Abstract
Background
Patients with thoracic aortic disease undergoing thoracic endovascular aortic repair (TEVAR) often have concomitant coronary artery disease and are at risk for perioperative adverse cardiac events. Despite this risk, the need for and extent of preoperative cardiac workup before TEVAR remain undefined. This study seeks to assess the adequacy of a limited cardiac evaluation before TEVAR, including assessment of cardiac symptoms, resting electrocardiography (ECG), and transthoracic echocardiography (TTE), as well as to estimate the incidence of perioperative cardiac events in patients undergoing TEVAR.
Methods
Retrospective analysis of a prospectively maintained Institutional Review Board-approved database was performed for all patients undergoing TEVAR at a single referral institution between May 2002 and June 2013. The analysis identified 463 TEVAR procedures. All procedures involving median sternotomy were excluded, and 380 procedures (343 patients) were included in the final analysis. Degree of cardiac workup was classified on the basis of the highest level of preoperative testing: no workup, resting ECG only, resting TTE, exercise/pharmacologic stress testing, or coronary angiography. Standard workup consisted of cardiac symptom assessment along with resting ECG or TTE, with further workup indicated for unstable symptoms, significantly abnormal findings on ECG or TTE, or multiple cardiac risk factors. Categorical and continuous variables were compared by Fisher’s exact test and analysis of variance, respectively.
Results
No preoperative cardiac workup was performed for 28 patients (7.4%); 127 patients (33.4%) had resting ECG only, 208 patients (54.7%) had resting echocardiography, 12 patients (3.2%) underwent stress testing, and five patients (1.3%) had coronary angiography. Patients undergoing stress testing or coronary angiography were older and had a higher incidence of known coronary artery disease (P < .01) and prior myocardial infarction (P = .01). Complex hybrid aortic repairs and TEVAR for aneurysmal disease were more likely to have an extensive workup, whereas nonelective procedures more commonly had no workup. A total of nine patients (2.4%) experienced a perioperative cardiac event (myocardial infarction or cardiac arrest), with no significant difference noted among all groups (P = .45), suggesting that the extent of cardiac workup was appropriate. The incidence of 30-day/in-hospital mortality (5.5%) and cardiac-specific mortality (0.8%) was similar among all groups.
Conclusions
The risk of a postoperative cardiac event after TEVAR is low (2.4%), and initial screening with either resting TTE or ECG, in addition to assessment of cardiac symptom status, appears adequate for most TEVAR patients. As such, we recommend resting TTE or ECG as the initial cardiovascular screening mechanism in patients undergoing TEVAR, with subsequent more invasive studies if initial screening reveals cardiovascular abnormalities.
The incidence of cardiac events such as arrhythmias, myocardial infarction (MI), and cardiac-related morbidity is increased in patients undergoing major vascular surgery with rates ranging from 5% to 15%.1–4 This increased incidence of cardiac morbidity and mortality is believed to be secondary to a high prevalence of underlying cardiac risk factors, such as coronary artery disease (CAD), congestive heart failure, hypertension, hyperlipidemia, and diabetes, among the vascular surgery population.2,5 Patients undergoing thoracic aortic surgery are no exception and represent one of the highest risk groups for perioperative cardiac events.6
Given this potential for cardiac morbidity and mortality after major vascular surgery, many studies have examined patient risk stratification and methods of prevention of adverse cardiac events.7,8 Previous literature focusing on risk stratification has used a variety of methods ranging from simple scoring systems, such as the Eagle or Lee criteria, to complex metrics, such as preoperative cardiopulmonary exercise testing.2,9,10 In addition, certain studies have focused on the need for assessment and treatment of coronary stenosis for prevention of perioperative cardiac events.11,12 However, the majority of these studies have included primarily abdominal vascular surgical procedures, only elective cases, or have grouped both open and endovascular repairs. Further, although patients undergoing thoracic aortic surgery are thought to be at particular risk for perioperative cardiac events,6 there are data suggesting that the incidence of such adverse events may be reduced with endovascular compared with open surgery (in abdominal aortic procedures),3 and to date a consensus algorithmic approach to cardiac workup before thoracic endovascular aortic repair (TEVAR) has not been defined. Further, the exact cardiac risk associated with TEVAR has not been well characterized.
