Abstract
The booming nanotech industry has raised public concerns about the environmental health and safety impact of engineered nanomaterials (ENMs). High-throughput assays are needed to obtain toxicity data for the rapidly increasing number of ENMs. Here we present a suite of high-throughput methods to study nanotoxicity in intact animals using Caenorhabditis elegans as a model. At the population level, our system measures food consumption of thousands of animals to evaluate population fitness. At the organism level, our automated system analyzes hundreds of individual animals for body length, locomotion speed, and lifespan. To demonstrate the utility of our system, we applied this technology to test the toxicity of 20 nanomaterials under four concentrations. Only fullerene nanoparticles (nC60), fullerol, TiO2, and CeO2 showed little or no toxicity. Various degrees of toxicity were detected from different forms of carbon nanotubes, graphene, carbon black, Ag, and fumed SiO2 nanoparticles. Aminofullerene and UV irradiated nC60 also showed small but significant toxicity. We further investigated the effects of nanomaterial size, shape, surface chemistry, and exposure conditions on toxicity. Our data are publicly available at the open-access nanotoxicity database www.QuantWorm.org/nano.
Keywords: Nanoparticles, nanomaterial, nanotoxicity, high-throughput, QuantWorm, C. elegans, surface chemistry, large scale screening
Introduction
Nanotechnology has generated remarkable scientific advances across many disciplines with applications in key sectors of the economy like healthcare, transportation, and energy. Worldwide research and commercial interests in engineered nanomaterials (ENMs) have led to rapid growth in their production, however, our limited understanding of the environmental health and safety aspect of ENMs (i.e., nano-EHS) remains a major barrier for future growth of nanotechnology.1 A key challenge in nano-EHS is the enormous diversity of ENM structures that could potentially adversely affect human health and the ecosystem.2 High-throughput toxicity screening is considered as a leading paradigm to tackle this challenge by prioritizing ENMs for more relevant but costly vertebrate models as well as developing quantitative structure-activity relationships, which lay the foundation for safe design.3 Several high-throughput screening (HTS) methods have been developed for ENM toxicity tests.4 While successful in generating toxicity data at a high speed, most of these HTS methods are biochemical or cell-based assays. These assays cannot detect complex toxicity such as organism-level toxicity that involves different tissues and organs or population-level toxicity that involves multiple animals. Therefore, it is necessary to develop high-throughput toxicity assays using intact animals.
The nematode Caenorhabditis elegans has become an emerging whole animal model for toxicology studies owing to its prevalence in the natural environment, extensive genetic and molecular toolbox, and easy and inexpensive maintenance. C. elegans toxicity assays have been validated as good predictors for the adverse effects of many chemicals in mammalian species.5 While high-throughput assays using C. elegans have been widely applied in genetic and chemical screens6-8, no C. elegans HTS has been reported on nanomaterial toxicity. Several low-throughput studies employed C. elegans to examine the toxicity of carbon and metal-based ENMs using endpoints like survival, lifespan, growth, reproduction, locomotion, metabolism, and gene expression (for details, see review by Zhao et al.9, and Table S1). Despite their initial success in the nano-EHS field, these studies used low-throughput methods to test a handful of ENMs at a time. While the qualitative conclusion of whether an ENM is toxic may be similar from different studies, the widely differed exposure methods and screening procedures (Table S1) make it difficult to integrate scattered literature data into a comprehensive toxicology library to enable quantitative toxicity comparison of all ENMs. Such library is critical for regulation and strategic prioritization. Furthermore, the bulk of ENMs tested in these studies were metal-based (Table S1). Only a few studies investigated the potential toxicity of carbon-based ENMs.10-12 Carbon nanotubes (CNTs) and hydroxylated fullerene were reported to pose toxicity toward C. elegans using lifespan, growth, and reproduction endpoints.10, 11, 13 On the other hand, graphite nano-platelets with a concentration up to 250 mg/L had no adverse effects on lifespan and reproduction.12 Considering the wide applications and fast-growing production of carbon-based ENMs, additional toxicity screening, particularly HTS, is critical.
In this study, we present a high-throughput system to measure population- and organism-level toxicity using C. elegans. The system quantifies population fitness, lifespan, growth, and locomotion. We applied this system to analyze 20 ENMs including 15 popular carbon-based ENMs such as single-walled (SWNT) and multi-walled carbon nanotubes (MWNT) with different size, shape, and surface functionality; fullerene C60 and its derivatives; graphene; graphene oxides; and carbon black. To our knowledge, this study is the first large-scale high-throughput toxicity screening of ENMs using an intact animal. Our results demonstrated the potential of our high-throughput system in generating cohesive dataset for nano-EHS. Our data are publicly available at www.QuantWorm.org/nano.
Materials and Methods
Nanomaterial Suspension Preparation
General information regarding the 20 ENMs tested in this study, including the source, size, and shape information provided by vendors, is summarized in Table 1. Aqueous suspensions of fullerol, graphene, graphene oxide (GO), reduced graphene oxide (RGO), TiO2 nanoparticles, TiO2 nanotubes, nano-Ag, fumed SiO2, CeO2, and aminofullerene were prepared at 1000 mg/L by sonicating the dry powder in deionized water generated by a Barnstead EPure system (Thermo Fisher Scientific, Waltham, MA) in a centrifuge tube using a sonicating cup horn (Sonics & Material, Newtown, CT) operated at 100W for 30 min. Suspensions of nC60 and UV irradiated nC60 were prepared using previously reported methods14 and later concentrated to 200 mg/L using 30K MWCO centrifugal filters (Amicon Ultra, Millipore, Massachusetts) (see supporting information for details). Aqueous CNT stock suspensions (1000 mg/L) were prepared by dispersing 400 mg nanomaterial powder in 400 mL deionized water using a sonicating probe (Vibra-Cell VCX 500, Sonics & Material, Newtown, CT) at 100W for 30 minutes in an ice bath. As several carbon-based nanomaterials (SWNT, MWNT-15-5, amine functionalized MWNT (MWNT-NH2), MWNT-30-5, MWNT-15-20, hydroxylated MWNT (MWNT-OH), and graphene) were unstable in deionized water, they were also prepared in 200 mg/L tannic acid (TA). Carbon black (CB) cannot be dispersed in either deionized water or TA solutions; it was prepared in 0.05% SDS at 1000 mg/L and subsequently diluted using deionized water to 200 mg/L (in 0.01% SDS). The stock suspensions were diluted to desired concentrations and autoclaved. The sterilized nanomaterial suspensions were stored in dark until use.
