Abstract
BACKGROUND
Mast cells have been associated with obliterative bronchiolitis (OB) in human pulmonary allografts, although their role in the development of OB remains unknown.
METHODS
In this study, we evaluated the role of mast cells in pulmonary allograft rejection using an orthotopic rat pulmonary allograft model that utilizes chronic aspiration of gastric fluid to reliably obtain OB. Pulmonary allograft recipients (n = 35) received chronic aspiration of gastric fluid with (n = 10) and without (n = 16) treatment with a mast cell membrane stabilizer, cromolyn sodium, or chronic aspiration with normal saline (n = 9) as a control.
RESULTS
The acute graft injury associated with long ischemic time in the model (6 hours total ischemic time; typical acute graft injury rate ~30%) was apparently blocked by cromolyn, because peri-operative mortality associated with the acute graft injury was not observed in any of the animals receiving cromolyn (p = 0.045). Further, the rats receiving cromolyn developed significantly fewer OB lesions than those treated with gastric fluid alone (p < 0.001), with a mean reduction of 46% of the airways affected.
CONCLUSIONS
These findings provide impetus for further studies aimed at elucidating the effects of cromolyn and the role of mast cells in pulmonary allotransplantation.
Keywords: mast cells, aspiration, pulmonary allograft, gastric fluid, obliterative bronchiolitis
Obliterative bronchiolitis (OB), the primary limiting factor of long-term graft survival after pulmonary transplantation, is characterized by the presence of dense, irreversible sub-mucosal scarring that partially or totally occludes small airways.1 Potential mechanisms of pathogenesis include alloimmune T-cell reactivity, donor-specific human leukocyte antigen (HLA) or non-HLA antibody responses; autoimmunity (e.g., Type 5 collagen reactivity, autoreactive T-helper 17 [Th17] response); and innate immunity.2
In this study, we utilized a well-established rat lung transplantation model3 to examine the hypothesis that mast cell degranulation exacerbates pulmonary allograft failure. The model involves development of OB over the course of 8 weeks in a manner dependent on donor–recipient mismatch and on chronic aspiration of gastric fluid.3 The model is associated with macrophage recruitment, extensive giant cell formation, and, in some but not all OB lesions, a mononuclear cell infiltrate that includes CD3-positive T cells.3 A clinically relevent ischemic period (5 hours cold and 1 hour warm) was used for all transplants in the current study because: (a) shorter ischemic times achieved in an experimental setting (<1 hour total ischemic time) result in significantly fewer OB lesions in this transplant model4; and (b) the study was designed to probe the role of mast cell function in the development of OB. Transplanted rats were either treated (n = 10) or not treated (n = 16) with the mast cell membrane stabilizer, cromolyn sodium. Cromolyn sodium effectively inhibits mast cell function,5 and has been widely used for decades in asthmatic patients, with few side effects.6 The effects of cromolyn on several outcomes were assessed, and compared with controls (n = 9), which received neither cromolyn sodium nor weekly aspirations with gastric fluid.
Methods
Animals
Male Wistar Kyoto (WKY; RT-1l) and Fischer 344 (F344; RT-1lv1) rats were purchased from Harlan Laboratories (Indianapolis, IN). All animals were housed in specific pathogen-free conditions in the animal care facilities at the Duke University Medical Center in accordance with institutional guidelines. All animal care and procedures were approved by the institutional animal care and use committee at Duke University.
Transplantation and aspiration procedure
Left lungs from WKY rats were orthotopically transplanted into F344 rats using a modification of the non-suture external cuff technique reported previously,7 with an ischemic time of 5 hours cold plus 1 hour warm ischemia. Gastric fluid (GF) was collected from F344 rats as previously described.4 Transplanted rats received aspirations of 0.5 ml/kg fluid (GF or normal saline) once weekly into the left (transplanted) lung for 8 weeks starting 1 week post-transplantation, as described previously.4
Study design and grouping
A total of 46 pulmonary allografts were performed, and 35 pulmonary graft recipients were utilized in the long-term arm of this study (11 could not be used due to acute graft failure prior to any aspiration; see Results). These 35 recipients were separated into 3 groups: animals receiving aspiration with GF (GF group; n = 16); animals receiving aspiration with GF and treatment with cromolyn sodium (Sigma-Aldrich, Inc., St. Louis, MO) (GF+C group; n = 10); and animals receiving aspiration with 0.9% normal saline (NS group; n = 9) as a control. All animals received weekly aspirations for 8 weeks. Cromolyn sodium (150 mg/kg) was administered subcutaneously to the GF+C recipients pre-operatively and then once daily post-operatively until the end of the experiment to block the systemic activation of mast cells, particularly those on the vascular side of the lung, where ischemia–reperfusion injury may be centered. The GF+C recipients also received aerosolized cromolyn (50 mg/ml solution aerosolized using an Invacare Stratos Portable desktop aerosol compressor with low flow nebulizer) for 20 minutes before each weekly GF aspiration. This additional drug delivery was performed to provide increased inhibition of mast cell function on the alveolar side of the lung, where aspiration injury is likely to occur.