From the inception of our institutional TEVAR program, we have used a limited cardiac evaluation before TEVAR including assessment of cardiac symptoms, resting electrocardiography (ECG), and transthoracic echocardiography (TTE), although this approach has not been systematically studied to date. Further, because unnecessary preoperative workup can lead to delays in surgery and increased health care costs, a better understanding of the optimal cardiac evaluation for TEVAR patients is essential. As such, the purpose of this study was to assess the efficacy of a limited preoperative cardiovascular workup before TEVAR as well as to assess the incidence of adverse cardiac events after TEVAR.
METHODS
Patients and data source
A retrospective review was performed of prospectively collected data from all patients undergoing TEVAR at a single referral institution. Preoperative, intraoperative, and postoperative variables were abstracted from the Duke Thoracic Aortic Surgery Database, a prospectively maintained clinical registry of all patients who have undergone thoracic aortic surgery at Duke University Medical Center (Durham, NC). The study was reviewed and approved by the Duke University Institutional Review Board, and the need for individual patient consent was waived.
Inclusion criteria included any patient undergoing TEVAR since program inception in May 2002 through June 2013. Any patient who underwent concomitant median sternotomy as part of the TEVAR procedure was excluded from analysis. After query of the database, 463 TEVAR procedures were identified, of which 380 (343 patients) met study criteria. Comorbidities and patient characteristics were defined by the Society of Thoracic Surgeons definitions.13 Patients were stratified on the basis of the degree of preoperative cardiac workup they received. Examination of patient medical records, including admission history and physical examination, clinic notes, radiology, cardiac catheterization, and transfer records, was used to determine the extent of preoperative workup performed. The groups identified, in order of invasiveness, were no workup, resting ECG, resting TTE, exercise/pharmacologic stress testing, and coronary angiography. In cases in which multiple procedures were performed, patients were assigned to the group according to the highest level of preoperative workup received. Only tests performed as part of the preoperative workup for the TEVAR procedure were included for analysis.
Preoperative cardiac evaluation
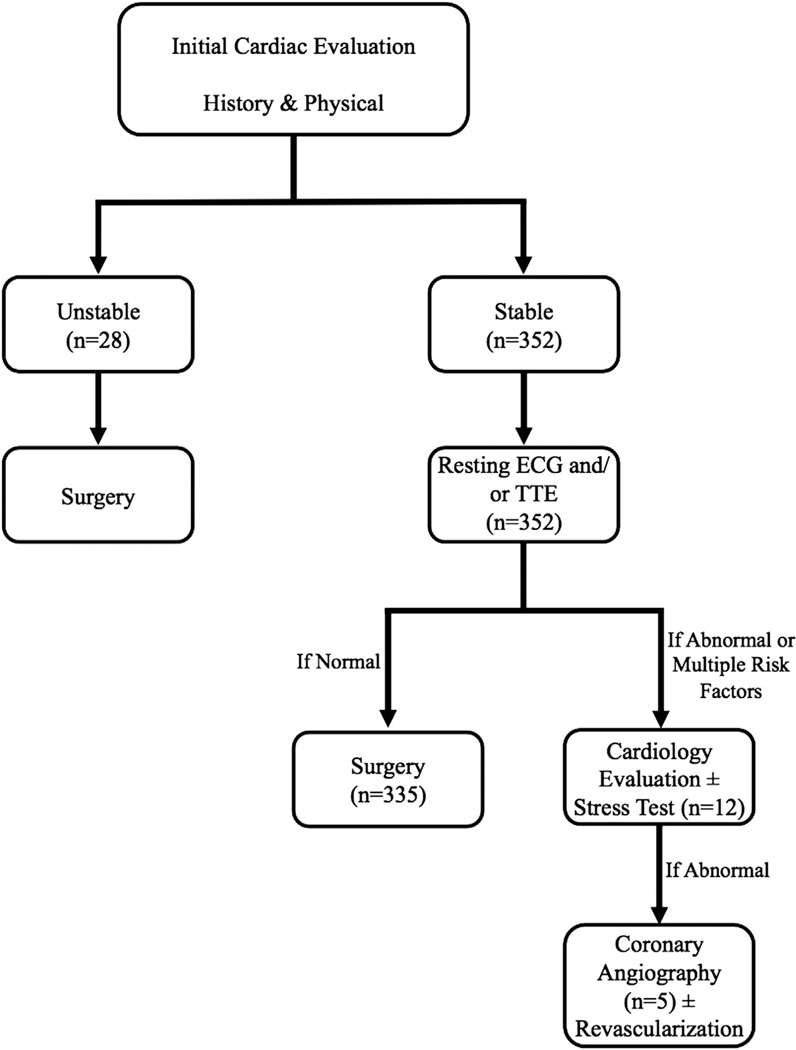