Table 1. List of Nanoparticles Studied.
| abbreviation | nanomaterial | size | aggregate size (nm)a | electrophoretic mobility μ μm/(V/cm)-s) | source |
|---|---|---|---|---|---|
| aminofullerene | amine functionalized fullerene | N/Ab | 212.8+60.3c 531.8+86.3d |
+0.52+0.04e -1.14±0.06d |
Prof. Pedro Alvarez's lab, Rice University |
| CB | carbon black | diameter: 42 nm | 272.2+21. 1f | -2.49+0.04e | Fisher Scientific #50-901-08775, Waltham, MA |
| CeO2 | cerium oxide nanopowder | diameter: < 25 nm | 90.9+6.0c | +2.41+0.16e | Aldrich #544841, St. Louis, MO |
| 688.3+93.3d | -1.10±0.09d | ||||
| fullerol | fullerol (C60(OH)x(ONa)y, x+y=24,y=6-8) | N/A | 148.5+22.0c | -0.82+0.04e | MER, Tucson, AZ |
| 376.1+38.5d | -0.23±0.05d | ||||
| GO | graphene oxide | N/A | 601.2+52.2c | -2.63+0.10e | Prof. Ajayan's lab, Rice University |
| graphene | graphene | N/A | 342.4+26.2d | -2.68+0.12d | Prof. Ajayan's lab, Rice University |
| MWNT-15-5 | multi-walled carbon nanotubes | outer diameter: 15+5 nm; length: 1-5 μm | 483.9+37. 1g | -2.67+0.05e | Nanolab, Waltham, MA |
| MWNT-15-20 | multi-walled carbon nanotubes | outer diameter: 15+5 nm; length: 5-20μm | 606.4+40.7g | -2.54+0.04e | Nanolab, Waltham, MA |
| MWNT-30-5 | multi-walled carbon nanotubes | outer diameter: 30+15 nm; length: 1-5μm | 411.6+50.2 g | -2.67+0.05 e | Nanolab, Waltham, MA |
| MWNT-COOH | carboxylated multi-walled carbon nanotubes | outer diameter: 15+5 nm; length: 1-5μm | 75.9+10.6c | -2.97+0.09e | Nanolab, Waltham, MA |
| MWNT-NH2 | amine functionalized multi-walled carbon nanotubes | outer diameter: 15+5 nm; length: 1-5μm | 361.1+18.7 g | -2.50+0.09 e | Nanolab, Waltham, MA |
| MWNT-OH | hydroxylated multi-walled carbon nanotubes | outer diameter: 13-18 nm; length: 1-12μm | 383.3+31.7 g | -2.63+0.08 e | Cheap Tubes, Brattleboro, VT |
| nano-Ag | nano Ag | diameter: 1-70 nm35 | 19.4+2.6 g 289.6+28.9d |
-2.38+0.25 e -0.39±0.02d |
Novacentrix, Austin, TX |
| nC60 | fullerene nanoparticles | N/A | 101.9+40.4c 684.4+90.4d |
-3.36+0.06e -2.53+0.10e |
MER, Tucson, AZ |
| RGO | reduced graphene oxide | N/A | 221.8±17.7c | -1.19+0.02d | Prof. Ajayan'slab, RiceUniversity |
| SiO2 | silica, fumed | diameter: 200-300 nm | 66.3±12.7d | -0.41+0.06d | Sigma #S5505, St. Louis, MO |
| SWNT | single-walled carbon nanotubes | outer diameter: ∼1.5 nm; length: 1-5 μm | 678.6±89.8c | -2.35+0.11e | Nanolab, Waltham, MA |
| TiO2nanoparticle | titanium dioxide, P25 | diameter: < 25 nm inner diameter: | 222.1+50.7c | -0.85+0.15e | Aldrich #637254, St. Louis, MO |
| 296.7+16.9d | -0.98+0.05 | ||||
| TiO2nanotube | titanium dioxide nanotubes | ∼200 nm; wall thickness: ∼20 nm; length: 10-100 μm | 408.2±77.0c | -0.15+0.15e | Prof. Jun Lou's lab, Rice University |
| 497.6±91.5c | -1.19+0.12d | ||||
| UV irradiated nC60 | UVA-irradiated fullerene nanoparticles | N/A | 109.4+7.2c14 | -1.13+0.05e | MER, Tucson, AZ |
All measurements were performed in the same background solution in which the stock ENM suspensions were prepared as well as in the S-media used in the toxicity tests.
Number mean hydrodynamic diameter (nm).
Not available.
Measured in deionized water.
Measured in S-media filtered through 0.22 μm syringe filters.
Measured in deionized water at ∼pH 5. Measurements at other pHs can be found in the SI and the online database.
Measured in 0.01% SDS;
Measured in 200 mg/L tannic acid; other materials were in deionized water.