Procurement of heart–lung block and measurement of compliance
Rats were euthanized 1 week after the final aspiration event at 9 to 10 weeks post-transplant. At the time of killing, the heart and lungs of transplanted animals were removed en bloc as previously described.7 Compliance data were collected from 9, 10 and 4 of the pulmonary allografts from the GF, GF+C and NS groups, respectively.
Histology
All fields in two sections from each lung (one from the upper part and another from the lower part of the lung) were analyzed. OB was defined as dense fibrosis in the sub-mucosa of membranous and respiratory bronchioles resulting in partial or complete luminal occlusion.8
The extent of pulmonary fibrosis in tissue sections was numerically graded according to a scoring system described previously.9
The amount of mast cells of the small bronchioles (including mucosa, sub-mucosa, smooth muscle and adventitia) was determined on slides stained with toluidine blue. The number of bronchiolar mast cells was reported as the total number of bronchiole-associated mast cells divided by number of small bronchioles per rat.
Cytokine and chemokine levels
Sera and lung tissue extracts were analyzed for multiple analytes using cytokine assay kits (Procarta; Affymetrix, Fremont, CA) and a BioPlex reader (Bio-Rad Laboratories, Hercules, CA). All assays were run as directed by the manufacturer.
Statistical analyses
Analysis of variance (ANOVA), unpaired t-tests and correlation analyses were performed using Prism 5.01 software (GraphPad Software, Inc., San Diego, CA), and an alpha = 0.05 was used. Two-tailed t-tests were used in all cases except that a 1-tailed t-test was used to evaluate the hypothesis that treatment with cromolyn sodium is associated with an increased compliance measurement. Contingency tables were constructed and assessed using the program designed by Kirkman.10 Cytokine and chemokine data were analyzed by ANOVA and post hoc t-tests.
Results
Intra- and peri-operative complications and death
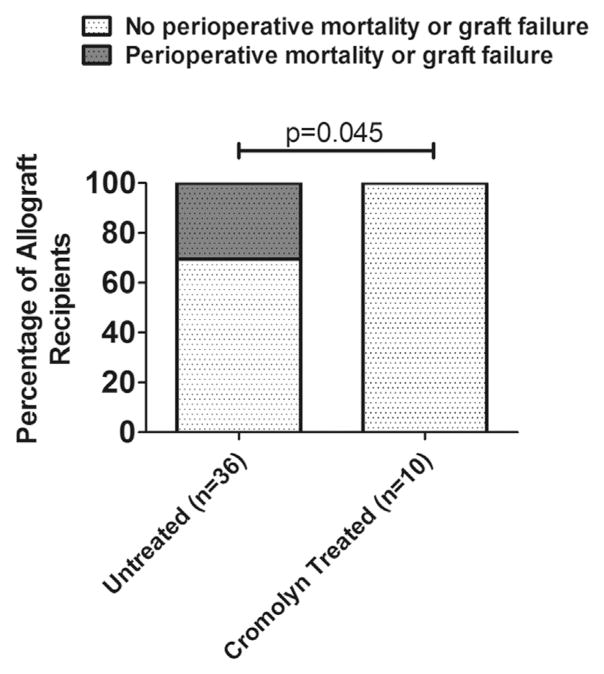
A total of 46 rat orthotopic left lung transplantations were performed in this experiment aimed at evaluating the role of mast cell function in pulmonary allograft rejection. As described in the Methods section, all recipients received pulmonary allografts subjected to a prolonged (5 hours cold and 1 hour warm) ischemic period, which is associated with an approximately 30% rate of acute graft loss in this model.4 Ten of the 46 recipients received a subcutaneous injection of 150 mg/kg cromolyn sodium pre-operatively. There were no intra-operative complications or deaths in any of the procedures. However, among the 36 recipients not treated with cromolyn sodium, 11 developed acute graft injury leading to death (10 developed hemoptysis and 1 was severely pale within 24 hours after the operation). Necropsy of these 11 rats revealed hemorrhagic consolidation of the pulmonary allografts with parenchymal necrosis and diffuse alveolar hemorrhage on histologic sections. In contrast, the 10 recipients treated with cromolyn sodium had no such peri-operative morbidity and mortality (Figure 1). Analysis using a 2 × 2 contingency table demonstrated a significant difference in peri-operative morbidity and mortality between the recipients with and without the treatment of cromolyn sodium (p = 0.045).
Figure 1.
Peri-operative morbidity and mortality of recipients treated with cromolyn sodium. In this experiment, 11 of 36 rats receiving lungs subjected to prolonged ischemia developed peri-operative morbidity and mortality with histologic evidence of parenchymal necrosis and diffuse alveolar hemorrhage. In contrast, no peri-operative morbidity or mortality developed in those recipients treated with pre-operative cromolyn, despite receiving lungs subjected to prolonged ischemia. Analysis using a 2 × 2 contingency table showed a significant improvement in peri-operative complications and death after treatment with cromolyn (p = 0.045).