Our institutional algorithm for pre-TEVAR cardiac workup consisted of assessment of cardiac symptoms with clinical history and physical examination in all patients. Patients undergoing elective cases were then subject to resting ECG or TTE. In cases of multiple cardiac risk factors, unstable patient symptoms such as low-level exertional or resting angina, or markedly abnormal findings on ECG or TTE, further evaluation of the patient’s cardiac status was undertaken (Fig). For nonelective procedures, only workup that would not delay surgery was done.
Fig.
Schematic of algorithmic approach to limited cardiac workup for thoracic endovascular aortic repair (TEVAR) patients. ECG, Electrocardiography; TTE, transthoracic echocardiography.
Outcomes
The primary study end point was the incidence of a perioperative cardiac event, defined as MI or cardiac arrest in the overall population as well as stratified by degree of cardiac workup performed. MI was defined by the occurrence of ST changes on ECG with accompanying rise of cardiac biomarkers.14 Secondary outcomes included 30-day/in-hospital and cardiac-specific mortality for both the overall population and stratified by degree of cardiac workup.
Statistical methods
After stratification of patients by the level of cardiac workup received, the Fisher’s exact test or analysis of variance, for categorical and continuous variables, respectively, was used for comparison of patient and procedural characteristics and the aforementioned postoperative outcomes. An affirmative decision was made a priori to set the significance level at α = .05 for all analyses. Statistical analysis was done with JMP Pro 10.0.2 (SAS Institute, Cary, NC) and R version 3.0.1 (Vienna, Austria).
RESULTS
The analysis identified 380 patients who met the study criteria. After stratification into the five predetermined groups, there were 28 patients (7.4%) who received no cardiac workup, 127 patients (33.4%) who underwent ECG alone, 208 patients (54.7%) with TTE, 12 patients (3.2%) with a stress test, and five patients (1.3%) with coronary angiography (Table I). Of the 12 patients who underwent a preoperative stress test, only one test result was positive for inducible ischemia (right coronary distribution) and did not lead to revascularization. Of the five patients undergoing coronary angiography, three had a positive functional study result leading to catheterization, whereas in two the decision was made to proceed directly to catheterization on the basis of preoperative cardiology consultation. Only one of the five patients undergoing coronary angiography had subsequent revascularization; this patient was status post two prior coronary bypass grafting operations and underwent multivessel percutaneous coronary intervention for a diseased saphenous vein graft as well as two native vessel stenoses.
Table I.
