Abstract
Background
A growing literature describes aneurysmal deterioration after implantation of the stentless porcine aortic Medtronic Freestyle bioprosthesis (MFB; Medtronic Inc, Minneapolis, MN), with some suggesting inadequate tissue fixation with immune response as a cause. However, disjointed reports make the significance of these findings difficult to interpret. We address this concern by aggregating available data.
Methods
We reviewed institutional data, the Food and Drug Administration’s Manufacturer and User Facility Device Experience registry, and the medical literature for mention of aneurysm or pseudoaneurysm after MFB. Case details were aggregated, and the rate of aneurysmal deterioration was estimated. Immunohistopathologic examination of institutional explanted specimens was performed to elucidate a cause.
Results
We found 42 cases of aneurysmal deterioration with adequate detail for analysis; all occurred with full root replacement and valve sizes ranging from 23 to 29 mm. The rate of aneurysmal deterioration considering all data sources was 1.1% (9 of 851; 95% confidence interval, 0.5% to 2.0%) vs 4.7% (4 of 86; 95% confidence interval, 1.3% to 11.5%) at our institution, where yearly surveillance imaging is performed. Rate of aneurysmal deterioration appeared constant until 5 years after the operation; however, events are reported out to 10 years. Consistent with previous reports, histopathology demonstrated an immune cell infiltrate in areas of MFB wall breakdown.
Conclusions
Aneurysmal deterioration is an increasingly described complication of MFB implantation as a full root, with an incidence as high as 4.7%. Given the observed immune reaction and lack of occurrence in smaller (19-mm and 21-mm) valve sizes, inadequate pressure fixation of larger valves is a potential etiology. Patients with MFB require annual surveillance imaging, and consideration of this complication should factor into preoperative decision making because treatment mandates redo root replacement, which may not be feasible in high-risk patients.
Aortic valve and root disease is a growing surgical problem in the United States due to increased disease recognition and an aging population [1]. For example, surgical repairs of the proximal thoracic aorta (root, ascending, arch) increased fivefold from 2004 to 2009, with 11,000 cases performed in 2009 in North America [2]. In addition, bioprosthetic valves, which allow patients to avoid long-term anticoagulation, now dominate valve replacement and account for more than 80% of valves implanted [3].
In use since 1992 and approved by the United States Food and Drug Administration (FDA) in 1997, the stentless aortic Medtronic Freestyle bioprosthesis (MFB; Medtronic Inc, Minneapolis, MN) is a full porcine root that has gained popularity due to flexibility as a valve and root replacement. The valve is glutaraldehyde-fixed with 2-amino oleic acid anticalcification treatment [4] and has excellent hemodynamic properties even in small sizes [5]. With these advantages and good long-term safety data, including rates of 10-year structural deterioration and reoperation of less than 10% [5], the MFB remains a commonly used aortic valve in a fast-growing area of cardiac surgery.
Despite these advantages, a growing number of reports in recent years [5–10] have detailed the complication of aneurysmal deterioration after MFB implantation, and our institution has noted 4 patients with aortic pseudoaneurysm after MFB implantation as a full root. Several studies have reported immune cell infiltrates within the porcine root [7, 8, 10], raising concerns of a host immune reaction to the foreign porcine material and leading to structural deficits in the root wall.
The present study reports our institutional experience with MFB implantation with detailed evaluation of institutional cases of aneurysmal deterioration, including immunohistopathologic examination of explanted specimens to elucidate a cause. Further, the study aggregates available noninstitutional data on aneurysmal deterioration after MFB implantation to determine the incidence and outcomes of this complication. By bringing further attention to this underappreciated problem, the study may help guide decision making around valve selection in cases where MFB use is being considered.
Material and Methods
Institutional Data
We used a prospectively maintained institutional database to examine all MFB cases from January 2007, when the valve was first used at our institution, through April 2013. Patient, operative, and outcome data were extracted from the Duke Thoracic Aortic Surgery Database, a clinical registry of thoracic aortic surgical patients at Duke University Medical Center (Durham, NC). Patients underwent annual follow-up examination at the Duke Center for Aortic Disease, with clinical assessment and computed tomography angiography, magnetic resonance angiography, or echocardiography, or a combination. Data on imaging follow-up and long-term mortality status were obtained through chart review, billing and clinical encounters, national death indices, and Duke Enterprise Data Unified Content Explorer–guided query [11]. Investigational Review Board approval was obtained prior to reviewing patient data.