Gross morphology and lung compliance of pulmonary allograft
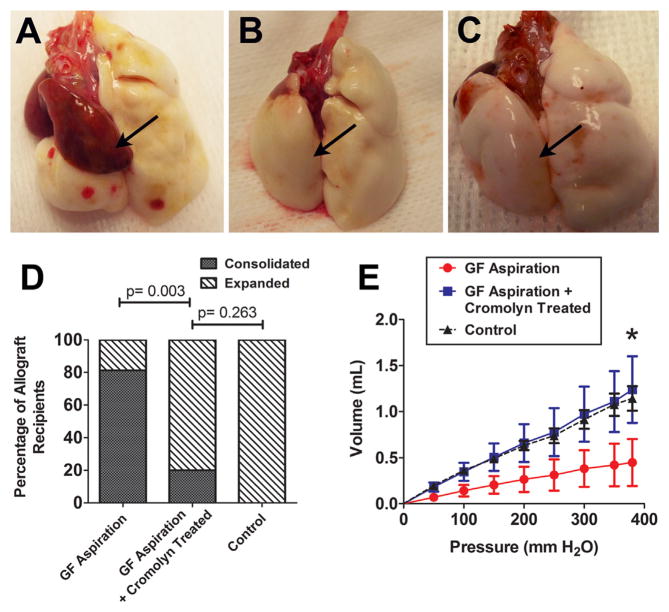
The majority of pulmonary allografts that received GF aspiration without cromolyn for 8 weeks developed discoloration, consolidation and shrinkage in size (Figure 2A). In contrast, 8 of 10 allografts treated with cromolyn sodium (GF+C group), despite exposure with GF for 8 weeks, had a normal contour and, based on subjective evaluation, expanded to at least 70% of the normal volume (the expansion volume of a native left lung) (Figure 2B). All pulmonary allografts exposed to 0.9% normal saline (NS group) were grossly normal and expanded to at least 70% of normal lung volume (Figure 2C). Analysis with a 2 × 2 contingency table revealed a significant difference in graft expansion between the animals with and without administration of cromolyn sodium (p = 0.003). There was no significant difference in graft expansion between animals in the GF+C and NS groups (p = 0.263) (Figure 2D).
Figure 2.
Gross morphology and lung compliance of pulmonary allografts 9 to 10 weeks after transplantation. After 8 weeks of gastric fluid (GF) aspiration, most of the transplanted lungs (arrow) had discoloration, consolidation and reduced size (A). On the other hand, the majority of allografts from recipients treated with cromolyn sodium expanded to >70% of normal lung volume (see Methods) and had normal lung contours (B), despite 8 weeks of GF aspiration. The allografts in the control group, receiving aspiration with normal saline, were grossly normal (C). The percentage of gross consolidation in the GF aspiration group was significantly higher than that in the GF aspiration plus cromolyn group (p = 0.003; analysis with a 2 × 2 contingency table) and the control group (p < 0.001; analysis with a 2 × 2 contingency table). There was no significant difference between the GF aspiration plus cromolyn group and the control group (p = 0.263; analysis with a 2 × 2 contingency table) (D). Pulmonary allografts in the GF aspiration plus cromolyn and control groups had similar compliance, whereas worse compliance was noted in allografts in the GF aspiration group (E). The volume of allografts treated with GF aspiration plus cromolyn at a pressure of 380 mm H2O was, on average, increased by >100% compared with grafts receiving aspiration without cromolyn (mean ± SE: 1.239 ± 0.361 ml vs 0.448 ± 0.255 ml; p = 0.049, 1-tailed t-test).
The compliance of the pulmonary allografts was measured ex vivo after 8 weekly GF aspirations. Figure 2E shows the volume of air that could be introduced into the pulmonary allografts at each pressure point in the 3 different groups. As can be seen, the transplanted lungs of animals receiving aspiration with GF plus treatment with cromolyn and of those receiving aspirations with NS had similar compliance, whereas worse compliance was noted in those receiving aspiration with GF but not treatment with cromolyn. The volume of allografts at a pressure of 380 mm H2O in the GF+C group was 1.239 ± 0.361 (mean ± SE) ml, compared with 0.448 ± 0.255 ml in the GF group (p = 0.049, 1-tailed t-test).