Patient characteristics
| Variable | Overall (N = 380) |
No workup (n = 28) |
ECG only (n = 127) |
TTE (n = 208) |
Stress test (n = 12) |
Coronary angiography (n = 5) |
P value |
|---|---|---|---|---|---|---|---|
| Age, years | 68 (55–75) | 52 (34–56) | 68 (53–75) | 69 (59–76) | 70 (63–74) | 73 (59, 76) | <.001 |
| Male sex | 232 (61.1) | 15 (53.6) | 89 (70.1) | 118 (56.7) | 6 (50) | 4 (80) | .081 |
| BMI | 27.8 (24.4–31) | 28 (24–31) | 29 (25–31) | 27 (24–30) | 29 (26–33) | 28 (24–30) | .692 |
| White race | 245 (64.5) | 13 (46.4) | 90 (70.9) | 130 (62.5) | 8 (66.7) | 4 (80) | .126 |
| Hypertension | 329 (86.6) | 14 (50) | 114 (89.8) | 187 (89.9) | 9 (75) | 5 (100) | <.001 |
| Hyperlipidemia | 220 (57.9) | 7 (25) | 76 (59.8) | 122 (58.7) | 10 (83.3) | 5 (100) | <.001 |
| Tobacco abuse | 230 (60.5) | 10 (35.7) | 67 (52.8) | 140 (67.3) | 10 (83.3) | 3 (60) | .001 |
| Diabetes mellitus | 57 (15) | 2 (7.1) | 18 (14.2) | 33 (15.9) | 3 (25) | 1 (20) | .53 |
| Peripheral vascular disease | 97 (25.5) | 3 (10.7) | 34 (26.8) | 54 (26) | 3 (25) | 3 (60) | .152 |
| Baseline creatinine level >1.5 mg/dL | 100 (26.3) | 4 (14.3) | 39 (30.7) | 53 (25.5) | 2 (16.7) | 2 (40) | .326 |
| History of stroke or TIA | 41 (10.8) | 2 (7.1) | 11 (8.7) | 24 (11.5) | 3 (25) | 1 (20) | .299 |
| COPD | 101 (26.6) | 4 (14.3) | 36 (28.3) | 53 (25.5) | 7 (58.3) | 1 (20) | .074 |
BMI, Body mass index; COPD, chronic obstructive pulmonary disease; ECG, electrocardiography; IQR, interquartile range; TIA, transient ischemic attack; TTE, transthoracic echocardiography.
Values are expressed as median (interquartile range) or number (%).
Comparison of patient characteristics revealed significant differences in age of the patients and incidence of hypertension, hyperlipidemia, and tobacco abuse among the groups (Table I). In all cases, as the age of the patient and number of cardiac risk factors increased, the degree of workup increased accordingly. Analysis of cardiac comorbid disease burden (Table II) demonstrated that patients with known CAD or history of prior MI were significantly more likely to undergo more extensive preoperative cardiac evaluation. Similarly, there was a trend toward greater workup in those with a history of preoperative congestive heart failure, lower ejection fraction, or prior coronary revascularization (Table II).
Table II.
Patient cardiac comorbidities
| Variable | Overall (N = 380) |
No workup (n = 28) |
ECG only (n = 127) |
TTE (n = 208) |
Stress test (n = 12) |
Coronary angiography (n = 5) |
P value |
|---|---|---|---|---|---|---|---|
| Known CAD | 109 (28.7) | 2 (7.1) | 39 (30.7) | 59 (28.4) | 5 (41.7) | 4 (80) | .005 |
| History of MI | 46 (12.1) | 1 (3.6) | 10 (7.9) | 29 (13.9) | 4 (33.3) | 2 (40) | .01 |
| Congestive heart failure | 24 (6.3) | 0 (0) | 9 (7.1) | 13 (6.2) | 1 (8.3) | 1 (20) | .298 |
| Ejection fraction | 55 (55-55) | 55 (55-55) | 55 (55-55) | 55 (55-55) | 55 (51–55) | 50 (48–52) | .425 |
| Prior coronary revascularization | .126 | ||||||
| None | 303 (79.7) | 28 (100) | 102 (80.3) | 162 (77.9) | 8 (66.7) | 3 (60) | |
| PCI | 34 (8.9) | 0 (0) | 16 (12.6) | 15 (7.2) | 2 (16.7) | 1 (20) | |
| CABG | 37 (9.7) | 0 (0) | 7 (5.5) | 27 (13) | 2 (16.7) | 1 (20) | |
| CABG and PCI | 6 (1.6) | 0 (0) | 2 (1.6) | 4 (1.9) | 0 (0) | 0 (0) |
CABG, Coronary artery bypass grafting; CAD, coronary artery disease; ECG, electrocardiography; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; TTE, transthoracic echocardiography.