Pathologic Examination
For the 3 patients who underwent reoperation with MFB explant for pseudoaneurysm at our institution, sections of porcine root were submitted for routine histology and immunohistochemical stains. Tissue was fixed in 10% buffered formalin, followed by standard processing and paraffin embedding. Immunohistochemical stains were performed using avidin biotinylated complex technique. Stains performed included cluster of differentiation (CD) 45, CD3, CD20, CD68, smooth muscle actin, muscle-specific actin, muscle-specific actin, and C4d.
Manufacturer and User Facility Device Experience Database
Available since 1993, the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) [12] database collects voluntary provider and industry adverse event reports related to medical devices. The MAUDE registry was queried for mentions of aneurysmal deterioration involving the MFB by using the MAUDE online search engine [13] terms “freestyle,” product code “LWR,” and “pseudoaneurysm” or “aneurysm.” Clinical data available in the MAUDE registry were extracted, including time from the operation to aneurysmal deterioration, location of aneurysmal defect, and death.
Literature Review
A literature review was performed, and incidence of MFB implantation and aneurysmal deterioration among studies was tabulated. By combining studies with overall population and incidence of aneurysm/pseudoaneurysm, including institutional data, an estimated rate of aneurysmal deterioration after MFB implantation was calculated. Because no pseudoaneurysm formations were noted after subcoronary or inclusion root MFB implantation, these cases were eliminated from analysis.
Statistical Analysis
Characteristics of patients with aneurysmal deterioration in the MAUDE and the literature searches were described using count and percentage for categoric variables and median and interquartile range for continuous variables. Incidence of aneurysmal deterioration was calculated as the percentage of formation among all patients at risk and also as a linearized rate per 100 patient-years of available follow-up. Finally, after combining patients from the MAUDE and literature searches, we developed a failure-free survival curve for patients with eventual aneurysmal deterioration to describe the time course of aneurysmal deterioration after MFB implantation. Stata 12.0 software (StataCorp LP, College Station, TX) and R 3.02 software (R Foundation for Statistical Computing, Vienna, Austria; 2008) were used for statistical analyses.
Results
Institutional Data
Between January 2007 and December 2012, 90 MFB were implanted at our institution, all as full root replacements. Four patients died, for a 30-day in-hospital mortality of 4.4%; therefore 86 patients were at risk for late pseudoaneurysm formation. During a median follow-up of 19 months (interquartile range, 5 to 37 months), an MFB pseudoaneurysm developed in 4 patients. All pseudoaneurysms were discovered on routine annual surveillance imaging. The location of the pseudoaneurysm was the left coronary sinus in 2 patients (Figs 1 and 2), the left and right coronary sinuses in 1, and in the left and non-coronary sinuses in 1. Successful reoperations were performed in 3 of the 4 patients, with 1 patient with end-stage renal disease and multiple comorbidities not offered reoperation. Patients are summarized in Table 1. Long-term outcomes from our institutional series of MFB implantations revealed 1-year and 5-year survival of 86.9% and 77.3%, respectively.
Fig 1.
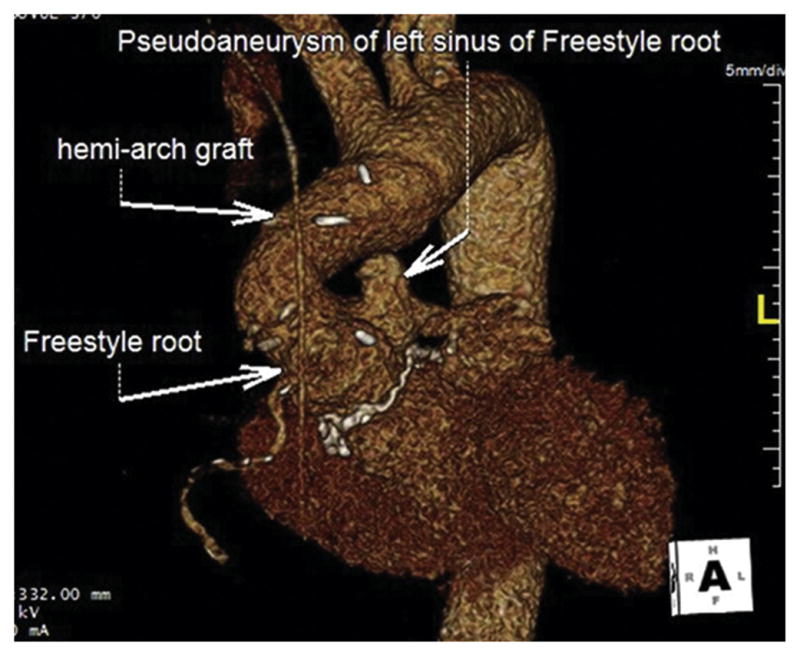
Computed tomography angiography three-dimensional reconstruction shows a pseudoaneurysm of the left coronary sinus after Medtronic Freestyle Bioprosthesis (Medtronic, Minneapolis, MN) full root replacement and hemiarch repair.