Obliterative bronchiolitis in small airways
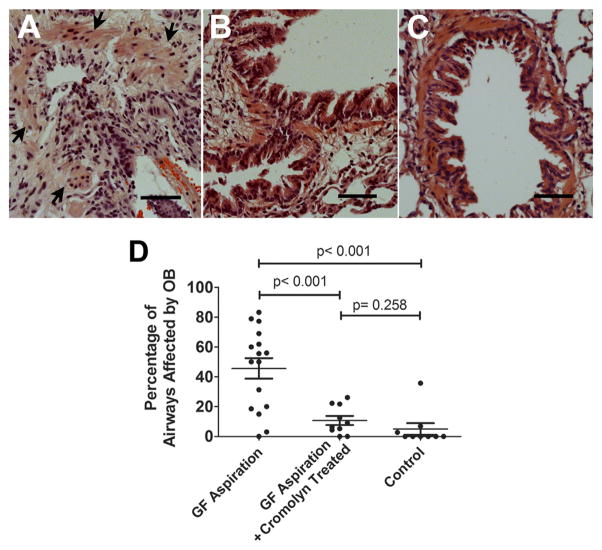
Near-complete obliteration caused by epithelial damage and sub-epithelial fibroproliferation developed in the majority of the pulmonary allografts receiving aspiration with GF but no treatment with cromolyn (Figure 3A). As described in the Methods section, the tissue sections of both the upper and lower halves of each transplant were systemically reviewed. Further, every bronchiole from each section was carefully examined by 2 investigators (J.C.C. and J.H.L.) in a blinded fashion, and the results shown are consistent with the observations of both assessments. The mean fraction of airways affected (ratio of the number of OB lesions to the number of total bronchioles per animal; see Methods) in the GF group was 0.456 ± 0.068 (mean ± SE). In contrast, most airways in the GF+C and NS groups lacked OB lesions (Figure 3B and C), with mean fractions of airways affected of 0.108 ± 0.030 and 0.050 ± 0.039, respectively. There was a significant difference between the GF and GF+C groups (p < 0.001), but not between the GF+C and NS groups (p = 0.258), indicating that treatment with cromolyn sodium was indeed associated with a decrease in the development of OB (Figure 3D). The drop from 45% of airways affected in the GF group down to 11% in the GF+C group suggests that the effect of the drug is profound, at least in this model.
Figure 3.
Histologic analysis of the small airways in the pulmonary allografts. An example of the OB lesions that developed in allografts exposed to 8 weekly aspirations of gastric fluid (GF) is shown (the arrows indicate the smooth muscle of this bronchiole). The lesions were characterized by epithelial damage and sub-epithelial fibroproliferation (A). In contrast, most bronchioles in lungs from the GF aspiration plus cromolyn and control (B, C) groups lacked characteristic features of OB. Quantitative analysis revealed that the fraction of airways affected was significantly higher in the GF aspiration group than in the GF aspiration plus cromolyn (p < 0.001) and the control (p < 0.001) groups. There was no significant difference between the percentage of airways affected in the allografts of the GF aspiration plus cromolyn group and the control group (p = 0.258) (D). Bar = 50 μm; hematoxylin and eosin stain.
Histologic fibrosis of allograft parenchyma
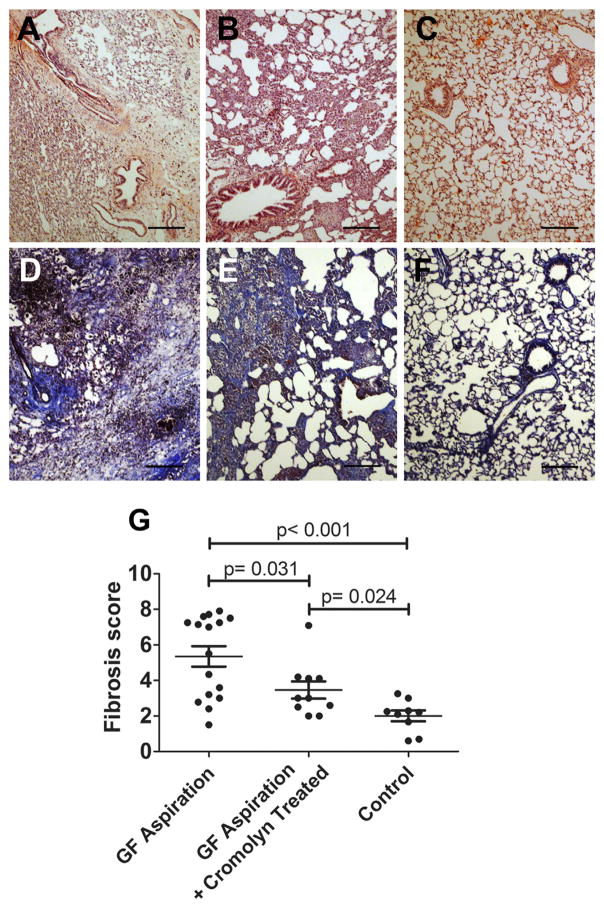
The architecture of the alveoli and of the interstitial spaces remained intact in the majority of allografts in the NS (control) group (Figure 4C and F). Most animals receiving long-term GF aspiration without cromolyn developed evident fibrosis in the parenchyma (Figure 4A and D), whereas the rats treated with cromolyn for 8 weeks had only mild to moderate parenchymal fibrosis, despite aspiration of GF for 8 weeks (Figure 4B and E). A semi-quantitative grading system, in which 0 denotes normal and 8 indicates total fibrosis, was utilized to evaluate the severity of parenchymal fibrosis of all pulmonary allografts. As shown in Figure 4G, the fibrosis score of the GF group was 5.354 ± 0.575 (mean ± SE), which was significantly higher than the scores of the GF+C (3.362 ± 0.483, p = 0.031) and NS (2.007 ± 0.301, p = 0.004) groups. Further, the NS group had a lower score on parenchymal fibrosis than did the GF+C group (p = 0.024).