ENM Characterization
Particle size and electrophoretic mobility of the nanomaterials were determined by dynamic light scattering (DLS) and phase analysis light scattering (PALS) using a ZEN 3600 ZetasizerNano (Malvern, Worcestershire, UK). All measurements were performed within 6 months after stock preparation. Immediately before the measurements, the stock suspensions were sonicated in the cup horn at 100W for 5 min, and diluted to 10 mg/L using the corresponding background solutions (deionized water, 0.01% SDS, 200 mg/L TA, and S-media15). For particle size measurement, each sample was analyzed at least 5 times consecutively with 10 runs in each measurement. Electrophoretic mobility was measured at unadjusted pH in the S-media, and at pH 3, 4, 5, 6, 7 and 8 in other background solutions, with the pH adjusted using HCl and NaOH. Three measurements were performed for each sample and each measurement consisted of 10 runs. It is noted that the light scattering from all the background solutions used (i.e., S-media, tannic acid and SDS) was negligible compared to that from the ENMs. Therefore, the size and electrophoretic mobility measured represent those of the dispersed ENMs.
C. elegans Cultivation
We used the wild-type N2 strain obtained from the Caenorhabditis Genetics Center (CGC). Worms were grown on NGM (Nematode Growth Medium) plates.15 Synchronized L1 larvae were obtained as described.15 The Escherichia coli strain OP50 was used as worm food.
ENM Exposure
Worms were exposed to nanomaterials in aqueous or solid media (Table 2). Nanomaterial suspensions were sonicated for 2 min at 35% amplitude (total ∼12,420 Joules applied) using a Vibra-Cell sonicator prior to application.
Table 2. Experimental Conditions and Toxicity Endpoints.
| assay | test condition exposure media | duration | [ENMa] | toxicity endpoints endpoints | unit |
|---|---|---|---|---|---|
| fitness | aqueous | 1 week | 1, 5, 25, and 50 ppm | optical density (OD595) fitness | N/Ab |
| N/A | |||||
| lifespan | solid | ∼4 weeks | 0.21, 0.52, 1.04, 2.08 μg/cm2 well surface | survival curve mean lifespan | % alive over time day |
| body size | aqueous solid | 3 daysc | 1, 5, 25, and 50 ppm | average body length | μm |
| 3 days | 0.21, 0.52, 1.04, 2.08 μg/cm2 well surface | ||||
| locomotion | solid | ∼2 weeks | 0.21, 0.52, 1.04, 2.08 μg/cm2 well surface | average speed | μm/sec |
Engineered nanomaterials.
Not available (no unit).
Chemicals with tannic acid were incubated for 4 days. Otherwise, incubation time was 3 days.
Tests in aqueous media were conducted in 24-well plates with 1 mL exposure media in each well. Overnight E. coli culture was centrifuged for 10 min at 3,700 g, and resuspended in S-medium15. Bacterial concentration was measured as optical density at 595 nm (OD595). The exposure media contained E. coli (OD595 = 1.5 for the fitness assays; OD595 = 1.0 for the body size assays) and nanomaterial (final concentration 1, 5, 25, or 50 ppm) in S-medium.
Assays on agar plates were conducted using 6-well plates. Each well was filled with 6 mL NGM agar and seeded with E. coli. 100 μL nanomaterial suspension of 20, 50, 100, or 200 ppm was added to each well. As most nanomaterials we tested were unlikely to diffuse through agar, we calculated the exposure concentration as μg/cm2 agar surface. The four tested concentrations were 0.21, 0.52, 1.04, and 2.08 μg/cm2.
Toxicity Assays
The fitness assay was adapted from Ramani et al.16 and used ∼50 L1 larvae/well. The plates were sealed with breathable films and placed on a 20°C shaker for 7 days. Aliquots were taken daily to measure OD595.
Three different controls were included in the assay: (1) nanomaterial alone without E. coli or worms, (2) nanomaterials with E. coli but without worms, and (3) E. coli and worms without nanomaterial. Some nanomaterials themselves have OD595 readings. Therefore, control (1) was used to establish the baseline for OD595 readings. The baseline reading was subtracted from measured OD595 of the test solutions, and the subtracted OD595 values were used in fitness calculations.
Lifespan and locomotion assays used ∼70 L1 larvae/well. When the animals reached the L4 larval stage, 25 nmol/L agar FUdR (5-fluorodeoxyuridine, catalog# AAAL16497-ME, VWR International) was added to kill the next generation of worms. Once the animals became adults, images and 30-second videos were taken daily using the QuantWorm system.17
The body size assay used ∼ 200 L1s/well for the aqueous media assay and 70 L1s/well for the solid media assay. Animals were cultured at 20°C for three days, transferred to unseeded agar plates, killed with 15 nL/well of 1 M sodium azide, and imaged using the QuantWorm system.
At least two independent trials were conducted for each assay. In each trial, at least two replicates were tested for each sample. Detailed experimental protocols can be found at www.QuantWorm.org/nano.
Results
Nanomaterial Characterization
The hydrodynamic diameters and electrophoretic mobility of ENMs are summarized in Table 1. In deionized water, the measured sizes of nano-Ag and fumed silica were close to their labeled monomer sizes. Other metal-based ENMs and some oxidized carbon-based ENMs (fullerenes and derivatives, carboxylated MWNT (MWNT-COOH), GO, and RGO) were relatively stable in deionized water, which could be attributed to their high surface charge and hydrophilicity. On the other hand, unfunctionalized carbon-based ENMs including SWNT, MWNT-15-5, MWNT-30-5, MWNT-15-20, and graphene were unstable in water due to their hydrophobic graphitic surfaces. MWNT-OH and MWNT-NH2 dispersed better than unfunctionalized nanotubes, but still aggregated fairly quickly. In general, the electrophoretic mobility of the ENMs was consistent with the surface functional groups or dispersant on the ENM surface. However, across different ENM samples, electrophoretic mobility was not a good indicator for the stability of ENM suspension (see detailed electrophoretic mobility data in Figure S1 and the online database), and in many cases, hydration forces induced by surface hydrophilicity seem to be the dominant stabilization mechanism, especially for stable ENMs with low surface charge.