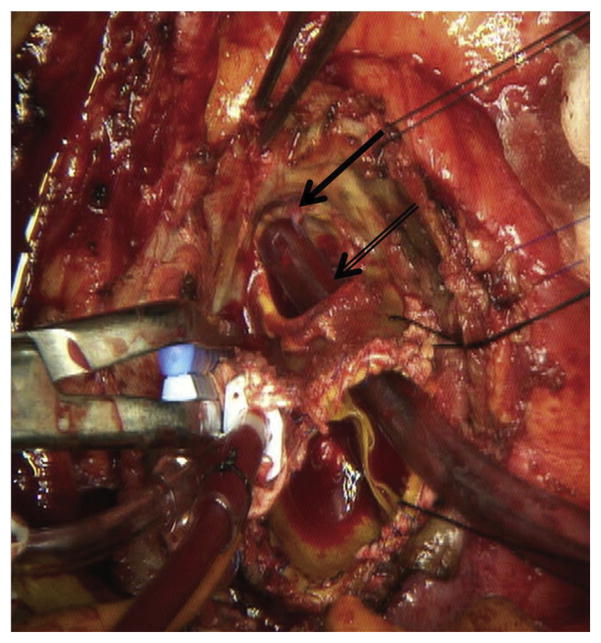
Fig 2.
Intraoperative photograph from same patient as Figure 1 demonstrates a large defect (black double arrow) in the left coronary sinus of the porcine root. The left coronary button (black solid arrow) has been displaced significantly away from the annulus toward the main pulmonary artery by the pseudoaneurysm.
Table 1.
Summary of Duke University Medical Center Patients With Pseudoaneurysm After Medtronic Freestyle Bioprosthesis Full Root Implantationa
| Age at MFB Implantation (y) | Sex | Durationb (mos) | Age at MFB Explantation (y) | Size (mm) | Location | Symptoms |
|---|---|---|---|---|---|---|
| 51 | M | 60 | 56 | 29 | Left CS | Chest pain |
| 63 | M | 36 | 66 | 29 | Left and right CS | None |
| 63 | M | 59 | 68 | 27 | Left CS | TIA |
| 38 | M | 19 | N/A | 29 | Left and non CS | None |
The pseudoaneurysm in all patients was found on routine follow-up imaging.
Time from MFB implantation until pseudoaneurysm diagnosis.
CS = coronary sinus; MFB = Medtronic Freestyle Bioprosthesis (Medtronic, Minneapolis, MN); N/A = not applicable; TIA = transient ischemic attack.
Histopathology
Microscopic examination of hematoxylin and eosin-stained sections of explanted MFB roots demonstrated a paucicellular elastic artery wall with areas of wall thinning, fibrosis with focal pannus formation, and thrombus. Focal areas within the media appeared to have retained smooth muscle nuclei, yet there was no associated inflammatory reaction. Focal areas of a mild mononuclear inflammatory infiltrate were noted within the media. These cells stained positively for the macrophage marker CD68 (Fig 3) and for the leukocyte common antigen CD45. No immunoreactivity was seen for smooth muscle actin, muscle-specific actin, CD3, CD20, or C4d, indicating a lack of evidence for retained porcine cellular components or a T-cell lymphocyte-mediated or antibody-mediated immune response. These results were consistent in the 3 explanted specimens, with final pathology indicating aortic wall degeneration without evidence of endocarditis.
Fig 3.
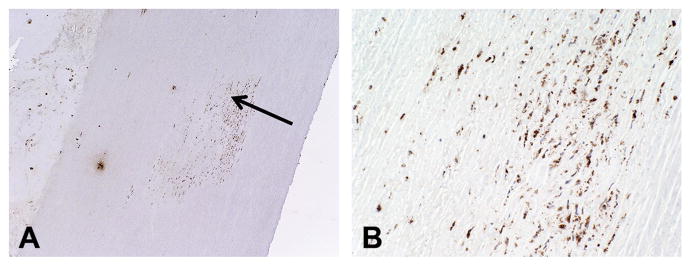
(A) Photomicrograph of mononuclear inflammatory cell infiltrate within the media (arrow) of the porcine root shows positive staining for macrophage marker cluster of differentiation (CD) 68. The lumen is seen to the right and the adventitia to the left (immunoperoxidase stain; original magnification ×4). (B) Macrophages magnified (original magnification ×20).
