for IA and TAA. Familial co-occurrence of TAA and IA also suggests a common genetic predisposition.7 Polymorphisms in certain genes, e.g., those related to collagen and transforming growth factor β receptors, confer increased risk of cerebral and aortic aneurysms, as documented in a recent comprehensive review.18 Genetic testing in young patients with aortic aneurysms or family history of aortopathies is occurring more frequently; similar testing may soon find a place in patients with IAs.
These results suggest that screening for BAVs in patients with IAs, or for IAs in patients with BAVs, is not warranted. Screening for TAAs in patients with IAs or IAs in patients with TAAs may be appropriate in a select population, although further study would be required to determine the subgroup of patients in whom such screening would be beneficial.
This study has several limitations. Retrospective selection of patients who underwent echocardiography may bias toward a larger prevalence of aortic disease. Conversely, the sensitivity of echocardiography is modest for BAV and TAA, and a minority of patients had chest CT, potentially causing us to underestimate the true prevalence of BAVs and TAAs. Despite using CT-defined age- and sex-based nomograms, the true prevalence of TAAs in a comparable normal population is unclear and varies according to the technique used. For clinical purposes, it is common to diagnose TAA when a diameter exceeds 4.0 cm, which was the case in most but not all the patients identified to have TAA by chest CT in this study. A prospective trial in patients with IAs and control subjects using imaging focused for evaluating TAAs and BAVs would surmount these issues.
ACKNOWLEDGMENT
The authors thank Dr. Alan Braverman for helpful discussions in preparing the manuscript. The authors also thank their anonymous reviewers who helped to improve the final manuscript.
GLOSSARY
- BAV
bicuspid aortic valve
- CI
confidence interval
- IA
intracranial aneurysm
- TAA
thoracic aortic aneurysm
- TEE
transesophageal echocardiogram
- TTE
transthoracic echocardiogram
AUTHOR CONTRIBUTIONS
Manu S. Goyal: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis, study supervision. Ravi Gottumukkala: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Sanjeev Bhalla: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval. Andrew Kates: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Gregory Zipfel: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Colin Derdeyn: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29:251–256. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Raissi SS, Maya MM, Velebir A. Screening for intracranial aneurysms in patients with bicuspid aortic valve. Neurology 2010;74:1430–1433. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 5.Connolly HM, Huston J, III, Brown RD, Jr, Warnes CA, Ammash NM, Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc 2003;78:1491–1499. [DOI] [PubMed] [Google Scholar]
- 6.Kuzmik GA, Feldman M, Tranquilli M, Rizzo JA, Johnson M, Elefteriades JA. Concurrent intracranial and thoracic aortic aneurysms. Am J Cardiol 2010;105:417–420. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Van Ginhoven G, Milewicz DM. Familial aggregation of both aortic and cerebral aneurysms: evidence for a common genetic basis in a subset of families. Neurosurgery 2005;56:655–661. [DOI] [PubMed] [Google Scholar]
- 8.Helgadottir A, Thorleifsson G, Magnusson KP, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet 2008;40:217–224. [DOI] [PubMed] [Google Scholar]
- 9.Kuzmik GA, Gunel M, Bulsara K, Tranquilli M, Elefteriades JA. Intracranial aneurysm patients may harbor thoracic aortic aneurysms. In: Aortic Symposium 2012. New York: American Association of Thoracic Surgeons; 2012. [Google Scholar]
- 10.Espinal M, Fuisz AR, Nanda NC, Aaluri SR, Mukhtar O, Sekar PC. Sensitivity and specificity of transesophageal echocardiography for determination of aortic valve morphology. Am Heart J 2000;139:1071–1076. [DOI] [PubMed] [Google Scholar]
- 11.Hager A, Kaemmerer H, Rapp-Bernhardt U, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg 2002;123:1060–1066. [DOI] [PubMed] [Google Scholar]
- 12.Lin FY, Devereux RB, Roman MJ, et al. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr 2008;2:298–308. [DOI] [PubMed] [Google Scholar]
- 13.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841–857. [DOI] [PubMed] [Google Scholar]
- 14.Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol 2005;30:470–522. [DOI] [PubMed] [Google Scholar]
- 15.Shaulov A, Leibowitz D, Rott D. Prevalence of bicuspid aortic valve in patients presenting with subarachnoid hemorrhage related to an intracerebral aneurysm. Int J Cardiol 2012;157:142–143. [DOI] [PubMed] [Google Scholar]
- 16.Svensjo S, Bengtsson H, Bergqvist D. Thoracic and thoracoabdominal aortic aneurysm and dissection: an investigation based on autopsy. Br J Surg 1996;83:68–71. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri V, Bella JN, Arnett DK, et al. Aortic root dilatation at sinuses of Valsalva and aortic regurgitation in hypertensive and normotensive subjects: the Hypertension Genetic Epidemiology Network Study. Hypertension 2001;37:1229–1235. [DOI] [PubMed] [Google Scholar]
- 18.Southerland AM, Meschia JF, Worrall BB. Shared associations of nonatherosclerotic, large-vessel, cerebrovascular arteriopathies: considering intracranial aneurysms, cervical artery dissection, moyamoya disease and fibromuscular dysplasia. Curr Opin Neurol 2013;26:13–28. [DOI] [PubMed] [Google Scholar]


