Abstract
Recent studies have demonstrated that erythropoietin (EPO) has extensive nonhematopoietic biological functions. However, little is known about how EPO regulates bone formation, although several studies suggested that EPO can affect bone homeostasis. In this study, we investigated the effects of EPO on the communication between osteoclasts and osteoblasts through the ephrinB2/EphB4 signaling pathway. We found that EPO slightly promotes osteoblastic differentiation with the increased expression of EphB4 in ST2 cells. However, EPO increased the expression of Nfatc1 and ephrinB2 but decreased the expression of Mmp9 in RAW264.7 cells, resulting in an increase of ephrinB2-expressing osteoclasts and a decrease in resorption activity. The stimulation of ephrinB2/EphB4 signaling via ephrinB2-Fc significantly promoted EPO-mediated osteoblastic differentiation in ST2 cells. EphB4 knockdown through EphB4 shRNA inhibited EPO-mediated osteoblastic phenotypes. Furthermore, in vivo assays clearly demonstrated that EPO efficiently induces new bone formation in the alveolar bone regeneration model. Taken together, these results suggest that ephrinB2/EphB4 signaling may play an important role in EPO-mediated bone formation.
Keywords: osteoblast, skeletal, osteogenesis, hematopoietic growth factors, hormones, cytokines
Introduction
Bone regeneration is a complex process between the bone-forming activity of osteoblasts and the bone-resorbing activity of osteoclasts. Many molecules are involved in the communication between osteoblasts and osteoclasts during bone remodeling (Hayden et al. 1995; Martin and Sims 2005; Boyce and Xing 2008). Receptor activator of nuclear factor κB ligand (RANKL) from osteoblasts interacts with receptor activator of nuclear factor κB (RANK) on osteoclasts and plays a critical role in the differentiation, biological function, and maintenance of osteoclasts—called the RANKL/RANK axis (Virk et al. 2009). However, tartrate-resistant acid phosphatase (TRAP) from osteoclasts contributes to the migration, proliferation, differentiation, and activation of osteoblasts (Hayman 2008), suggesting that the proliferation and differentiation of osteoblasts clearly require signals from osteoclasts. Thus, communication between osteoblasts and osteoclasts plays critical roles during bone remodeling.
It was recently found that ephrinB2 (a transmembrane ligand expressed by osteoclasts) and EphB4 (a tyrosine kinase receptor expressed by osteoblasts) are involved in the control of bone homeostasis (Mundy and Elefteriou 2006; Zhao et al. 2006). Uniquely, EphB4 and ephrinB2 interactions produce bidirectional signals that affect both EphB4-expressing osteoblasts and ephrinB2-expressing osteoclasts (Zhao et al. 2006), leading to enhanced formation of osteoblasts and suppressed formation of osteoclasts, which together result in bone formation.
Erythropoietin (EPO) is a well-known hormone that regulates red blood cell generation (Beckman et al. 1999). Recent studies have found that EPO also plays roles in bone homeostasis. EPO can enhance bone formation by increasing the expression of vascular endothelial growth factor (Holstein et al. 2011) and bone morphogenetic protein 2 (Shiozawa et al. 2010). In addition, EPO regulates bone formation through mTOR signaling (Kim et al. 2012). In this study, we investigated the effect of EPO on the communication of osteoclasts and osteoblasts through the ephrinB2/EphB4 signaling pathway. Our results demonstrate that EPO increases osteoblastic activity through EphB4 signaling while increasing the number of ephrinB2-expressing osteoclasts, but dampening their resorptive activity. Together, bidirectional signals activated by EPO through ephrinB2/EphB4 signaling resulted in a bone formation.
Materials and Methods
Cell Cultures
Mouse bone marrow–derived stromal cell line ST2 (RIKEN CELL BANK, Ibaraki, Japan) and rat bone marrow mesenchymal stem cells (BMSCs) were grown in Minimum Essential Medium Alpha Modified (α-MEM, Gibco, Life Technologies, USA), supplemented with 10−8 M dexamethasone (Sigma-Aldrich, Milwaukee, WI, USA), 50 mg/L of ascorbic acid (Sigma-Aldrich), and 10 mM β-glycerol phosphate sodium (Sigma-Aldrich), known as osteogenic medium. The RAW264.7 cell line was derived from mouse monocyte macrophage cell (CAS CELL BANK, Shanghai, China) and grown in low-glucose Dulbecco’s Modified Eagle’s Medium (L-DMEM, Invitrogen, CA, USA), supplemented with 50 ng/mL of recombinant mouse RANKL (R&D System, Minneapolis, MN, USA), known as osteoclastogenic medium. The following supplements were included in both cell cultures: 10% fetal bovine serum (Gibco, CA, USA), 100 U/mL of penicillin G, and 100 μg/mL of streptomycin. To stimulate ephrinB2/EphB4 signaling in ST2 cell culture, ephrinB2-Fc was used. Briefly, 6- or 96-well plates were coated with poly-L-lysine (0.01% solution, Sigma-Aldrich) for 4 h at room temperature before culture; then, ephrinB2-Fc or Fc fragments (R&D System) were either clustered with anti-Fc antibody or coated on poly-L-lysine cover plates at 4 mg/mL, unless otherwise indicated (Zhao et al. 2006). The plates were incubated at 37 °C in a 5% CO2 incubator for 2 h. After 2 h, ST2 cells were seeded in the pretreated 6- or 96-well plate with osteogenic medium.