Literature Review
Of 12 studies (including the current study) that evaluated the MFB and contained an adequate pathologic description to evaluate for aneurysmal deterioration, one was a case report without a denominator, and two involved overlapping cohorts reported in other included studies (Table 2). Therefore, eight studies involving 851 patients and 9 patients with aneurysmal deterioration were combined to estimate an incidence of this complication at 1.1% (95% confidence interval, 0.5% to 2.0%). After exclusion of the 4 patients with short-term death, the rate of pseudoaneurysm formation in our institutional series, where yearly surveillance computed tomography angiography or magnetic resonance angiography is performed on nearly all patients, was 4.7% (4 of 86; 95% confidence interval, 1.3% to 11.5%). When calculated based on patient-years of follow-up, institutional incidence was 2.3 pseudoaneurysms/100 patient-years. For comparison, no aortic root pseudoaneurysms have been observed in 169 patients undergoing aortic root replacement at our institution with Dacron (DuPont, Wilmington, DE) bioprosthetic or mechanical valved conduits during the study period.
Table 2.
Selected Literature Review of Medtronic Freestyle Bioprosthesis Implantation
| Study (First Author) | Study Years | MFB Implantsa | Aneurysm/Pseudoaneurysm |
|---|---|---|---|
| DUMC Study | 2007–2013 | 86 | 4 |
| Mazzola [14] | 2001–2010 | 77 | 0 |
| David [10] | NR | 298 | 2 |
| Nairb [8] | 2003–2009 | 279 | 2 |
| Butanyb [7] | 2003–2005 | 430 | 2 |
| Smith [15] | 2005–2009 | 18 | 0 |
| LeMaire [16] | 2001–2007 | 47 | 0 |
| Bach [5] | 1997–2004 | 178 | 1 |
| Ozaki [6] | 1999–2002 | 61 | 2 |
| El-Hamamsy [17] | 1997–2005 | 86 | 0 |
| Mizuno [9] | 2008 | Case report | 1 |
Patients who died perioperatively and were thus not at risk for aneurysm/pseudoaneurysm formation were excluded.
Indicates studies not included in incidence calculation due to overlapping patient cohorts.
DUMC = Duke University Medical Center; MFB = Medtronic Free-style Bioprosthesis (Medtronic Inc, Minneapolis, MN).
MAUDE Database Inquiry
The MAUDE online search revealed 325 reports of adverse events with the MFB. Of these, 30 (9.2%) contained a report of aneurysm or pseudoaneurysm. Among aneurysmal complications, time to presentation varied widely, from 2.5 to 127 months after implantation, with a median time to presentation of 26.5 months (interquartile range, 18 to 47 months). Data from the MAUDE registry, medical literature, and our institutional series were combined to describe characteristics of patients developing aneurysms/pseudoaneurysms (Table 3). In 15.4% of cases, aneurysmal deterioration resulted in patient death. The combined data, containing all patients with available information on time from operation to aneurysmal deterioration, showed no evidence of repeat entries. In Kaplan-Meier analysis of event-free survival among patients who would eventually develop aneurysmal deterioration, the rate of aneurysm/pseudoaneurysm formation appeared constant until 5 years after the operation; however, events continued up to 10 years (Fig 4).
Table 3.
Details of Medtronic Freestyle Bioprosthesis Aneurysmal Deterioration
| Variable | Resulta |
|---|---|
| Pseudoaneurysm, No. | 42 |
| MAUDE, No. (%) | 30 (71.4) |
| Medical literature, No. (%) | 8 (19) |
| DUMC, No. (%) | 4 (9.5) |
| Time to pseudoaneurysm, months | |
| Mean (SD) | 37.5 (27.6) |
| Median (IQR) | 34 (19, 50) |
| Range | 2.5–127 |
| Female, No. (%) | 4 (36.4) |
| Age, median (IQR) y | 59 (40, 63) |
| Valve size, No. (%) | |
| 23 mm | 2 (25) |
| 25 mm | 2 (25) |
| 27 mm | 1 (12.5) |
| 29 mm | 3 (37.5) |
| Pseudoaneurysm location, No. (%) | |
| Left coronary sinus | 9 (45) |
| Right coronary sinus | 6 (30) |
| Noncoronary sinus | 8 (40) |
| Death, No. (%) | 4 (15.4) |
Percentages represent only patients with nonmissing data for given variable. Some patients had pseudoaneurysms of >1 coronary sinus.
DUMC = Duke University Medical Center; IQR = interquartile range; MAUDE = Manufacturer and User Facility Device Experience; SD = standard deviation.
Fig 4.