Figure 4.
Histologic analysis of pulmonary parenchyma in the pulmonary allografts. Pulmonary allografts exposed to 8 weekly aspirations of gastric fluid (GF) developed severe fibrosis and destruction of the parenchyma (A, D). Recipients given GF aspiration plus cromolyn developed mild to moderate fibrosis of pulmonary parenchyma in the allografts (B, E). Expansion of the parenchyma and normal architecture of the allografts were noted in the control group, which was exposed to 8 weekly aspirations of normal saline (C, F). Semi-quantitative analysis of parenchymal fibrosis revealed that allografts treated with GF aspiration plus cromolyn had significantly less parenchymal fibrosis than allografts treated with GF aspiration without cromolyn (p = 0.031) (G). Bar = 200 μm; (A–C) hematoxylin and eosin stain; (D–F) Masson trichrome stain.
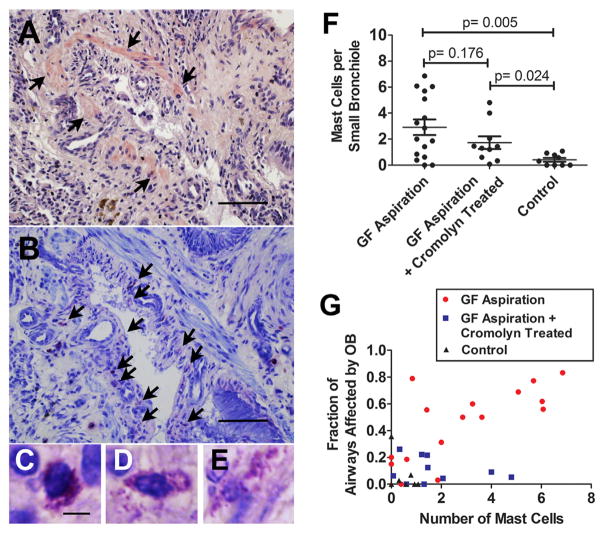
Mast cells in bronchioles of pulmonary allografts
Mast cells were frequently observed in OB lesions of the pulmonary allografts that were exposed long term to GF (Figure 5A and B). Different stages of degranulation of mast cells could be seen in OB lesions under high-power magnification (Figure 5C–E). Quantitative analysis showed that the number of mast cells per small bronchiole increased significantly in the animals aspirated with GF (GF vs NS, p = 0.005). Surprisingly, there was no significant difference between the number of mast cells per bronchiole in animals with and without treatment with cromolyn sodium (p = 0.176). Further, the number of mast cells per small bronchiole increased significantly in the GF+C group compared with the NS group (p = 0.024) (Figure 5F). A positive correlation between the fraction of airways affected by OB lesions and the number of mast cells per small bronchiole was observed only in the allografts from the GF group (r = 0.709, p = 0.002), and not in the GF+C (r = −0.225, p = 0.532) or NS (r = −0.297, p = 0.439) groups (Figure 5G).
Figure 5.
Mast cells in the small bronchioles. Serial sections using hematoxylin and eosin stain (A) and toluidine blue stain (B) show an OB lesion in a pulmonary allograft exposed to 8 weekly aspirations of gastric fluid (GF). In (A), arrows denote the smooth muscle of the bronchiole, and in (B) arrows indicate mast cells associated with the bronchiole. Different stages of degranulation of mast cells were noted in the bronchiole using toluidine blue staining (C–E). The number of mast cells per small bronchiole increased significantly in animals aspirated with GF, regardless of cromolyn treatment (GF aspiration vs control: p = 0.005; GF aspiration plus cromolyn vs control: p = 0.024). There was no significant difference between the number of mast cells per bronchiole in animals with and without treatment with cromolyn (GF aspiration vs GF aspiration plus cromolyn: p = 0.176) (F). A positive correlation was observed between OB and the number of mast cells in allografts from the GF aspiration group (r = 0.709, p = 0.002), but not in allografts from the GF aspiration plus cromolyn group (r = −0.225, p = 0.532) or the control group (r = −0.297, p = 0.439) (G). Bar = 200 μm in (A) and (B) and 5 μm in (C)–(E).
Cytokine and chemokine levels in sera and in lungs of transplanted animals
To probe the effects of cromolyn on various components of the immune system in our transplant model, we evaluated the levels of a variety of cytokines and chemokines in the sera (Table 1) and in the lung tissue extracts (Table 2) of the transplanted animals. Aspiration resulted in a significant change in the average levels of 13 and 4 of the 29 molecules analyzed in the serum and the lung, respectively (comparing GF aspiration with controls). The effects of cromolyn on the system were apparently complex, with several prominent features. First, the effects of cromolyn were widespread, affecting the serum and the lung differently, and affecting innate immune, Th1-, Th2-, and Th17-related factors. Second, cromolyn did not tend to “reverse” the effects of aspiration. In some cases (e.g., C–C ligand 7 [CCL7] and tumor necrosis factor-alpha [TNF-α] in the serum; interleukin-1alpha [IL-1α], beta-nerve growth factor [β-NGF], CCL3 and C–X–C ligand [CXCL2] in the lung), changes induced by aspiration may actually be enhanced by cromolyn. A third observation was that cromolyn affected several factors (e.g., CCL2 in the sera and IL-5 and colony stimulating factor-3 [CSF-3] in the lung) that were unaffected by aspiration.