Alkaline Phosphatase and Alizarin Red Assays
ST2 cells and BMSCs were seeded at 5 × 104 cells per well in a 6-well plate or 3 × 103 cells per well in a 96-well plate and cultured with osteogenic medium for 24 h. Then, 20 U/mL of EPO (epoetin alfa, Amgen, Thousand Oaks, CA, USA) was added into EPO groups; the same volume of phosphate buffered saline (PBS) was added into control group. On days 7 and 14, alkaline phosphatase (ALP) activity was analyzed with ALP substrate (Sigma-Aldrich) and read with a microplate reader (Gen5, Biotek Synergy, Winooski, VT, USA) under 520 nm according to the manufacturer’s instructions. After 2 and 3 wk, cells were fixed with 95% ethanol and stained with 2% alizarin red (Sigma-Aldrich). The stained samples were then eluted with 10% cetylpyridinium chloride. The absorbance was measured at 550 nm with a spectrometer.
EPHB4 Inhibition Assay
ST2 cells were seeded at 1.2 × 105 cells per well in 6-well plates. After 24 h, cells were transfected with mouse EphB4 shRNA (OriGene Technologies, TG500619), which included 4 unique 29-mer shRNA constructs in a retroviral GFP plasmid, the GFP cloning plasmid (pGFP-V-RS), used herein as control A, and a noneffective 29-mer scrambled shRNA cassette in pGFP-V-RS vector, used herein as control B (0.2 μg/well), via Lipofectamine 2000 (Invitrogen). For EPO- or ephrinB2-Fc-treated groups, 20 U/mL of EPO or ephrinB2-Fc preclustered with anti-Fc antibody was added 24 h after transfection.
TRAP Staining and Bone Resorption Assays
RAW264.7 cells were seeded at 1.2 × 105 cells per well in 6-well plates and cultured with osteoclastogenic medium. After 9 d, cells were fixed and stained with a Leukocyte (TRAP) Kit (Sigma-Aldrich) according to the manufacturer’s instructions. For bone resorption assay, RAW264.7 cells were seeded on bone slices in 24-well plates and cultured with osteoclastogenic medium for 14 d. The area of bone resorption from 5 randomly selected fields was delineated and calculated with Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) and normalized to TRAP-positive cells.
Quantitative Reverse Transcription Polyermerase Chain Reaction
Total RNA from cell cultures was extracted with Trizol (Invitrogen), and 1 mg of total RNA was used for reverse transcription reaction via the PrimerScript RT Reagent Kit with gDNA Eraser (TaKaRa, Shijodori Kyoto, Japan) to synthesize cDNA. Sequences of primers are listed in the Table. All quantitative polyermerase chain reaction assays were performed in the MX3005P system (Agilent, Santa Clara, CA, USA) with SYBR Premix Ex Taq (TaKaRa) under the following conditions: 95 °C for 30 s, 95 °C for 5 s, 55 °C for 30 s, and 72 °C for 1 min, for 40 cycles. β-actin was used as the internal control.
Table.
Primers Used for Quantitative Reverse Transcription Polyermerase Chain Reaction Assays in This Study.
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) |
|---|---|---|---|
| Actb | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA | 171 |
| Runx2 | GCACAAACATGGCCAGATTCA | AAGCCATGGTGCCCGTTAG | 126 |
| Sp7 | AAGTTATGATGACGGGTCAGGTACA | AGAAATCTACGAGCAAGGTCTCCAC | 129 |
| Col1a1 | GACATGTTCAGCTTTGTGGACCTC | GGGACCCTTAGGCCATTGTGTA | 119 |
| Ephb4 | AGTGGCTTCGAGCCATCAAGA | CTCCTGGCTTAGCTTGGGACTTC | 186 |
| Efnb2 | ACGGTCCAACAAGACGTCCA | GCTGTTGCCATCGGTGCTA | 107 |
| C-fos | ACGTGGAGCTGAAGGCAGAAC | AGCCACTGGGCCTAGATGATG | 66 |
| Nfatc1 | CAAGTCTCACCACAGGGCTCACTA | TCAGCCGTCCCAATGAACAG | 144 |
| Mmp9 | GCCCTGGAACTCACACGACA | TTGGAAACTCACACGCCAGAAG | 85 |
| Ctsk | CACCCAGTGGGAGCTATGGAA | GCCTCCAGGTTATGGGCAGA | 123 |
bp, base pair.
Western Blots
Whole cell lysates were prepared from cells, separated on 10% SDS-polyacrylamide gel and transferred to PVDF membranes. The membranes were incubated with 5% milk for 1 h and incubated with primary antibodies β-actin (1:200; Santa Cruz, CA; Dallas, TX, USA), EphB4 (1:200; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4 °C. Blots were incubated with HRP-conjugated secondary antibodies (1:1000; Beyotime, Beijing, China) for 1 h, and protein expression was detected with DAB. Quantification of the results was performed using software Image J 1.48p.