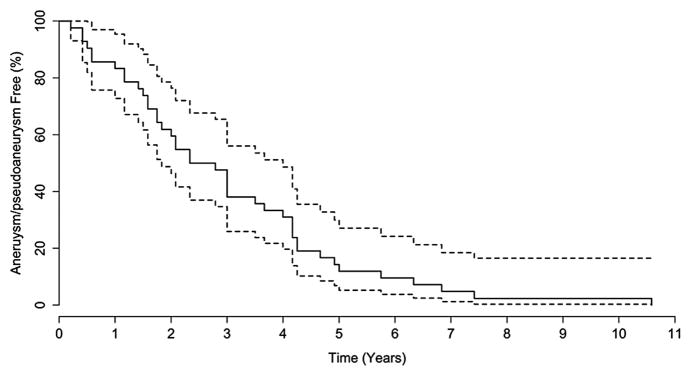
The solid line represents the aneurysm/pseudoaneurysm-free survival curve for 36 patients who develop eventual aneurysmal deterioration and the dotted lines represent the 95% confidence intervals.
Comment
Aneurysmal deterioration is a rare but serious and increasingly described complication of the aortic root MFB. Because it is unlikely that adequate data sources will become available to provide more definitive results on this complication, the current study provides several key insights for surgeons treating these patients: First, eventual aneurysmal deterioration has been estimated in 1.1% of MFB full-root cases; however, based on institutional data, this rate may be higher than 4% in patients under close imaging surveillance.
Second, there does not seem to be a clear group of patients at risk for this complication based on valve size, although cases have not been reported with 19 or 21 mm MFB.
Finally, this complication can occur over a wide range of postoperative intervals, with consistent rates of reporting from 2.5 months to 5 years after the operation, and continued event reporting exceeding 10 years after implantation.
The MFB has demonstrated good long-term outcomes, with several studies reporting outcomes more than 5 years from the operation [5, 18, 19]. Increasingly, however, reports of aneurysmal deterioration after MFB implantation as a full root have been published. Although rare, the potential sudden and catastrophic outcome of this complication raises concerns about how to surveil and manage this potential problem.
Several published reports describe patients with asymptomatic pseudoaneurysm formation found on routine surveillance imaging, despite aneurysm diameters of more than 40 mm [6]. Other patients experienced significant symptoms, including heart failure from extramural compression of the right ventricular outflow tract [9] or from aortopulmonary fistula [20], transient ischemic attacks related to emboli from pseudoaneurysm thrombus, or cardiopulmonary arrest and death [6].
All pseudoaneurysms in our institutional series were found on routine imaging surveillance, although 2 of 4 patients had symptoms attributable to the pseudoaneurysm. Lifelong annual surveillance follow-up imaging with computed tomography angiography or magnetic resonance angiography appears mandatory after MFB full root implantation, especially because the pseudoaneurysms were not noted on transthoracic echocardiography in any of the patients from our institutional series.
MFB sizes reported in our institutional series are larger (27 to 29 mm) compared with those previously reported (23 to 25 mm); however, previous reports came from Japan, where use of smaller sizes may be more frequent [6, 9]. All reported cases of aneurysmal deterioration involved full root replacement, likely because the native aortic wall remains intact around the implanted valve with the subcoronary or inclusion techniques. As such, any inflammation within the implanted porcine valve tissue would lead only to structural valve deterioration rather than pseudoaneurysm formation.
Our institutional experience of no pseudoaneurysms observed in 169 aortic root replacements performed with Dacron bioprosthetic or mechanical valved conduits during the study period, as well as other reports [21] describing a rate of pseudoaneurysm formation 10-fold lower than the current study, would suggest this to be a problem specific to biologic porcine full root implants, although porcine root experience is limited due to the St. Jude Medical Toronto Root never being brought to market. That no aneurysmal deterioration in 19 or 21 mm MFB roots has been described is noteworthy. The differential pressures exerted on these valves during pressure fixation and after implantation require further study to understand the implications of this observation in the search for a cause of this complication. Finally, the MAUDE registry reported pseudoaneurysm formation evenly distributed among the coronary sinuses, whereas all of our institutional cases of pseudoaneurysm involved the left coronary sinus, with one also involving the right and one the noncoronary sinus.
