Abstract
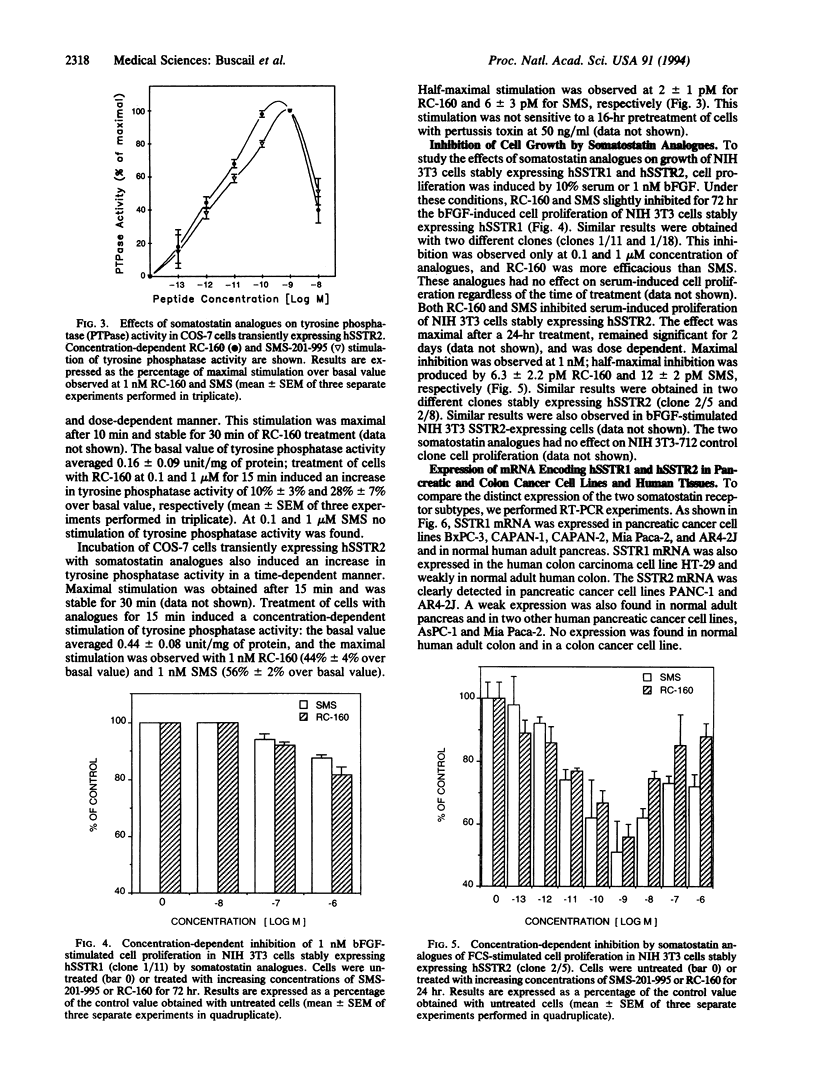
The effects of somatostatin analogues RC-160 and SMS-201-995 on tyrosine phosphatase and cell proliferation were investigated in COS-7 and NIH 3T3 cells expressing human somatostatin receptor subtype 1 or 2 (SSTR1 or SSTR2). Binding experiments were performed on membranes from COS-7 cells expressing human SSTR1 or SSTR2 using 125I-labeled [Tyr11]S-14 or [Tyr3]SMS-201-995, respectively. The somatostatin analogues RC-160 and SMS-201-995 exhibited low affinity for SSTR1 (IC50 of 0.43 and 1.5 microM, respectively) and high affinity for SSTR2 (IC50 of 0.27 and 0.19 nM). Addition of these analogues to cells expressing either SSTR1 or SSTR2 did not result in an inhibition of adenylate cyclase activity. In SSTR2-expressing cells, both analogues induced a rapid stimulation of a tyrosine phosphatase activity (EC50: RC-160, 2 pM; SMS-201-995, 6 pM) and an inhibition of serum-stimulated proliferation (EC50: RC-160, 6.3 pM; SMS-201-995, 12 pM). In SSTR1-expressing cells, only RC-160 induced stimulation of a tyrosine phosphatase activity. Both analogues caused an inhibition of cell proliferation at a concentration higher than 10 nM in accordance with their affinities for the SSTR1 receptor subtype. A good correlation between the affinities of RC-160 and SMS-201-995 for each receptor subtype and their potencies to inhibit cell proliferation suggests the involvement of these receptors in cell growth regulation. Tyrosine phosphatase was stimulated by both these analogues in SSTR2 and by RC-160 in SSTR1 at affinities similar to their ability to inhibit growth and bind to receptors, implicating tyrosine phosphatase as a transducer of the growth inhibition signal. We also found that mRNAs of receptor subtypes were variably expressed in different pancreatic and colon cancer cell lines, indicating the necessity of a precise analysis of receptor subtypes in target tissues before therapy with analogues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Reisine T. Molecular biology of somatostatin receptors. Trends Neurosci. 1993 Jan;16(1):34–38. doi: 10.1016/0166-2236(93)90050-v. [DOI] [PubMed] [Google Scholar]
- Bensaïd M., Tahiri-Jouti N., Cambillau C., Viguerie N., Colas B., Vidal C., Tauber J. P., Estève J. P., Susini C., Vaysse N. Basic fibroblast growth factor induces proliferation of a rat pancreatic cancer cell line. Inhibition by somatostatin. Int J Cancer. 1992 Mar 12;50(5):796–799. doi: 10.1002/ijc.2910500522. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruno J. F., Xu Y., Song J., Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colas B., Cambillau C., Buscail L., Zeggari M., Esteve J. P., Lautre V., Thomas F., Vaysse N., Susini C. Stimulation of a membrane tyrosine phosphatase activity by somatostatin analogues in rat pancreatic acinar cells. Eur J Biochem. 1992 Aug 1;207(3):1017–1024. doi: 10.1111/j.1432-1033.1992.tb17138.x. [DOI] [PubMed] [Google Scholar]
- Dukas K., Sarfati P., Vaysse N., Pradayrol L. Quantitation of changes in the expression of multiple genes by simultaneous polymerase chain reaction. Anal Biochem. 1993 Nov 15;215(1):66–72. doi: 10.1006/abio.1993.1555. [DOI] [PubMed] [Google Scholar]