Table 1.
Cytokine and Chemokine Levels in Sera
| GF aspiration (pg/ml) | GF aspiration + cromolyn-treated (pg/ml) | Control (pg/ml) | p-value | |
|---|---|---|---|---|
| IL-1α | 3.91 ± 0.16 | 3.87 ± 0.21 | 3.06 ± 0.21 | 0.0072b,c |
| IL-1β | 14.11 ± 1.00 | 12.84 ± 1.18 | 9.56 ± 1.26 | 0.0250b |
| IL-2 | 2.56 ± 0.32 | 1.97 ± 0.50 | 1.71 ± 0.60 | 0.3919 |
| IL-4 | 5.47 ± 0.28 | 5.40 ± 0.34 | 4.46 ± 0.42 | 0.0898 |
| IL-5 | 58.29 ± 1.01 | 56.64 ± 1.33 | 50.32 ± 2.73 | 0.0066b |
| IL-6 | 139.0 ± 11.5 | 144.8 ± 9.4 | 120.3 ± 11.7 | 0.3334 |
| IL-10 | 46.49 ± 1.25 | 47.23 ± 2.14 | 40.28 ± 2.54 | 0.0380 |
| IL-13 | 3.95 ± 0.28 | 3.60 ± 0.33 | 2.63 ± 0.45 | 0.0334b |
| IL-12 (p40) | 66.45 ± 3.29 | 53.55 ± 2.32 | 46.92 ± 5.31 | 0.0025b |
| IL-12 (p70) | 52.63 ± 1.91 | 51.84 ± 2.47 | 40.45 ± 2.97 | 0.0022b,c |
| IL-17A | 21.91 ± 0.68 | 21.83 ± 0.77 | 18.24 ± 1.10 | 0.0106b |
| β-NGF | 40.23 ± 1.86 | 37.17 ± 4.08 | 35.32 ± 3.18 | 0.4742 |
| Eotaxin/CCL11 | 141.0 ± 1.7 | 149.4 ± 9.0 | 131.5 ± 8.9 | 0.2044 |
| G-CSF/CSF-3 | 6.74 ± 0.22 | 6.58 ± 0.45 | 5.38 ± 0.39 | 0.0188b |
| GM-CSF | 3.36 ± 0.40 | 2.73 ± 0.60 | 2.03 ± 0.52 | 0.1663 |
| Gro-α/CXCL1 | 160.0 ± 4.6 | 158.1 ± 7.8 | 134.2 ± 8.8 | 0.0247b |
| ICAM | 243.2 ± 15.8 | 294.6 ± 24.6 | 249.8 ± 34.6 | 0.2949 |
| IFN-γ | 7.56 ± 0.28 | 7.99 ± 0.42 | 6.05 ± 0.57 | 0.0089b,c |
| IP-10/CXCL10 | 34.92 ± 1.27 | 35.33 ± 1.32 | 29.32 ± 1.45 | 0.0087b,c |
| Leptin | 0.692 ± 0.422 | 0.031 ± 0.031 | 1.96 ± 0.82 | 0.0543 |
| MCP-1/CCL2 | 82.56 ± 2.24 | 98.89 ± 8.46 | 85.94 ± 5.69 | 0.0985 |
| MCP-3/CCL7 | 98.29 ± 3.04 | 117.7 ± 7.9 | 92.54 ± 3.95 | 0.0053a,c |
| MIP-1α/CCL3 | 7.16 ± 0.43 | 6.50 ± 0.41 | 5.17 ± 0.67 | 0.0297b |
| MIP-2/CXCL2 | 35.49 ± 2.13 | 32.37 ± 3.09 | 25.24 ± 3.63 | 0.0483b |
| sRankl/TNFSF11 | 53.38 ± 2.81 | 51.76 ± 4.09 | 40.36 ± 5.15 | 0.0566 |
| RANTES/CCL5 | 50.64 ± 2.75 | 62.86 ± 4.02 | 47.50 ± 3.45 | 0.0110a,c |
| TNF-α | 22.33 ± 0.93 | 23.03 ± 1.10 | 18.86 ± 1.13 | 0.0261c |
| sVCAM-1/CD106 | 81.15 ± 2.70 | 83.19 ± 4.40 | 82.58 ± 11.00 | 0.9700 |
| VEGF-α | 25.42 ± 1.35 | 23.30 ± 1.36 | 19.74 ± 2.15 | 0.0559 |
The mean, standard error and p-values obtained using 1-way ANOVA are shown. GF aspiration, n = 13; GF aspiration + cromolyn treatment, n = 9; and control, n = 9. Values significant at p < 0.05 are indicated in bold. Post hoc Bonferroni’s multiple comparison test results are as follows:
p < 0.05, GF aspiration vs GF aspiration + cromolyn-treated.
p < 0.05, GF aspiration vs control.
p < 0.05, GF aspiration + cromolyn-treated vs control.