Due to the high ionic strength, all CNTs aggregated and settled quickly in the S-media despite the use of tannic acid; other carbon-based ENMs except fullerol and aminofullerene also experienced significant aggregation and notable sedimentation within 24 hours. Nano-Ag, SiO2, CeO2, TiO2 nanoparticles, TiO2 nanotubes, fullerol, and aminofullerene were stable with no noticeable sedimentation in the S-media within 24 hours. However, with the exception of SiO2, their mean particle sizes were notably larger than those in deionized water. These changes were in general consistent with the changes in their electrophoretic mobility as a result of double layer compression due to the high ionic strength and the adsorption of citrate on the ENM surface. It is noted that, the particle size and electrophoretic mobility measured in the S-media still may not represent those during the exposure to C. elegans as the presence of E. coli could significantly alter ENM dispersion. Accurately determining ENM dosage and aggregation state under complex exposure conditions remains a major challenge.
Because leaching of metal impurities in CNTs may interfere with toxicity assays, the toxicity of CNT leachate was tested using E. coli. No discernible toxic effect was found (Figure S2) suggesting negligible toxicity by leachable impurities.
High-Throughput Toxicity Assays
To fully exploit C. elegans as a high-throughput nanotoxicity model, we developed a pipeline to quantify ENM toxicity in C. elegans (Figure 1). To monitor the toxicity at the population level, we used a fitness assay to evaluate the overall health of a population of thousands of animals by measuring their food intake, which can be quantified in liquid media using the optical density of E. coli. To measure the toxicity at the organism level, we selected three endpoints (Table 2) to evaluate different aspects of ENM toxicity: body size to screen for development and metabolism effects, locomotion to identify behavioral effects, and lifespan to monitor long-term effects. To enable high-throughput measurements of these endpoints, we used the QuantWorm imaging system to automatically analyze these parameters.17 To facilitate toxicity comparison across different endpoints, values for all endpoint measurements were normalized by the control values.
Figure 1.
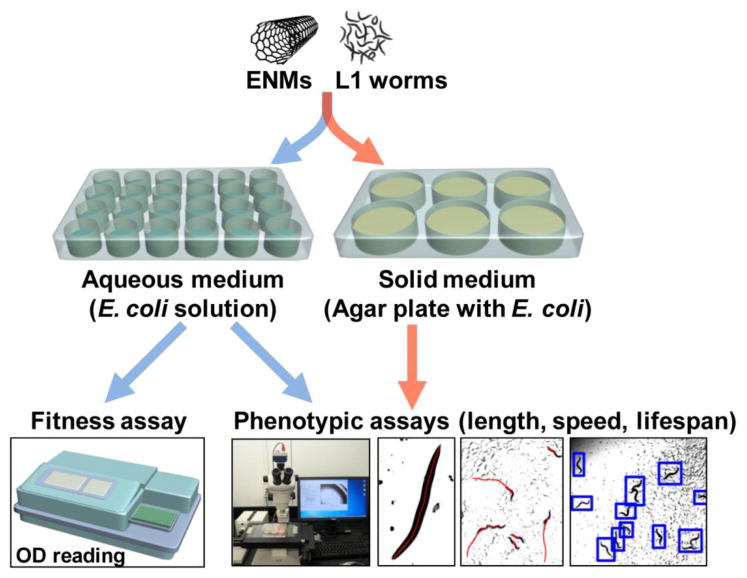
A high-throughput pipeline for toxicity assays. First larval stage (L1) worms were exposed to nanomaterials (ENMs) in aqueous or solid media. A microplate reader was used for the fitness assay. The QuantWorm system was used for imaging and measuring body length, locomotion, and lifespan.
The Fitness Assay is Highly Effective in Toxicity Detection
The rationale behind the fitness assay was that healthier animal populations generate more progeny and consume more food.16 Therefore food consumption reflected the fitness of a population (Figure 2A). Unconsumed bacterial food was measured by OD595, and fitness was thus calculated as decrease in OD595 values over time. If a nanomaterial negatively impacted the health of the population, worms consumed less food, and a smaller decrease in OD595 values was observed (Figure 2B). The fitness index F was defined as F = ΔODENM / ΔODControl, where ΔODENM and ΔODControl were the decrease of OD595 values over time for the ENM-exposed and control group respectively.
Figure 2.

C. elegans populational fitness is a sensitive endpoint for nanotoxicity. (A) The fitness assay is based on the concept that healthier populations have more progeny and eat more food. Brown indicates the presence of bacterial food. Without ENMs, all food was consumed by Day 6; With toxic ENMs, little food was consumed. (B) OD595 changed over time in two independent fitness assays. Bars and error bars indicate means and standard deviations. Sample size was n = 3 wells for SWNT or n = 12 wells for control in both trials. (C) F values for the two trials in B. Bars and error bars represent mean and 95% confidence intervals. (D) Toxicity survey of 20 different nanomaterials at four concentrations. Fitness index F displayed as a heat map with blue and yellow indicating fitness lower and higher than control values respectively. Red box marks the data point shown in B and C. Same heat map scale is used throughout the paper.
Despite its success as a genetic screen,16 the fitness assay has not been reported in toxicity studies. Therefore, it was necessary to evaluate its utility as a toxicity assay. We found the fitness assay to be both sensitive and reproducible, generating similar toxicity measurements in multiple independently conducted trials. For example, 50 ppm SWNT significantly reduced fitness in two trials (Student's t-test p < 0.0001, Figure 2C). Fitness was also the most sensitive endpoints among all the endpoints we examined (see data in Figure 3 and online database).
Figure 3.