In Vivo Animal Experiments
Animal experiments in this study were approved by the Jilin University Animal Care and Use Committee and conforms to the ARRIVE guidelines. We used a classical animal model—the alveolar bone regeneration model (Elsubeihi and Heersche 2004)—to evaluate the effects of EPO on bone regeneration in vivo. To successfully create this model, the crown of the left mandibular incisor was repeatedly cut at the gingival level with a high-speed turbine drill under anesthesia (60 mg/kg of ketamine and 8 mg/kg of xylazine, intramuscularly) at days 10, 8, 6, 4, and 2, before this mandibular incisor was fully extracted. The mandibular incisors of rodents keep erupting over their lives. Once the crown of the mandibular incisor is removed, the tooth will erupt more quickly, resulting in edema of the periodontal ligaments (Shimizu et al. 1996; Kertesz et al. 2006), which can facilitate the extraction of mandibular incisors. On day 0, the left mandibular incisor was carefully extracted. Seventy-two male Wistar rats (210 ± 10 g, 7 to 8 wk old) were randomly divided into 3 groups: a control group administered with 10 µL of PBS (n = 24), an experimental group administered with 10 U of EPO into the tooth socket (n = 24), and another experimental group administered with 20 U of EPO into the tooth socket (n = 24). The defects were closed by periodontal dressing.
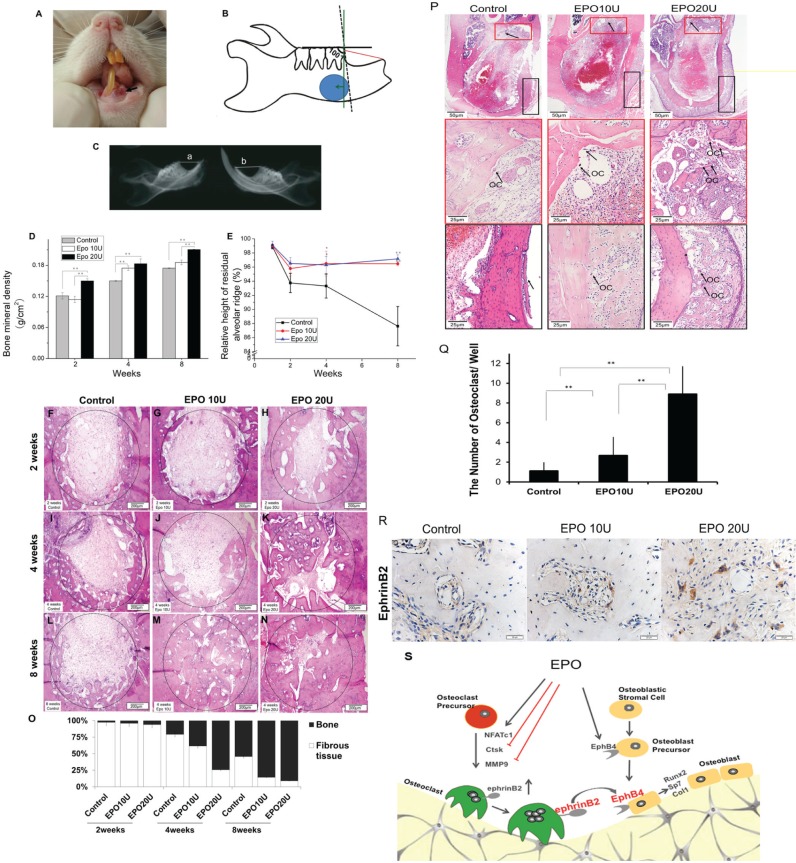
Rats were anesthetized and euthanized by heart perfused fixation with 4% paraformaldehyde solution 2, 4, and 8 wk postoperation. The mandible samples were examined with a LEXXOS Digital 2D Densitometer (DMS, Nanterre, France) for bone mineral density (BMD) and soft X-ray (Astra-4800, UMAX, China) for relative height of residual alveolar ridge. The relative height of the residual alveolar ridge was used to evaluate the effects of EPO on bone regeneration and resorption. The relative height of the residual alveolar ridge was defined as the ratio of distance between left and right, which was from the infradental point (apex of alveolar ridge of mandibular incisor) to the highest point of mesial alveolar bone of lower first molar (Fig. 5C). All measurements were done by Image-Pro Plus 6.0 software (Media Cybernetics). To measure BMD, 0.33 cm2 of round area was selected in the anterior aspect of the molar region of the mandible near the inferior border (see Fig. 5B). The anterior border of the circle was determined to be approximately 100° anterior to a flat line plane across the occlusal surface of the molar teeth as shown in the drawing. The circle encompasses the incisor tooth socket that was injected with PBS or EPO. Then, the left mandible from which the incisor had been extracted was decalcified, embedded in paraffin, and stained with hematoxylin and eosin (HE) staining. For immunohistochemistry staining, tissue sections were blocked for 30 min at room temperature with 3% normal goat serum, incubated with 2 μg/L of rabbit IgG anti-ephrin-B2 and normal rabbit IgG (Santa Cruz Biotechnology, Dallas, TX, USA) as a negative control at 4 °C overnight, washed, and incubated with secondary goat anti-rabbit labeled with biotin at room temperature for 2 h. Then, sections were stained with DAB substrate (DAKO, Glostrup, Denmark) and counterstained with hematoxylin.
Figure 5.