Values are expressed as median (interquartile range) or number (%).
With regard to operative characteristics (Table III), patients with more extensive cardiac workup were more likely to have aneurysmal disease, whereas more than half of aortic transection patients (13 of 23) did not receive any preoperative cardiac workup. In addition, patients undergoing more extensive endovascular procedures (hybrid thoracoabdominal aortic aneurysm and hybrid arch repair) were more likely to have a more extensive cardiac workup with stress test or coronary angiography, and there was a trend toward more extensive workup in those with larger preoperative aortic diameters. Patients with no cardiac workup were more likely to be nonelective case status (86%) and American Society of Anesthesiologists class 4. There was no significant difference among the groups with regard to concomitant vascular procedures performed or the number of endografts implanted (Table III).
Table III.
Operative characteristics
| Variable | Overall (N = 380) |
No workup (n = 28) |
ECG only (n = 127) |
TTE (n = 208) |
Stress test (n = 12) |
Coronary angiography (n = 5) |
P value |
|---|---|---|---|---|---|---|---|
| Nonelective procedure status | 148 (38.9) | 24 (85.7) | 54 (42.5) | 66 (31.7) | 3 (25) | 1 (20) | <.001 |
| Procedure performed | .005 | ||||||
| Isolated descending | 288 (75.8) | 26 (92.9) | 107 (84.3) | 145 (69.7) | 7 (58.3) | 3 (60) | |
| Hybrid TAAA | 65 (17.1) | 1 (3.6) | 16 (12.6) | 42 (20.2) | 5 (41.7) | 1 (20) | |
| Hybrid arch | 27 (7.1) | 1 (3.6) | 4 (3.1) | 21 (10.1) | 0 (0) | 1 (20) | |
| Aortic disease | <.001 | ||||||
| Aneurysm | 230 (60.5) | 7 (25) | 75 (59.1) | 131 (63) | 12 (100) | 5 (100) | |
| Acute dissection | 54 (14.2) | 6 (21.4) | 20 (15.7) | 28 (13.5) | 0 (0) | 0 (0) | |
| Chronic dissection | 73 (19.2) | 2 (7.1) | 24 (18.9) | 47 (22.6) | 0 (0) | 0 (0) | |
| Traumatic transection | 23 (6.1) | 13 (46.4) | 8 (6.3) | 2 (1) | 0 (0) | 0 (0) | |
| ASA class | <.001 | ||||||
| 2 | 3 (0.8) | 0 (0) | 1 (0.8) | 1 (0.5) | 0 (0) | 1 (20) | |
| 3 | 226 (59.5) | 7 (25) | 66 (52) | 146 (70.2) | 6 (50) | 1 (20) | |
| 4 | 151 (39.7) | 21 (75) | 60 (47.2) | 61 (29.3) | 6 (50) | 3 (60) | |
| Number of endografts implanted | 2 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 2 (1–2) | 2 (2-2) | .202 |
| Maximal aortic diameter | 5.8 (4.8–6.6) | 5 (4–7) | 6 (4–6) | 6 (5–7) | 6 (5–6) | 7 (7-7) | .056 |
| Concomitant procedure | 156 (41.1) | 8 (28.6) | 45 (35.4) | 94 (45.2) | 6 (50) | 3 (60) | .181 |
ASA, American Society of Anesthesiologists; ECG, electrocardiography; TAAA, thoracoabdominal aortic aneurysm; TTE, transthoracic echocardiography.
Values are expressed as median (interquartile range) or number (%).