In a comprehensive examination of this complication, data from MFB implantations at the University of Toronto were detailed in a series of articles describing the clinical, imaging, and pathology results of explanted specimens [7, 8, 10]. These studies noted 3 specimens with aneurysmal deterioration without infective endocarditis etiology. In an in-depth immunohistopathologic examination of these specimens, the authors found macrophage, T-lymphocyte and B-lymphocyte, and antibody-mediated immune reaction in the porcine roots. Animal studies have also demonstrated a host immune reaction to glutaraldehyde-treated bioprosthetic roots, a reaction that was reduced with steroid administration [22]. In addition, other groups have described the α-Gal epitope (Gal-alpha1–3Galbeta1-(3)4GlcNAc-R), which is prominent on the cells of most nonprimate mammals (including porcine cells) and not fully removed by glutaraldehyde fixation, as a possible source of immunogenicity due to anti-α-Gal antibodies in patients with porcine valves [23].
Results from our histologic examination demonstrated small foci of macrophages within the media of the porcine roots, consistent with previous studies. Contrary to the University of Toronto reports, we were unable to find evidence of a T-cell or B-cell lymphocyte inflammatory infiltrate. We were not able to confirm or exclude incomplete decellularization in areas of the media where smooth muscle nuclei were still apparent. However, these foci lacked an associated inflammatory infiltrate, making incomplete decellularization less likely. Deposition of the complement product C4d within the capillaries of solid organ allografts can assist in the diagnosis of antibody-mediated rejection. This stain was performed on several sections of porcine roots with abundant nonspecific staining and was uninformative. Further studies to elucidate the etiology of this complication are needed.
The potential sequelae of pseudoaneurysm formation can be catastrophic and include transient ischemic attack, stroke, heart failure, and sudden death [6, 8]. These sequelae, particularly sudden death, may lead to significant underreporting of this complication, especially among those not undergoing imaging surveillance or autopsy. Given the results of this and previous studies, we recommend rigorous surveillance and careful consideration of patients who are selected for MFB implantation. Because symptoms may be minimal or absent, these patients require annual imaging, as noted previously, to recognize and replace valves with this complication.
Consideration of this complication should also factor into preoperative decision making, because its correction mandates redo aortic root replacement, a technically challenging operation that may not be feasible in certain high-risk patients. Given the possible immune reaction responsible for this complication, replacing this type of structural deterioration of the MFB with a second MFB may be ill advised [6].
Reoperation in these patients can be technically challenging, and several points merit mention. First, unlike Dacron grafts, which are typically encased in a dense peel of fibrous tissue at reoperation, all of the MFB roots were completely free of adhesions and easily separated from surrounding cardiac structures (Fig 5). The major challenges of redo root replacement were because in all 3 patients, the measured diameter of the aortic annulus at reoperation was significantly smaller (6 to 8 mm smaller) than at the time of original valve implant. This differs from reoperation after prior mechanical or stented bio-prosthetic valve implantation, where the diameter of the residual annulus after valve explant is generally identical to that of the previously implanted valve due to the rigid frame of the mechanical or stented bioprosthetic valve. In the case of the stentless MFB, however, we postulate that the inflammation and scarring related to the immune response to the porcine tissue, in conjunction with the lack of a rigid frame to hold the annulus open, lead to the observed reduction in annular size. This finding is important because it may necessitate mechanical or supraannular stented bioprosthetic valved conduit implantation to achieve adequate effective orifice area at the time of reoperation.
Fig 5.
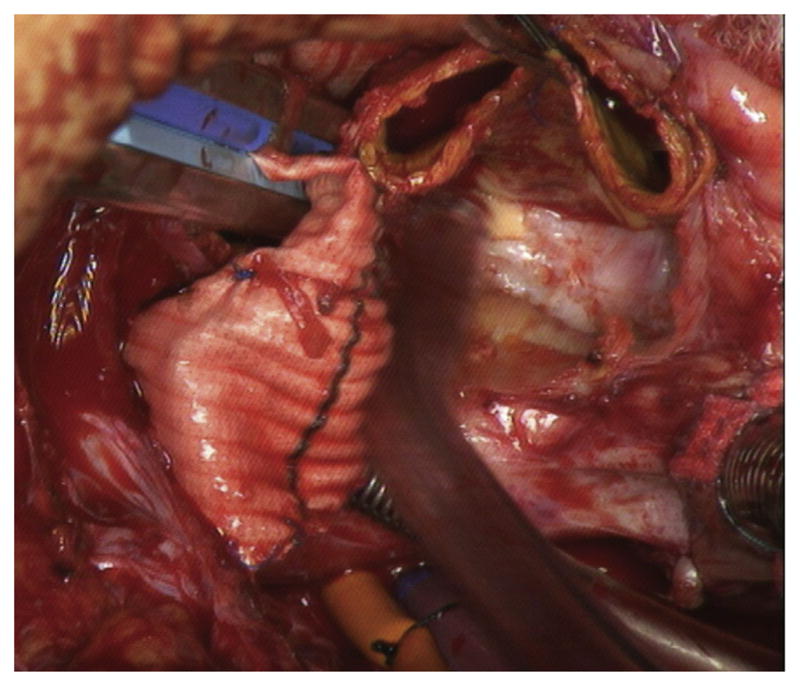
Intraoperative photograph demonstrates lack of adhesions to stentless porcine root, which is easily separated from surrounding cardiac structures.