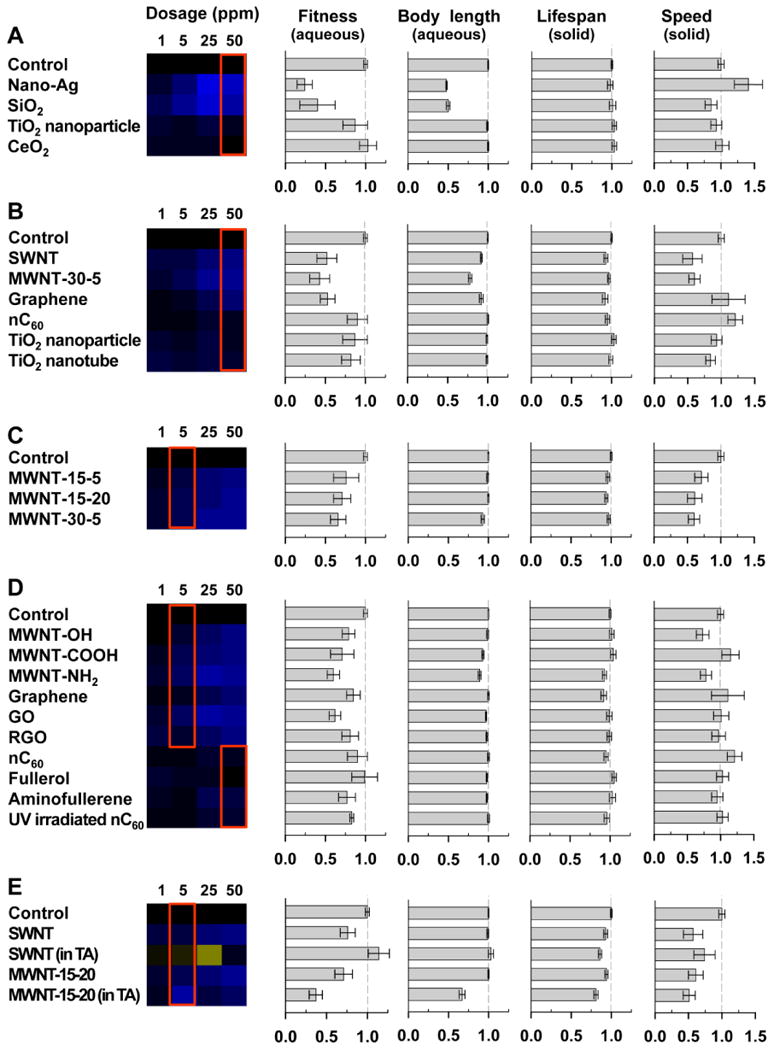
Different chemical properties affect nanomaterial toxicity. Toxicity was impacted by nanomaterial constituent elements (A), shape (B), size (C), surface chemistry (D), and exposure media (E). Heat maps represent fitness at four different concentrations of nanomaterials. Bar charts represent means and 95% confidence intervals for each endpoint at selected exposure concentrations: the concentrations for the fitness and body length charts are marked by a red box in each heat map; the concentrations for all lifespan and speed charts is 2.08 μg/cm2 well surface. Aqueous and solid indicate the type of exposure media for each assay. Sample size: n > 5 wells for fitness, n > 101 worms for body length, n > 195 worms for lifespan, and n > 71 tracks for locomotion.
A Large Number of ENMs Have Toxicity
Using the high-throughput technologies, we examined the toxicity of twenty nanomaterials at four concentrations. Only five nanomaterials, nC60, fullerol, TiO2 nanoparticles, TiO2 nanotubes, and CeO2, showed little or no toxicity under all test conditions. The fitness assay, our most sensitive assay, detected dose-dependent toxicity for most nanomaterials including CB, SWNT, MWNTs, graphene, GO, nano-Ag, and fumed SiO2 (Figure 2D). Such toxicity was often confirmed by other endpoints (Figure 3).
Effects of Constituent Elements, Shape, and Size on Nanotoxicity
As an example of toxicity affected by nanomaterial constituent elements, we found that nano-Ag and fumed SiO2 were more toxic than TiO2 and CeO2 nanoparticles. Nano-Ag and fumed SiO2 greatly reduced fitness and body length at 50 ppm and altered locomotion speed at 2.08 μg/cm2 (Figure 3A). In contrast, TiO2 and CeO2 nanoparticles showed little or no effects on all endpoints (Figure 3A).
Both TiO2 nanoparticles and TiO2 nanotubes showed no significant toxicity in all tests (Figure 3B), suggesting that toxicity in this case was determined by the constituent elements not the shape of the nanomaterials. A different case was observed in carbon-based nanomaterials. While the semi-spherical nC60 showed no significant toxicity, SWNT, MWNT, and graphene showed strong toxicity in fitness, body length, and speed (Figure 3B).
We also compared MWNTs of three different sizes (Figure 3C). Since MWNTs had poor dispersion in the absence of surfactants, we compared their toxicity at the lower exposure concentration of 5 ppm to minimize the effects of aggregation. At 5 ppm, MWNT-30-5 showed slightly more toxicity than MWNT-15-5 and MWNT-15-20 in mean fitness and body length, however, the differences were not significant (Student's t-test, p > 0.05). Therefore, a wider range of inner/outer diameters and lengths needs to be tested to understand the relationship between toxicity and MWNT size.
Effects of Surface Chemistry on Nanotoxicity
Toxicities of MWNT-15-5 with the functional group of -OH, -COOH, or -NH2 were compared (Figure 3D). Amine functionalized MWNT showed the most toxicity whereas hydroxylated MWNT had the least toxicity. Significant differences in fitness, body length, and lifespan were found between MWNT-OH and MWNT-NH2 (p < 0.01, student's t-test).
We also compared toxicities of graphene, GO, and RGO (Figure 3D). Fitness assays revealed that GO was the most toxic (p = 0.02 between GO and RGO, student's t-test), and RGO was similar to graphene. No significant differences were detected in other assays.
In addition, we compared the nanotoxicity among fullerene and its derivatives: fullerol, aminofullerene, and UV irradiated nC60 (Figure 3D). While nC60 and fullerol showed little or no toxicity, aminofullerene and UV irradiated nC60 showed small but significant toxicity (Student's t-test p < 0.0001) at the highest test concentration of 50 ppm in fitness assays. Such subtle toxicity was not detected using other endpoints.