Table 2.
Cytokine and Chemokine Levels in Lung Tissue Extracts
| GF aspiration (pg/ml) | GF aspiration + cromolyn- treated (pg/ml) | Control (pg/ml) | p-value | |
|---|---|---|---|---|
| IL-1α | 213.5 ± 51.7 | 340.5 ± 56.4 | 63.32 ± 19.27 | 0.0046c |
| IL-1β | 3,157 ± 725 | 3428 ± 368 | 1,871 ± 931 | 0.3405 |
| IL-2 | 0.25 ± 0.12 | 0.41 ± 0.26 | 0.27 ± 0.22 | 0.8218 |
| IL-4 | 0.36 ± 0.02 | 0.32 ± 0.02 | 0.41 ± 0.05 | 0.1389 |
| IL-5 | 12.02 ± 0.51 | 9.78 ± 0.48 | 12.13 ± 0.37 | 0.0036a,c |
| IL-6 | 29.93 ± 2.45 | 84.94 ± 63.79 | 25.30 ± 2.96 | 0.3933 |
| IL-10 | 7.56 ± 1.47 | 5.33 ± 0.38 | 8.40 ± 1.60 | 0.3207 |
| IL-13 | 2.01 ± 0.31 | 2.20 ± 0.21 | 2.15 ± 0.57 | 0.9195 |
| IL-12(p40) | 832.4 ± 121.0 | 532.3 ± 41.2 | 394.3 ± 80.6 | 0.0124b |
| IL-12(p70) | 3.14 ± 0.75 | 3.02 ± 0.61 | 2.14 ± 0.71 | 0.6094 |
| IL-17A | 2.00 ± 0.48 | 0.90 ± 0.26 | 3.28 ± 1.00 | 0.0507c |
| β-NGF | 62.23 ± 17.10 | 147.3 ± 29.05 | 27.38 ± 1.43 | 0.0012a,c |
| Eotaxin/CCL11 | 300.1 ± 91.39 | 352.4 ± 104.6 | 433.5 ± 84.55 | 0.6253 |
| G-CSF/CSF-3 | 0.30 ± 0.02 | 0.16 ± 0.03 | 0.33 ± 0.04 | 0.0008a,c |
| GM-CSF | 4.20 ± 0.48 | 3.63 ± 0.48 | 5.43 ± 1.02 | 0.1962 |
| Gro-α/CXCL1 | 29.87 ± 1.28 | 30.65 ± 1.33 | 32.45 ± 2.58 | 0.5593 |
| ICAM | 2,259 ± 376 | 2,247 ± 415 | 1,470 ± 417 | 0.3474 |
| IFN-γ | 1.57 ± 0.40 | 0.87 ± 0.06 | 1.82 ± 1.04 | 0.5068 |
| IP-10/CXCL10 | 61.26 ± 11.76 | 29.39 ± 2.66 | 109.4 ± 41.15 | 0.0576 |
| Leptin | 77.59 ± 4.75 | 75.16 ± 6.01 | 116.4 ± 21.1 | 0.0266b |
| MCP-1/CCL2 | 375.4 ± 76.9 | 688.3 ± 90.7 | 461.4 ± 204.3 | 0.1733 |
| MCP-3/CCL7 | 109.9 ± 23.5 | 215.7 ± 29.4 | 146.7 ± 66.0 | 0.1444 |
| MIP-1α/CCL3 | 40.30 ± 6.37 | 47.87 ± 2.89 | 8.34 ± 3.09 | <0.0001b,c |
| MIP-2/CXCL2 | 78.32 ± 20.64 | 128.0 ± 24.5 | 27.08 ± 7.42 | 0.0124c |
| sRankl/TNFSF11 | 31.02 ± 5.46 | 35.23 ± 2.72 | 25.70 ± 7.44 | 0.5487 |
| RANTES/CCL5 | 365.4 ± 60.3 | 367.1 ± 50.0 | 475.9 ± 84.6 | 0.4487 |
| TNF-α | 10.23 ± 1.15 | 10.32 ± 1.14 | 7.72 ± 1.52 | 0.3193 |
| sVCAM-1/CD106 | 335.5 ± 34.6 | 315.3 ± 17.9 | 321.7 ± 28.7 | 0.8860 |
| VEGF-α | 194.7 ± 50.3 | 270.8 ± 84.0 | 468.5 ± 53.8 | 0.0152b |
Mean, standard error and p-values obtained using a 1-way ANOVA are shown. GF aspiration, n = 15; GF aspiration + cromolyn treatment, n = 10; and control, n = 9. Values significant at p < 0.05 are indicated in bold. Post hoc Bonferroni’s multiple comparison test results are as follows:
p < 0.05, GF aspiration vs GF aspiration + cromolyn-treated.
p < 0.05, GF aspiration vs control.
p < 0.05, GF aspiration + cromolyn-treated vs control.