Erythropoietin (EPO) increased bone mineral density (BMD) and bone formation and inhibited resorption of residual alveolar ridge in an alveolar bone regeneration model. (A) A photo of the rat incisor tooth extraction site. (B) A diagram of an alveolar bone defect model. The solid blue area was selected for the BMD analysis, which is in the anterior aspect of the molar region of the mandible near the inferior border. The red line is relative height of the mandibular residual ridge. The green line represents the direction of the tissue section processed for histological analysis. (C) Photo of soft X-ray. The left photo is the mandible of the EPO-treated side of the tooth socket; the right photo is the mandible of the nontreated side of the tooth socket. Both are from the same rat. The relative height of residual alveolar ridge = (a/b) × 100%. (D) BMD results. (E) The relative height of residual alveolar ridge measurement. (F–N) Hematoxylin and eosin (HE) staining. (O) Quantitative analysis of HE staining. (P, Q) HE staining on day 5 and the number of osteoclasts. (R) Immunohistochemistry staining. (S) Model for the role of ephrinB2-EphB4 signaling in EPO-mediated communication between osteoclast and osteoblast. EPO increases the number of osteoclasts, which are higher in expression of ephrinB2 but lower in their ability to resorb bone. Conversely, EPO induces enhanced expression of EphB4 by osteoblasts, which, when activated by ephrinB2 expressed by osteoclasts, leads to enhanced osteoblast activity. Data shown are mean ± SD. *P < 0.05; **P < 0.01.
Statistical Analysis
All the in vitro experiments were conducted in triplicate and repeated 3 times. In vivo experiments were repeated twice. Results were presented as mean ± standard deviation. One-way analyses of variance, following by a Tukey test, were used to determine the statistical significance of differences. Values of P < 0.05 were considered significant.
Results
EPO and Ephrinb2 Synergistically Induced Osteoblastic Phenotype
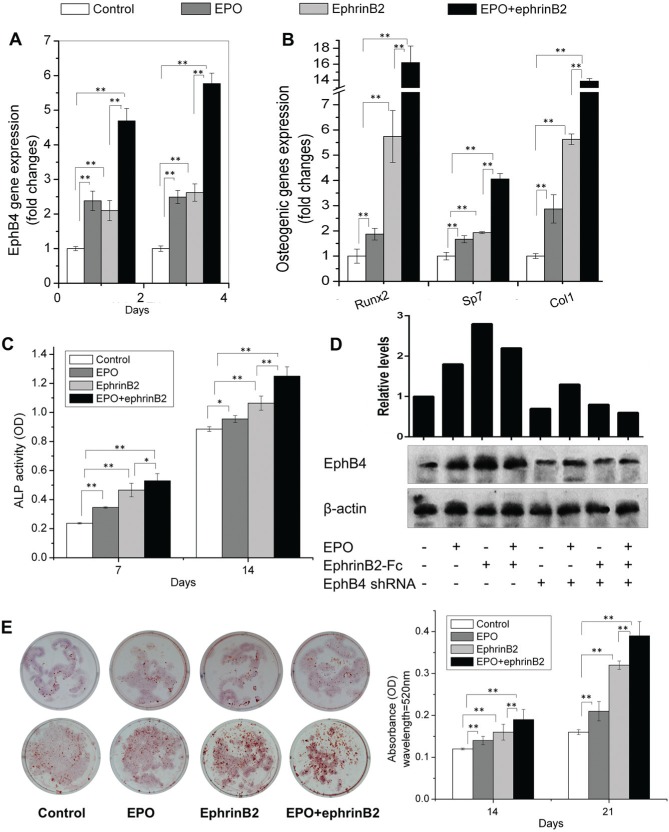
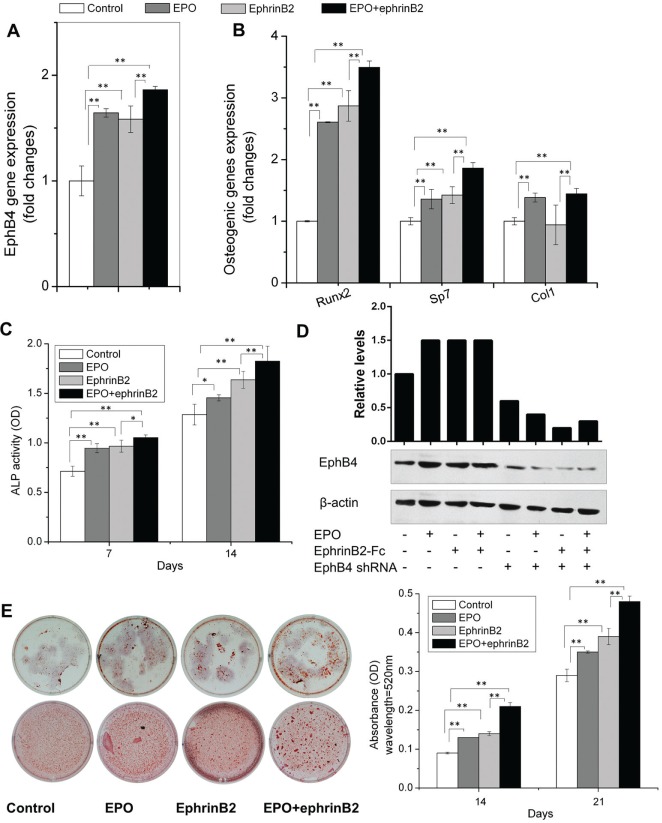
To explore our hypothesis that EPO activates ephrinB2/EphB4 signaling to alter bone homeostasis, mRNA levels for bone specific markers were examined following EPO stimulation. qRT-PCR and Western blot demonstrated that EPO significantly increased the expression of EphB4 receptor on ST2 cells (Fig. 1A, D). In addition, EPO alone slightly stimulated the expression of Runx2, Sp7, and Col1 (Fig. 1B). After stimulation of ephrinB2/EphB4 signaling using ephrinB2-Fc, EPO dramatically upregulated the expression of EphB4, Runx2, Sp7, and Col1 (Fig. 1A, B). Figure 1C demonstrates that while EPO alone induces ALP activity in ST2 cells, activation of ephrinB2/EphB4 signaling using ephrinB2-Fc further increases ALP activity. Alizarin red staining demonstrates the same pattern as ALP activity (Fig. 1E). Figure 2 showed that EPO had the similar anabolic effects on BMSCs. These data demonstrated that EPO can induce osteoblastic differentiation, especially when ephrinB2/EphB4 signaling is stimulated.