Toxicity Interactions Between Tannic Acid and Carbon Nanotubes
To examine the effects of dispersion on nanotoxicity, we tested carbon nanotubes with and without tannic acid (TA). TA is a naturally occurring polyphenol that is known to stabilize certain carbon nanotubes (CNTs), and often used as a surrogate of dissolved organic matter.18, 19 For nanomaterial samples with TA, a background solution with the same concentration of TA was used as the control in all toxicity assays. A concentration of 5 ppm nanomaterials was chosen for comparison as significant aggregation was observed in higher concentrations even with TA.
The presence of TA significantly enhanced the toxicity of MWNT but reduced the toxicity of SWNT on fitness and body size (Figure 3E). The dosage response curve also lost its linearity in the presence of TA (Figure 4A).
Figure 4.
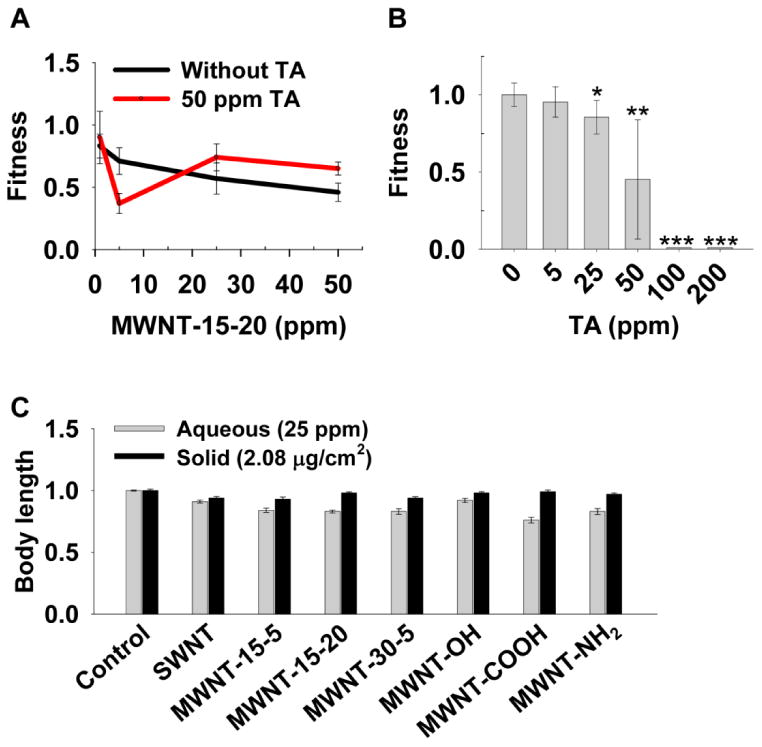
Exposure media affect nanotoxicity. (A) The effects of four concentrations of MWNT-15-20 with and without tannic acid (TA) on animal fitness. Sample size was n > 6 wells for each condition. (B) Toxic effect of TA on animal fitness. Sample size was n = 8 wells for each condition. * p < 0.05; ** p < 0.01; *** p < 0.001; p value, Student's t-test. (C) Comparison of body length between aqueous and solid medium exposure. Bars and error bars stand for mean and 95% confidence intervals.
The complicated effect of TA was partly because TA itself is toxic to worms. TA has been reported to be toxic to C. elegans, the barnacle Balanus amphitrite, rats, mice, and rabbits.20-22 TA (50 ppm) was used as our dispersant and itself significantly decreased the animal fitness (Figure 4B). Therefore, the combined toxicity in our study was from both TA and the nanomaterial. The combined toxicity could be a result from TA-CNT interactions: TA could have enhanced CNT toxicity by increasing CNT dispersion, or CNTs could have absorbed TA, resulting in less available TA in solution18 and thus reducing toxicity from TA.
The different effects of TA on MWNT and SWNT toxicity (Figure 3E) could be caused by their different capabilities to absorb TA, different dispersion statuses, and/or different toxicity mechanisms.
Effects of Solid and Aqueous Media on Nanotoxicity
The exposure media can affect nanotoxicity in multiple ways. For example, in comparison to aqueous media, solid media can promote worm-nanoparticle contact and thus increase toxicity, but solid media can also promote aggregation and decrease toxicity. Our body size assay was in both aqueous and solid media, enabling us to evaluate the overall effects of exposure media on toxicity. Most of the nanomaterials used in this study likely accumulated on the agar surface instead of diffusing throughout the agar, making it difficult to assess their effective concentration. For example, all CNTs we used had lengths greatly exceeding the agar pore size23, 24 (Table 1). If they could penetrate a 1 mm layer of the agar, the effective exposure concentration for the highest concentration we tested (2.08 μg/cm2) would be 23.4 ppm. We thus compared CNT toxicity between the 25 ppm group in aqueous media and the 2.08 μg/cm2 group on solid media. The two concentrations were also comparable using other measurements such as mass per well (25 μg/well aqueous vs. 20 μg/well solid), mass per worm (0.13 μg/worm aqueous vs. 0.29 μg/worm solid). More severe toxicity was observed when worms were exposed to nanomaterials in aqueous media than solid media (Figure 4C). Such difference could be a result of more CNT aggregation on solid media, or higher bioavailability and enhanced interactions between nanotubes and internal structures of the worms in aqueous media. It is also possible that worms were more stressed in liquid culture and that stress exacerbates CNT toxicity.
Open-Access Nanotoxicity Database
All of our data are publicly available at http://www.QuantWorm.org/nano. The website provides toxicity data, nanomaterial characterization data, experimental protocols, and software downloads (Figure S3, S4).