Discussion
In this study, use of cromolyn prevented acute graft failure associated with prolonged ischemic time and ameliorated aspiration-induced OB and parenchymal fibrosis. Given the effective inhibition of mast cell function by cromolyn, our findings point toward a potentially important role of mast cells in pulmonary transplantation. However, cromolyn affects other immune processes, including those associated with neutrophils, T cells and B cells,11,12 and further studies regarding the role of mast cells in pulmonary transplantation are warranted.
The immunologic effects of cromolyn, as probed by a broad-spectrum analysis of cytokines and chemokines in the sera and the lung, were revealing. First, as expected, the effects of cromolyn were not associated with reversal of the effects caused by aspiration. Rather, some effects of aspiration on the immune system were possibly enhanced by cromolyn, and some immune factors unaffected by aspiration were strongly affected by cromolyn. These observations point toward a complex milieu of immune factors involved in pulmonary transplantation, and suggest that immune suppression of a limited number of immune components (e.g., use of drugs targeted at mast cells) results in effects that may be described as a redirection of immune function rather than a suppression of immune function. Based on the cytokine and chemokine profiles, a number of specific hypotheses can be formulated. For example, changes in the concentration of CCL7 in the serum suggest that cromolyn may alter systemic macrophage function in the model. As another example, increases in IL-1α induced by cromolyn in the lung tissue suggest the possibility that cromolyn induces at least some pro-inflammatory changes, despite the overtly positive effects of the drug on graft outcome. It remains to be determined whether these complex changes are due to direct or downstream effects of cromolyn.
Consistent with previous results aimed at evaluating the effects of long ischemic time in this model,4 we observed that about 30% of the animals receiving pulmonary allografts with a prolonged ischemic time in this study developed peri-operative morbidity and mortality (necrosis and intrapulmonary hemorrhage of lung grafts). Over the course of several experiments, we have now performed 70 rat lung allotransplantations with prolonged ischemic times, 21 (30%) of which developed hemorrhagic consolidation with histologic evidence of diffused alveolar hemorrhage and necrosis of parenchyma. Analysis with a 2 × 2 contingency table revealed that the recipients treated with pre-operative cromolyn sodium had significantly fewer peri-operative complications and death than those without cromolyn sodium treatment (p = 0.038). This result indicates that use of cromolyn reduces peri-operative insults due to prolonged ischemia of pulmonary grafts. Consistent with this finding, Barr et al reported that adding lodoxamide tromethamine, a derivative of cromolyn sodium, to lung preservation solutions resulted in better post-ischemic function in cold-stored rat lungs after reperfusion.13
In conclusion, we have demonstrated that cromolyn sodium ameliorates the development of OB and parenchymal fibrosis in pulmonary allografts, potentially by mediating mast cell function without affecting mast cell recruitment. The findings provide a good starting point to understand the role that mast cells play in the development of OB, and point toward a potential treatment strategy for clinical lung transplantation. It is encouraging that cromolyn is already used in the treatment of asthma, inflammatory bowel disease and systemic mastocytosis, although the method of delivery and the dose of cromolyn for lung transplant recipients remains to be determined.
Acknowledgments
We thank Susanne Meza-Keuthen for helpful discussion.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Davis RD, Pasque MK. Pulmonary transplantation. Ann Surg. 1995;221:14–28. doi: 10.1097/00000658-199501000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–8. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614–21. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JC, Leung JH, Tang T, et al. In the face of chronic aspiration, prolonged ischemic time exacerbates obliterative bronchiolitis in rat pulmonary allografts. Am J Transplant. 2012;12:2930–7. doi: 10.1111/j.1600-6143.2012.04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein IL. Cromolyn sodium. Chest J. 1985;87(suppl):68S–73. doi: 10.1378/chest.87.1_supplement.68s. [DOI] [PubMed] [Google Scholar]
- 6.Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42:79–89. [PubMed] [Google Scholar]
- 7.Tang T, Chang JC, Xie A, et al. Aspiration of gastric fluid in pulmonary allografts: effect of pH. J Surg Res. 2013;181:e31–8. doi: 10.1016/j.jss.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–70. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkman TW. [Accessed July 22, 2013];Statistics to use. 1996 http://www.physics.csbsju.edu/stats/
- 11.Skedinger MC, Augustine NH, Morris EZ, et al. Effect of disodium cromoglycate on neutrophil movement and intracellular calcium mobilization. J Allergy Clin Immunol. 1987;80:573–7. doi: 10.1016/0091-6749(87)90009-1. [DOI] [PubMed] [Google Scholar]
- 12.Holen E, Elsayed S. The effect of disodium-cromoglycate (DSCG) on in-vitro proliferation of CD4(+) CD8(+), and CD19(+) cell-populations derived from allergic and healthy donors. Allergy. 1995;50:249–56. doi: 10.1111/j.1398-9995.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 13.Barr ML, Carey JN, Nishanian GP, et al. Addition of a mast cell stabilizing compound to organ preservation solutions decreases lung reperfusion injury. J Thorac Cardiovasc Surg. 1998;115:631–6. doi: 10.1016/S0022-5223(98)70328-9. [DOI] [PubMed] [Google Scholar]