The second difficulty was that because the pseudoaneurysms arose from the left coronary sinus of Valsalva, the left coronary ostium was pushed away from the annulus by the pseudoaneurysm (Figs 2 and 6A), and dense scarring in this area made remobilization challenging. As such, we found use of the “legs” technique [24] useful in 2 of the 3 cases, whereby a short (1 cm) 8-mm Dacron interposition graft was placed between the ostium of the left coronary and the Dacron valved conduit to allow reimplantation without tension (Fig 6B). Because the interposition graft is very short, the risk of late thrombosis should be minimal compared with the longer standard Cabrol limb [25].
Fig 6.
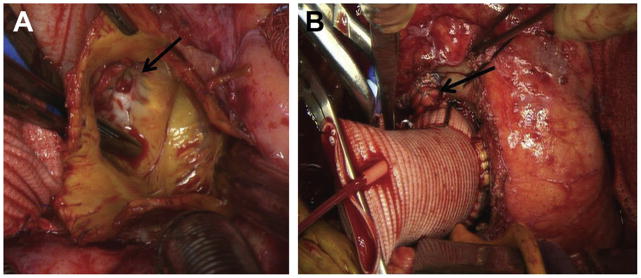
(A) Intraoperative photograph demonstrates another example of the left coronary button displaced away from the annulus by a left porcine sinus pseudoaneurysm and also clearly demonstrates the left coronary button anastomosis (arrow) is intact and that the pseudoaneurysm arises directly from the left porcine sinus wall lateral to the button. (B) Completion of redo root replacement from same patient demonstrating use of the “legs” technique, whereby a short interposition Dacron (DuPont, Wilmington, DE) graft (arrow) is used to anastomose the displaced left coronary button to the Dacron valved conduit.
Given these challenges, we now restrict use of full root MFB implantation to cases of a small annulus, whereby suboptimal hemodynamics would be obtained with implantation of a small mechanical or stented bioprosthetic valve, and generally limit use to the 19-mm and 21-mm valves given the lack of reported pseudoaneurysm with these sizes. However, larger sizes are still occasionally used in cases of endocarditis given the known increased resistance of the MFB bioprosthesis to recurrent infection.
This study has several limitations, including its retrospective nature and lack of complete data across the multiple sources collated. The rarity of this complication makes a more robust study unlikely; therefore, the potential for unmeasured confounders and imperfect estimates will continue to plague research on this subject. Owing to lack of standard surveillance and unknown cause of death in some patients from data sources outside of our institutional series, the rate of aneurysmal deterioration is almost certainly underestimated. Although immunohistopathologic examination revealing chronic inflammation and possible evidence of porcine cells make inadequate fixation an interesting hypothesis, we lack data to establish firm causality.
In conclusion, aneurysmal deterioration is a rare but potentially catastrophic complication after MFB implantation as a full root. A synthesis of currently available data suggests that this complication will eventually develop in approximately 1% (and potentially >4%) of patients receiving a MFB full root implant, although the time course is variable. As changes in population demographics, aortic disease prevalence, and frequency of aortic operations potentially increase use of the MFB, appropriate patient selection and adequate surveillance will be critical to minimize the sequelae of aneurysmal deterioration.
Footnotes
Presented at the Poster Session of the Fiftieth Annual Meeting of The Society of Thoracic Surgeons, Orlando, FL, Jan 25–29, 2014.