Figure 1.
Erythropoietin (EPO) and ephrinB2 induce an osteoblastic phenotype. ST2 cells were cultured in osteogenic medium with EPO and/or ephrinB2-Fc. RNA was extracted on day 4 or 6, and expression of EphB4 (A), Runx2, Sp7, and Col1 (B) in ST2 cells was quantified by qRT-PCR. Alkaline phosphatase (ALP) activity was analyzed on days 7 and 14 (C). Protein levels of EphB4 were analyzed by Western Blot and quantified by Image J 1.48p (D). Alizarin red staining was performed on days 14 and 21; the stained samples were eluted with 10% cetylpyridinium chloride; and the absorbance was measured (E). Data shown are mean ± SD. These assays were repeated 3 times. *P < 0.05; **P < 0.01.
Figure 2.
Erythropoietin (EPO) EPO and ephrinB2 induce an osteoblastic phenotype. Bone marrow mesenchymal stem cells were cultured in osteogenic medium with EPO and/or ephrinB2-Fc. RNA was extracted on day 4, and expression of EphB4 (A), Runx2, Sp7, and Col1 (B) in bone marrow mesenchymal stem cells were quantified by qRT-PCR. Alkaline phosphatase (ALP) activity was analyzed on days 7 and 14 (C). Protein levels of EphB4 were analyzed by Western blot and were quantified by Image J 1.48p (D). Alizarin red staining was performed on days 14 and 21; the stained samples were eluted with 10% cetylpyridinium chloride; and the absorbance was measured (E). Data shown are mean ± SD. These assays were repeated 3 times. *P < 0.05; **P < 0.01.
EPHB4 Knockdown Blocked EPO-mediated Osteoblastic Phenotype
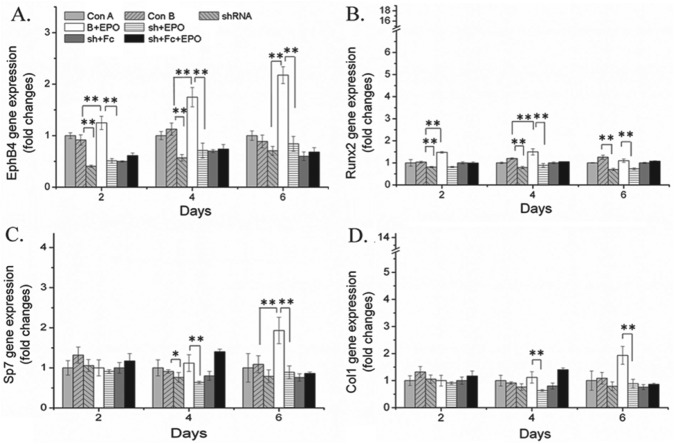
To further confirm the specific effects of EPO on osteoblast differentiation and the communication of osteoclast and osteoblast through the ephrinB2/EphB4 signaling pathway, the expression of EphB4 was knocked down by EphB4 shRNA. When EphB4 was knocked down, EPO did not alter the expression of osteoblastic marker genes (Fig. 3). Moreover, when ephrinB2-Fc was added to stimulate the ephrinB2/EphB4 signaling pathway, EPO did not upregulate the expressions of EphB4 (Figs. 1D, 2D), Runx2, Sp7, and Col1.
Figure 3.
Knockdown of EphB4 blocked erythropoietin (EPO)–induced osteoblastic activity. ST2 cells were transfected with mouse EphB4 shRNA and the corresponding controls with Lipofectamine 2000. After 24 h, cells were cultured in osteogenic medium with EPO and/or ephrinB2-Fc. RNA was extracted on day 4, and expressions of EphB4, Runx2, Sp7, and Col1 in ST2 cells were quantified by quantitative reverse transcription polyermerase chain reaction. Data shown are mean ± SD. These assays were repeated 3 times. *P < 0.05; **P < 0.01.
EPO Increased Formation of Ephrinb2 Expressing Osteoclasts
Next we explored the role that EPO signaling has on osteoclast activity. Figure 4A demonstrates that EPO increased the expression of ephrinB2 in RAW264.7 cells. In addition, EPO significantly increased the expression of Nfatc1 but decreased the expression of Mmp9 and Ctsk (Fig. 4B). TRAP staining indicative of active osteoclasts showed that EPO increased the number of TRAP-positive osteoclasts (Fig. 4C). When cells were treated with RANKL, EPO significantly increased the number of TRAP-positive osteoclasts. Moreover, EPO enhanced the formation of osteoclasts in a RANKL-dependent or RANKL-independent manner. The bone resorption assays showed that EPO decreased RANKL-mediated resorption activity of osteoclasts (Fig. 4D), suggesting that while EPO increased the number of osteoclasts, which express higher levels of ephrinB2, resorption activity was uncoupled from cell number.
Figure 4.