Discussion
Fitness Assay for Toxicity Assessment
The fitness assay provided toxicology data of intact animals at the throughput of cell-based assays. However, our fitness assay has several advantages over cell-based assays in evaluating nanotoxicity. First, this assay examined nanotoxicity on an extremely large animal population. The starting control group population of 50 worms normally grew to over 10,000 animals by the end of the assay, enabling detection of toxicity on a small percentage of animals. Second, the fitness assay measured long-term exposure over multiple generations of worms. As C. elegans has a life cycle of 3 days, the 6-day assay was enough time for not only the initial animals and their children to become reproducing adult worms, but also the third generation to hatch to L1 worms. Finally, fitness was a highly inclusive endpoint. The assay captured not only lethality but also sublethal effects such as reproductive issues, developmental defects, and eating disorders. All these features made the fitness assay extremely sensitive in detecting nanotoxicity.
Although the fitness assay does not distinguish whether the primary toxicity was on worms or bacteria, several factors indicate that it likely reflects a direct toxicity on worms. The S-medium used in the assay does not support bacteria proliferation, as bacteria OD595 remained at constant levels in S-medium. OD595 also stayed flat over time in all our controls where E. coli was placed with nanomaterials without worms, suggesting that even if nanomaterials had toxicity on bacteria, the toxicity was not reflected on OD595 readings. Therefore, the measured OD595 changes completely depend on worm consumption. Furthermore, bacteria killed by ENMs were unlikely to affect worm fitness. Worms fed on dead bacteria have similar appearances and brood sizes as those fed on live bacteria and show similar lifespan as those fed on non-dividing live bacteria.25, 26
Nanomaterial Toxicity
Some of the twenty nanomaterials tested have been investigated previously in different toxicology assays.27, 28 Overall, the results are consistent with ours, confirming the accuracy of our high-throughput system. For example, nano-Ag was generally more toxic than TiO2 nanomaterials in bacteria, algae, crustaceans, and fish,27 and nC60 was less toxic than SWNT, MWNT, and SiO2 nanoparticles to alveolar macrophages in a cytotoxicity assay.29 While previous studies compared only a handful of ENMs, this study allowed quantitative toxicity comparison across all 20 ENMs because high-throughput technologies enabled toxicity screening of large sample set under the same experimental conditions. The low-cost high-throughput approach is also critical for constructing the nano-EHS database (see our online database) for regulation and green design.
The screening data suggested that the adverse effects of nanomaterials were determined by multiple chemical properties, as well as their dispersion state. Chemical composition and dissolution proved to be the two major factors controlling toxicity of metal-based ENMs. Metal-based ENMs with intrinsically toxic constitutes that can be readily dissolved in aqueous phase were more toxic to C. elegans. Nano-Ag is a prime example, and similar results have been reported by several studies.30-32 On the other hand, TiO2 nanoparticles and TiO2 nanotubes, despite their size and shape differences, both have low toxicity in tested conditions likely owing to their relatively safe chemical composition. CNTs were found to be toxic in C. elegans at low dosages, in line with literature reports.11 There is no clear correlation between their size/shape and toxicity. This could be partially attributed to the formation of aggregates in aqueous phase, which masks their differences in physical dimensions. Surface functionality of carbon-based ENMs influences both their dispersion state and the interactions between ENMs and worms. Among MWNTs with similar dimensions (i.e., MWNT-15-5, MWNT-OH, MWNT-COOH, and MWNT-NH2), only MWNT-COOH forms suspension with long-term stability. Nevertheless, it had similar toxicity as MWNT-15-5 and MWNT-OH. On the other hand, MWNT-NH2 was significantly more toxic than other MWNTs, indicating the important role of ENM-worm interactions. Similarly, amide-functionalized CNTs were reported to facilitate the cellular uptake by C. elegans.11 Another category of carbon-based ENMs, fullerene and its derivatives, has limited toxicity in C. elegans despite the surface functionality. Fullerol with sizes around 4.7 and 40.1 nm at 100 mg/L was reported to induce apoptosis-mediated toxicity in C. elegans, significantly reducing survival, body size, and reproduction.33 The low toxicity of fullerol found in this work is likely caused by the large particle size (148.5 ± 22.0 nm) and low exposure concentrations. The toxicity of graphenes correlates well with their extent of surface oxidation (GO > RGO > graphene). GO was reported to disturb functions of both primary and secondary targeted organs through oxidative stress, enhanced intestinal permeability, and suppressed defecation in C. elegans34 Losing of surface oxides could alter the interactions between GO and bio-interfaces, potentially reducing its translocation across biological barriers. Consistently, graphite nano-platelets were non-toxic in C. elegans12
Supplementary Material
Acknowledgments
We thank Joaquina Nunez, Charles Ho, and Alma Hinojos for technical support. We thank the China Scholarship Council for Tianxiao Wang's graduate fellowship. This research was funded by the National Institutes of Health grants (HG004724 and DA018341) and by a Searle Scholar grant to WZ.