References
- 1.Gillum RF. Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol. 1995;48:1289–98. doi: 10.1016/0895-4356(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 2.Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol. 2012;60:1156–62. doi: 10.1016/j.jacc.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Schoen FJ, Levy RJ. Mechanism of efficacy of 2-amino oleic acid for inhibition of calcification of glutaraldehyde-pretreated porcine bioprosthetic heart valves. Circulation. 1994;90:323–9. doi: 10.1161/01.cir.90.1.323. [DOI] [PubMed] [Google Scholar]
- 5.Bach DS, Kon ND, Dumesnil JG, Sintek CF, Doty DB. Ten-year outcome after aortic valve replacement with the free-style stentless bioprosthesis. Ann Thorac Surg. 2005;80:480–6. doi: 10.1016/j.athoracsur.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki N, Hino Y, Hanafusa Y, et al. Perforation of the Val-salva sinus after implantation of Medtronic Freestyle aortic bioprosthesis. Ann Thorac Surg. 2006;82:2282–5. doi: 10.1016/j.athoracsur.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 7.Butany J, Zhou T, Leong SW, et al. Inflammation and infection in nine surgically explanted Medtronic Freestyle stentless aortic valves. Cardiovasc Pathol. 2007;16:258–67. doi: 10.1016/j.carpath.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Nair V, Law KB, Li AY, Phillips KR, David TE, Butany J. Characterizing the inflammatory reaction in explanted Medtronic Freestyle stentless porcine aortic bioprosthesis over a 6-year period. Cardiovasc Pathol. 2012;21:158–68. doi: 10.1016/j.carpath.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno T. Wall rupture of Medtronic Freestyle stentless porcine aortic root bioprosthesis. Interact Cardiovasc Thorac Surg. 2008;7:1129–30. doi: 10.1510/icvts.2008.183624. [DOI] [PubMed] [Google Scholar]
- 10.David TE, Armstrong S, Maganti M, et al. Postimplantation morphologic changes of glutaraldehyde-fixed porcine aortic roots and risk of aneurysm and rupture. J Thorac Cardiovasc Surg. 2009;137:94–100. doi: 10.1016/j.jtcvs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Horvath MM, Winfield S, Evans S, et al. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266–76. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. [Accessed February 27, 2013];Manufacturer and User Facility Device Experience Database - (MAUDE) Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/ReportingAdverseEvents/ucm127891.htm.
- 13.U.S. Food and Drug Administration. [Accessed February 27, 2013];MAUDE-Manufacturer and User Facility Device Experience Online Search. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm.
- 14.Mazzola A, Di Mauro M, Pellone F, et al. Freestyle aortic root bioprosthesis is a suitable alternative for aortic root replacement in elderly patients: a propensity score study. Ann Thorac Surg. 2012;94:1185–90. doi: 10.1016/j.athoracsur.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Smith CR, Stamou SC, Hooker RL, et al. Stentless root bio-prosthesis for repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2013;145:1540–4. doi: 10.1016/j.jtcvs.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 16.LeMaire SA, Green SY, Sharma K, et al. Aortic root replacement with stentless porcine xenografts: early and late outcomes in 132 patients. Ann Thorac Sur. 2009;87:503–12. doi: 10.1016/j.athoracsur.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 17.El-Hamamsy I, Clark L, Stevens LM, et al. Late outcomes following freestyle versus homograft aortic root replacement: results from a prospective randomized trial. J Am Coll Cardiol. 2010;55:368–76. doi: 10.1016/j.jacc.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi S, Baillot R, Voisine P, Mathieu P, Dagenais F. Structural deterioration of the Freestyle aortic valve: mode of presentation and mechanisms. J Thorac Cardiovasc Surg. 2006;132:401–6. doi: 10.1016/j.jtcvs.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Ennker JA, Albert AA, Rosendahl UP, et al. Ten-year experience with stentless aortic valves: full-root versus sub-coronary implantation. Ann Thorac Surg. 2008;85:445–52. doi: 10.1016/j.athoracsur.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Kameda Y, Mizuguchi K, Kuwata T, Mori T, Taniguchi S. Aortopulmonary fistula due to perforation of the aortic wall of a freestyle stentless valve. Ann Thorac Surg. 2004;78:1827–9. doi: 10.1016/j.athoracsur.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Pacini D, Ranocchi F, Angeli E, et al. Aortic root replacement with composite valve graft. Ann Thorac Surg. 2003;76:90–8. doi: 10.1016/s0003-4975(03)00265-0. [DOI] [PubMed] [Google Scholar]
- 22.Manji RA, Zhu LF, Nijjar NK, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–27. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 23.Naso F, Gandaglia A, Bottio T, et al. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013;2:252–61. doi: 10.1111/xen.12044. [DOI] [PubMed] [Google Scholar]
- 24.Mills NL, Morgenstern DA, Gaudiani VA, Ordoyne F. “Legs” technique for management of widely separated coronary arteries during ascending aortic repair. Ann Thorac Surg. 1996;61:869–74. doi: 10.1016/0003-4975(95)01185-4. [DOI] [PubMed] [Google Scholar]
- 25.Cabrol C, Pavie A, Mesnildrey P, et al. Long-term results with total replacement of the ascending aorta and reimplantation of the coronary arteries. J Thorac Cardiovasc Surg. 1986;91:17–25. [PubMed] [Google Scholar]