- Kluxen F. W., Bruns C., Lübbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuhtsen S., Esteve J. P., Bernadet B., Vaysse N., Susini C. Molecular characterization of the solubilized receptor of somatostatin from rat pancreatic acinar membranes. Biochem J. 1988 Sep 15;254(3):641–647. doi: 10.1042/bj2540641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. T., Liebow C., Kamer A. R., Schally A. V. Effects of epidermal growth factor and analogues of luteinizing hormone-releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyrosine residues of specific protein substrates in various tumors. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1656–1660. doi: 10.1073/pnas.88.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M. J. The somatostatin receptor in the GI tract. Annu Rev Physiol. 1992;54:455–468. doi: 10.1146/annurev.ph.54.030192.002323. [DOI] [PubMed] [Google Scholar]
- Liebow C., Lee M. T., Schally A. Antitumor effects of somatostatin mediated by the stimulation of tyrosine phosphatase. Metabolism. 1990 Sep;39(9 Suppl 2):163–166. doi: 10.1016/0026-0495(90)90237-7. [DOI] [PubMed] [Google Scholar]
- Liebow C., Reilly C., Serrano M., Schally A. V. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Pan M. G., Florio T., Stork P. J. G protein activation of a hormone-stimulated phosphatase in human tumor cells. Science. 1992 May 22;256(5060):1215–1217. doi: 10.1126/science.256.5060.1215. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rens-Domiano S., Law S. F., Yamada Y., Seino S., Bell G. I., Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992 Jul;42(1):28–34. [PubMed] [Google Scholar]
- Rens-Domiano S., Reisine T. Biochemical and functional properties of somatostatin receptors. J Neurochem. 1992 Jun;58(6):1987–1996. doi: 10.1111/j.1471-4159.1992.tb10938.x. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Coy D. H., De Neef P., Camus J. C., Cauvin A., Waelbroeck M., Christophe J. Interaction of vasoactive intestinal peptide (VIP) and N-terminally modified VIP analogs with rat pancreatic, hepatic and pituitary membranes. Eur J Biochem. 1986 Aug 15;159(1):45–49. doi: 10.1111/j.1432-1033.1986.tb09831.x. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Strnad J., Eppler C. M., Corbett M., Hadcock J. R. The rat SSTR2 somatostatin receptor subtype is coupled to inhibition of cyclic AMP accumulation. Biochem Biophys Res Commun. 1993 Mar 31;191(3):968–976. doi: 10.1006/bbrc.1993.1312. [DOI] [PubMed] [Google Scholar]
- Tahiri-Jouti N., Cambillau C., Viguerie N., Vidal C., Buscail L., Saint Laurent N., Vaysse N., Susini C. Characterization of a membrane tyrosine phosphatase in AR42J cells: regulation by somatostatin. Am J Physiol. 1992 Jun;262(6 Pt 1):G1007–G1014. doi: 10.1152/ajpgi.1992.262.6.G1007. [DOI] [PubMed] [Google Scholar]
- Viguerie N., Tahiri-Jouti N., Ayral A. M., Cambillau C., Scemama J. L., Bastié M. J., Knuhtsen S., Estève J. P., Pradayrol L., Susini C. Direct inhibitory effects of a somatostatin analog, SMS 201-995, on AR4-2J cell proliferation via pertussis toxin-sensitive guanosine triphosphate-binding protein-independent mechanism. Endocrinology. 1989 Feb;124(2):1017–1025. doi: 10.1210/endo-124-2-1017. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]