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information: Additional figures, tables, and descriptions. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Qu XL, Brame J, Li QL, Alvarez PJJ. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Accounts Chem Res. 2013;46(3):834–843. doi: 10.1021/ar300029v. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CR, George S, Horst AM, Ji ZX, Miller RJ, Peralta-Videa JR, Xia TA, Pokhrel S, Madler L, Gardea-Torresdey JL, Holden PA, Keller AA, Lenihan HS, Nel AE, Zink JI. Nanomaterials in the environment: From materials to high-throughput screening to organisms. ACS Nano. 2011;5(1):13–20. doi: 10.1021/nn1034857. [DOI] [PubMed] [Google Scholar]
- 3.Damoiseaux R, George S, Li M, Pokhrel S, Ji Z, France B, Xia T, Suarez E, Rallo R, Madler L, Cohen Y, Hoek EMV, Nel A. No time to lose-high throughput screening to assess nanomaterial safety. Nanoscale. 2011;3(4):1345–1360. doi: 10.1039/c0nr00618a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, Zhang H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Accounts Chem Res. 2012;46(3):607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106(1):5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5(5):387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 7.Boyd WA, McBride SJ, Freedman JH. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: Evaluation of a novel, high-throughput screening assay. PLoS ONE. 2007;2(12):e1259. doi: 10.1371/journal.pone.0001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomotto J, Ségalat L. High-throughput screening and small animal models, where are we? Br J Pharmacol. 2010;160(2):204–216. doi: 10.1111/j.1476-5381.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao YL, Wu QL, Li YP, Wang DY. Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans. RSC Adv. 2013;3(17):5741–5757. [Google Scholar]
- 10.Wu QL, Li YX, Li YP, Zhao YL, Ge L, Wang HF, Wang DY. Crucial role of the biological barrier at the primary targeted organs in controlling the translocation and toxicity of multi-walled carbon nanotubes in the nematode Caenorhabditis elegans. Nanoscale. 2013;5(22):11166–11178. doi: 10.1039/c3nr03917j. [DOI] [PubMed] [Google Scholar]
- 11.Chen PH, Hsiao KM, Chou CC. Molecular characterization of toxicity mechanism of single-walled carbon nanotubes. Biomaterials. 2013;34(22):5661–5669. doi: 10.1016/j.biomaterials.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 12.Zanni E, De Bellis G, Bracciale MP, Broggi A, Santarelli ML, Sarto MS, Palleschi C, Uccelletti D. Graphite nanoplatelets and Caenorhabditis elegans: Insights from an in vivo model. Nano Lett. 2012;12(6):2740–2744. doi: 10.1021/nl204388p. [DOI] [PubMed] [Google Scholar]
- 13.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34(3):J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Qu XL, Hwang YS, Alvarez PJJ, Bouchard D, Li QL. UV irradiation and humic acid mediate aggregation of aqueous fullerene (nC(60)) nanoparticles. Environ Sci Technol. 2010;44(20):7821–7826. doi: 10.1021/es101947f. [DOI] [PubMed] [Google Scholar]
- 15.Stiernagle T. WormBook. The C elegans Research Community. WormBook; 2006. Maintenance of C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramani Arun K, Chuluunbaatar T, Verster Adrian J, Na H, Vu V, Pelte N, Wannissorn N, Jiao A, Fraser Andrew G. The majority of animal genes are required for wild-type fitness. Cell. 2012;148(4):792–802. doi: 10.1016/j.cell.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Jung SK, Aleman-Meza B, Riepe C, Zhong W. QuantWorm: A comprehensive software package for Caenorhabditis elegans phenotypic assays. PLoS ONE. 2014;9(1):e84830. doi: 10.1371/journal.pone.0084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, Xing B. Tannic acid adsorption and its role for stabilizing carbon nanotube suspensions. Environ Sci Technol. 2008;42(16):5917–5923. doi: 10.1021/es800329c. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Liljestrand HM, Shimizu Y, Morita M. Effects of physical-chemical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ Sci Technol. 2003;37(12):2646–2657. doi: 10.1021/es026405w. [DOI] [PubMed] [Google Scholar]
- 20.Lau SC, Qian PY. Inhibitory effect of phenolic compounds and marine bacteria on larval settlement of the barnacle Balanus amphitrite amphitrite Darwin. Biofouling. 2000;16(1):47–58. [Google Scholar]
- 21.Barnes J, Rossiter R. Toxicity of tannic acid. Lancet. 1943;242(6260):218–222. [Google Scholar]
- 22.Boyd EM. The acute toxicity of tannic acid administered intragastrically. Can Med Assoc J. 1965;92(25):1292. [PMC free article] [PubMed] [Google Scholar]
- 23.Righetti PG, Brost BC, Snyder RS. On the limiting pore size of hydrophilic gels for electrophoresis and isoelectric focussing. J Biochem Biophys Methods. 1981;4(5):347–363. doi: 10.1016/0165-022x(81)90075-0. [DOI] [PubMed] [Google Scholar]
- 24.Pernodet N, Maaloum M, Tinland B. Pore size of agarose gels by atomic force microscopy. Electrophoresis. 1997;18(1):55–58. doi: 10.1002/elps.1150180111. [DOI] [PubMed] [Google Scholar]
- 25.Gems D, Riddle DL. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics. 2000;154(4):1597–1610. doi: 10.1093/genetics/154.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161(3):1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahru A, Dubourguier HC. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269(2-3):105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Du J, Wang S, You H, Zhao X. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environ Toxicol Pharmacol. 2013;36(2):451–462. doi: 10.1016/j.etap.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39(5):1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 30.Roh Jy, Sim SJ, Yi J, Park K, Chung KH, Ryu Dy, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43(10):3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Jiang C, Hsu-Kim H, Badireddy AR, Dykstra M, Wiesner M, Hinton DE, Meyer JN. Silver nanoparticle behavior, uptake, and toxicity in Caenorhabditis elegans: Effects of natural organic matter. Environ Sci Technol. 2014;48(6):3486–3495. doi: 10.1021/es404444n. [DOI] [PubMed] [Google Scholar]
- 32.Ellegaard-Jensen L, Jensen KA, Johansen A. Nano-silver induces dose-response effects on the nematode Caenorhabditis elegans. Ecotoxicol Environ Saf. 2012;80(0):216–223. doi: 10.1016/j.ecoenv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Cha YJ, Lee J, Choi SS. Apoptosis-mediated in vivo toxicity of hydroxylated fullerene nanoparticles in soil nematode Caenorhabditis elegans. Chemosphere. 2012;87(1):49–54. doi: 10.1016/j.chemosphere.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Yin L, Li X, Tang M, Zhang T, Wang D. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale. 2013;5(20):9934–9943. doi: 10.1039/c3nr02084c. [DOI] [PubMed] [Google Scholar]
- 35.Zodrow K, Brunet L, Mahendra S, Li D, Zhang A, Li Q, Alvarez PJJ. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43(3):715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