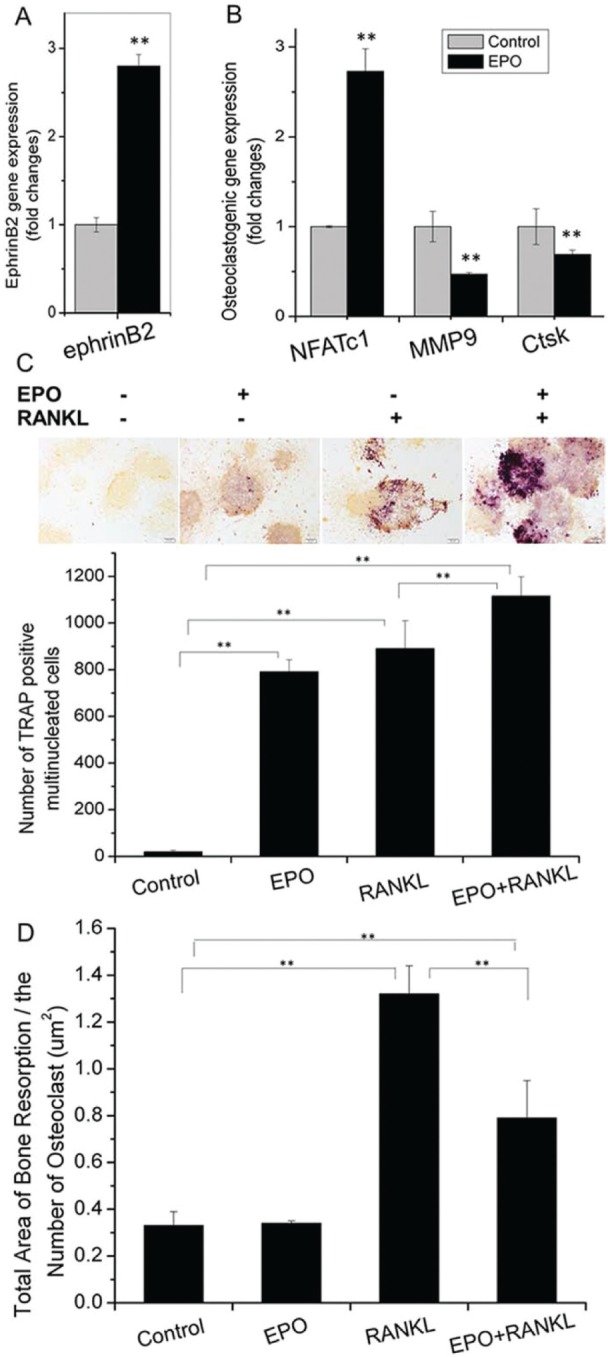
Erythropoietin (EPO) increases ephrinB2-expressing osteoclasts while reducing their functional activity. RAW264.7 cells were cultured in osteoclastogenic medium, with or without EPO. RNA was extracted on day 4, and expressions of ephrinB2 (A), Nfatc1, Mmp9, and Ctsk (B) in RAW264.7 cells were quantified by quantitative reverse transcription polyermerase chain reaction. RAW264.7 cells were cultured with receptor activator of nuclear factor κB ligand (RANKL) and/or EPO. Tartrate-resistant acid phosphatase (TRAP) staining was performed on day 9 and the number of TRAP-positive cells was counted (C). RAW264.7 cells were cultured on bone slices with RANKL and/or EPO. After 14 d, bone resorption lacunae area was delineated, calculated with Image-Pro Plus (IPP) 6.0 software, and normalized to TRAP-positive cells. Data shown are mean ± SD. These assays were repeated 3 times. *P < 0.05; **P < 0.01.
EPO Increased Bone Formation and Inhibited Resorption of Alveolar Ridge in an Animal Model
Thus far, the in vitro data suggest that EPO signaling through Eph receptors activates bone formation by stimulating osteoblast activity while reducing osteoclast activity. To prove that these observations are relevant in vivo, an alveolar bone resorption model was used (Fig. 5A–C). Results of BMD analysis demonstrated that the density of alveolar bone in the EPO-treated groups were significantly higher than in the control groups at 2, 4, and 8 wk, particularly at the higher concentration (Fig. 5D). Measurement of relative height of the residual alveolar ridge demonstrated that there were no differences among the 3 groups at 2 wk (Fig. 5E). The values of EPO-treated groups, however, were significantly higher compared to the control group at 4 and 8 wk, which indicated that more bone was formed in EPO-treated group relative to the control group (Fig. 5E). These data suggest that EPO may protect and possibly restore bone mass in this model. Further analysis by histology demonstrated that fibrous connective tissue in the defect area was significantly decreased in the EPO-treated groups compared to controls at 2, 4, and 8 wk postsurgery, especially in the higher concentration of EPO used (Fig. 5F–O). Importantly, in both EPO-treated groups, beginning at 8 wk, new bone tissue was markedly increased in the defect area (Fig. 5F–O). These tissues composed of newly formed bone with scattered osteoblasts was identified at the edge of tooth sockets and along the direction of bone trabeculae extending inward toward the center of the defect area in both EPO-treated groups (Fig. 5F–O). New bone trabeculae, however, ran parallel to the edge of the extraction sockets in the PBS control group (Fig. 5F–O). At 8 wk, bone deposition continued to increase, and the new bone tissue became more mature in EPO-treated groups (Fig. 5F–O). At 5 d, HE staining demonstrated that a dose-dependent increase in osteoclasts was present in the EPO-treated groups, corresponding to the in vitro data (Fig. 5P, Q). At 5 d, immunohistochemistry of the regenerating tissue proved that delivery of EPO induces upregulation of ephrinB2 on the osteoclasts in vivo (Fig. 5R).
Discussion
EPO possesses multiple biological functions besides regulation of red blood cell generation, such as contributing to the brain’s response to neuronal injury, to the process of wound healing, and to promoting the migration, proliferation, and differentiation of endothelial progenitor cells. The effects of EPO on bone homeostasis, however, remain controversial (Garcia et al. 2011; Singbrant et al. 2011; Kim et al. 2012; McGee et al. 2012; Sun et al. 2012). Herein, we investigated the role of EPO-mediated differentiation of osteoblasts and osteoclasts on their communication through ephrinB2/EphB4 signaling in vitro and in an in vivo wound model.
We found that EPO alone slightly induced osteoblastic differentiation in ST2 cells and BMSCs, as indicated by the expression of Runx2, Sp7, and Col1; ALP activity; and alizarin red staining (Figs. 1, 2). It is known that the ephrinB2/EphB4 signaling pathway through overexpression of EphB4 receptor promotes differentiation of osteoblasts (Zhao et al. 2006). We observed that EPO alone upregulated the expression of EphB4 receptor in ST2 cells and BMSCs. Thus, when investigating the effects of EPO on ST2 cells and BMSCs under the condition of stimulation of ephrinB2/EphB4 signaling using ephrinB2-Fc, we found that EPO further significantly induced osteoblastic differentiation (Figs. 1, 2). EPO directly promoted the differentiation of osteoblasts, while at the same time ephrinB2 interacted with the EphB4 receptor activated by EPO on ST2 cells and BMSCs to drive differentiation (Figs. 1, 2). Furthermore, studies using EphB4 shRNA blocked these effects (Fig. 3). Previous reports demonstrate that EPO can contribute to bone formation directly and indirectly (Holstein et al. 2011; Singbrant et al. 2011; Kim et al. 2012). Interestingly, our results also suggest that EPO promoted differentiation of osteoblasts directly and indirectly through the ephrinB2/EphB4 signaling pathway. EPO not only synergizes with ephrinB2 to induce osteoblastic differentiation but also can influence osteoblastic differentiation through potentially other signaling mechanisms.
In RAW264.7 cells, EPO significantly increased the expression of Nfatc1 (Fig. 4B), which is an essential transcriptional factor for osteoclastogenesis and which regulates the differentiation and fusion of osteoclasts; in addition, EPO significantly decreased the expression of Mmp9 and Ctsk (Fig. 4B), which is involved in osteoclast-mediated resorption of bone organic components (Delaissé et al. 2000; Andersen et al. 2004). These observations suggest that EPO increases the formation of osteoclasts but uncoupled their ability to resorb bone, which was confirmed by TRAP staining and bone resorption assay (Fig. 4C, D). Moreover, EPO increased the expression of ephinB2 on RAW264.7 cells, suggesting that EPO increases the number of osteoclasts, which were less able to resorb bone but express a higher level of ephrinB2. These observations within the limits of the model system suggest that there may be major differences in Eph signaling in osteoblasts versus osteoclasts.
In the clinic, the height and width of the residual alveolar ridge frequently decrease after tooth extraction. Therefore, an alveolar bone regeneration animal model was used to mimic conditions encountered during reconstructive surgery. In this study, we locally delivered EPO to the extraction defect area. Our in vivo studies demonstrate that EPO promotes new bone formation (Fig. 5). While the precise mechanism remains unclear as to how bone formation predominated bone resorption, the in vitro data are consistent with these observations.
It has been reported that EPO inhibits proinflammatory pathways and apoptosis, improves vascularization, promotes endochondral ossification, and enhances osteoblastogenesis (Holstein et al. 2007; Mihmanli et al. 2009; Holstein et al. 2011). Soft X-ray demonstrated that the density of alveolar bone significantly increased in EPO-treated groups as compared to the control group after 2 wk. We observed that the relative height of the residual alveolar ridge in the EPO-treated groups was significantly higher than that of control group after 4 wk, which indicated that there was more bone formation in EPO-treated groups than in the control group. HE staining revealed that EPO markedly increased new bone formation in the defect area. Together the data suggest that EPO increases the proliferation and differentiation of osteoblasts, resulting in the stimulation of new bone formation. Moreover, these results are also inconsistent with previous studies, which demonstrated that EPO treatment enhances bone formation (Holstein et al. 2011).
It was recently recognized that EPO is involved in bone formation by direct (Holstein et al. 2011) and indirect pathways (Singbrant et al. 2011), for example, by mTOR (mammalian target of rapamycin) signaling (Kim et al. 2012). Bone remodeling is a complex process, which includes cell-cell contact or molecular communication between osteoblasts and osteoclasts. To our knowledge, this work represents the first to demonstrate that EPO alters the communication between osteoclasts and osteoblasts through the ephrinB2/EphB4 signaling pathway.
One of the limitations of our investigations is the rodent model. Our model mimics fairly well the wound healing following a tooth extraction; however, rodents are generally believed to heal faster than humans. Thus, it is possible that the use of EPO in a rodent wound-healing model may not reflect what is possible in humans. However, a recent prospective double-blind study was performed in which 60 patients received local injection of either EPO or a placebo into the site of a tibiofibular fracture 2 wk after surgical fixation (Bakhshi et al. 2013). Patients who were treated with local EPO injections demonstrated 2-wk-faster union and lower nonunion rates compared to controls (Bakhshi 2013). While the sites of the skeletal defects are different, our data are consistent with a possible therapeutic for bone healing in humans.
In conclusion, EPO not only directly promotes osteoblastic differentiation but also indirectly stimulates osteoblastic phenotypes through communication of osteoclast-osteoblast contact through the ephrinB2/EphB4 signaling pathway, leading to bone formation (Fig. 5S). These new findings are useful for understanding EPO-mediated bone remodeling and will lead to the development of new ways to overcome the difficulties of bone defect treatment.
Author Contributions
C. Li, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; C. Shi, contributed to data acquisition, drafted and critically revised the manuscript; J. Kim, contributed to data analysis and interpretation, critically revised the manuscript; Y. Chen, S. Ni, L. Jiang, D. Li, J. Hou, contributed to data acquisition, critically revised the manuscript; C. Zheng, contributed to data analysis and interpretation, drafted and critically revised the manuscript; R.S. Taichman, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; H. Sun, contributed to conception, design, and data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by grants from the National Natural Science Foundation of China (No. 81271111), the National Institutes of Health (DE022493, DE020721), and the Ministry of Education of China (No. 20120061130010).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Andersen TL, del Carmen Ovejero M, Kirkegaard T, Lenhard T, Foged NT, Delaissé JM. 2004. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 35:1107–1119. [DOI] [PubMed] [Google Scholar]
- Bakhshi H, Kazemian G, Emami M, Nemati A, Karimi Yarandi H, Safdari F. 2013. Local erythropoietin injection in tibiofibular fracture healing. Trauma Mon. 17:386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman DL, Lin LL, Quinones ME, Longmore GD. 1999. Activation of the erythropoietin receptor is not required for internalization of bound erythropoietin. Blood. 94:2667–2675. [PubMed] [Google Scholar]
- Boyce BF, Xing L. 2008. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 473:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé JM, Engsig MT, Everts V, del Carmen Ovejero M, Ferreras M, Lund L, Vu TH, Werb Z, Winding B, Lochter A, et al. 2000. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta. 291:223–234. [DOI] [PubMed] [Google Scholar]
- Elsubeihi ES, Heersche JN. 2004. Quantitative assessment of post-extraction healing and alveolar ridge remodelling of the mandible in female rats. Arch Oral Biol. 49:401–412. [DOI] [PubMed] [Google Scholar]
- Garcia P, Speidel V, Scheuer C, Laschke MW, Holstein JH, Histing T, Pohlemann T, Menger MD. 2011. Low dose erythropoietin stimulates bone healing in mice. J Orthop Res. 29:165–172. [DOI] [PubMed] [Google Scholar]
- Hayman AR. 2008. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 41:218–223. [DOI] [PubMed] [Google Scholar]
- Hayden JM, Mohan S, Baylink DJ. 1995. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 17:93S-98S. [DOI] [PubMed] [Google Scholar]
- Holstein JH, Menger MD, Scheuer C, Meier C, Culemann U, Wirbel RJ, Garcia P, Pohlemann T. 2007. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 80:893–900. [DOI] [PubMed] [Google Scholar]
- Holstein JH, Orth M, Scheuer C, Tami A, Becker SC, Garcia P, Histing T, Mörsdorf P, Klein M, Pohlemann T, et al. 2011. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 49:1037–1045. [DOI] [PubMed] [Google Scholar]
- Kertesz N, Krasnoperov V, Reddy R, Leshanski L, Kumar SR, Zozulya S, Gill PS. 2006. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4–EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 107:2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS. 2012. Erythropoietinmediated bone formation is regulated by mTORsignaling. J Cell Biochem. 113:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. 2005. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 11:76–81. [DOI] [PubMed] [Google Scholar]
- McGee SJ, Havens AM, Shiozawa Y, Jung Y, Taichman RS. 2012. Effects of erythropoietin on the bone microenvironment. Growth Factors. 30:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihmanli A, Dolanmaz D, Avunduk MC, Erdemli E. 2009. Effects of recombinant human erythropoietin on mandibular distraction osteogenesis. J Oral Maxillofac Surg. 67:2337–2343. [DOI] [PubMed] [Google Scholar]
- Mundy GR, Elefteriou F. 2006. Boning up on ephrin signaling. Cell. 126:441–443. [DOI] [PubMed] [Google Scholar]
- Shimizu R, Komatsu N, Nakamura Y, Nakauchi H, Nakabeppu Y, Miura Y. 1996. Role of c-junin the inhibition of erythropoietin receptor-mediated apoptosis. Biochem Biophys Res Commun. 222:1–6. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, et al. 2010. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 5(6):e10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singbrant S, Russell MR, Jovic T, Liddicoat B, Izon DJ, Purton LE, Sims NA, Martin TJ, Sankaran VG, Walkley CR. 2011. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 117:5631–5642. [DOI] [PubMed] [Google Scholar]
- Sun H, Jung Y, Shiozawa Y, Taichman RS, Krebsbach PH. 2012. Erythropoietin Modulates the Structure of Bone Morphogenetic Protein 2–Engineered Cranial Bone. Tissue Eng Part A. 18:2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, Dougall WC, Lieberman JR. 2009. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 44:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. 2006. Bidirectional ephrinB2–EphB4 signaling controls bone homeostasis. Cell Metab. 4:111–121. [DOI] [PubMed] [Google Scholar]