The overall incidence of perioperative MI or adverse cardiac event (MI or cardiac arrest) was 2.4% (n = 9) for both. All adverse cardiac events involved MI, and one of those patients also had a postoperative cardiac arrest. Of the nine MIs, two were treated with revascularization procedures (percutaneous coronary intervention), whereas the rest were managed medically. Patients who suffered a cardiac event had an average of 4.2 cardiac risk factors (hypertension, hyperlipidemia, smoking, diabetes, CAD, history of MI, and peripheral vascular disease); those without an event had an average of 2.8 risk factors (P < .01). Operative factors with prolonged operative time and extensive blood loss appeared to play a role in two of the nine cases (22%) of perioperative cardiac events. All but one of the MIs occurred in the TTE group (n = 8; 3.8%). The same held true for cardiac events, with all but one of the cardiac events occurring in the TTE group (n = 8; 3.8%), and all patients suffering a cardiac event had an abnormal finding on preoperative ECG or TTE. However, the abnormalities were generally nonspecific and mild and, consequently, did not lead to referral for a higher level of preoperative testing.
Only three instances (0.8%) of 30-day/in-hospital cardiac-specific mortality occurred, with one event (0.8%) in the ECG-only group and two occurrences in the TTE group (1.0%). Two of the three cardiac deaths followed nonelective procedures. Overall, 5.5% (n = 21) of all patients died within 30 days or in the hospital. Of the 18 patients who died of a noncardiac event, the majority of deaths were secondary to multisystem organ failure due to complications of the underlying aortic disease or the procedure itself. Notably, there were no instances of adverse cardiac events or cardiac-specific or any-cause mortality in the no-workup, stress test, or coronary angiography groups. Further, there were no significant differences between subgroups of workup extent for any of the analyzed outcomes (Table IV).
Table IV.
Perioperative outcomes
| Variable | Overall (N = 380) |
No workup (n = 28) |
ECG only (n = 127) |
TTE (n = 208) |
Stress test (n = 12) |
Coronary angiography (n = 5) |
P value |
|---|---|---|---|---|---|---|---|
| Postoperative cardiac eventa | 9 (2.4) | 0 (0) | 1 (0.8) | 8 (3.8) | 0 (0) | 0 (0) | .449 |
| Postoperative MI | 9 (2.4) | 0 (0) | 1 (0.8) | 8 (3.8) | 0 (0) | 0 (0) | .449 |
| Thirty-day/in-hospital death | 21 (5.5) | 0 (0) | 9 (7.1) | 12 (5.8) | 0 (0) | 0 (0) | .668 |
| Thirty-day/in-hospital cardiac death | 3 (0.8) | 0 (0) | 1 (0.8) | 2 (1) | 0 (0) | 0 (0) | .999 |
ECG, Electrocardiography; MI, myocardial infarction; TTE, transthoracic echocardiography.
Values are expressed as number (%).
Defined as MI or cardiac arrest.
DISCUSSION
Because of the significant incidence of cardiac morbidity and mortality after major vascular surgery, preoperative cardiac risk stratification is thought to be important6,9,15 as it may allow identification of those patients at highest risk for a perioperative cardiac event. Further, the information gained through the risk stratification process can help guide patient management during and after surgery, which also may lead to better outcomes. However, unwarranted cardiac workup not only increases health care costs but has the potential to delay surgery. Previous data examining methods of risk stratification have focused primarily on abdominal or peripheral vascular surgery or elective surgery and have not assessed the extent of cardiac workup required for patients undergoing TEVAR procedures. The current study demonstrates that the incidence of adverse cardiac events after TEVAR is low (2.4%) and that a limited cardiac workup, consisting of a preoperative assessment with history and physical examination along with resting ECG and TTE in asymptomatic patients, appears adequate in most individuals.
Given the importance of preventing cardiac events in patients undergoing vascular surgery, a significant amount of literature has been devoted to the topic. The 2010 American College of Cardiology/American Heart Association guidelines for thoracic aortic disease recommend additional studies to determine the presence of significant CAD in the setting of symptoms of myocardial ischemia as well as additional testing to quantitate a patient’s comorbid state before thoracic aortic procedures.15 These recommendations, however, do not provide a clear algorithmic approach to cardiac workup before TEVAR and apply to both open and endovascular procedures. Given the decreased perioperative cardiac risk associated with endovascular vs open surgery,16 it is unclear if guidelines for patients undergoing TEVAR should differ from those for patients undergoing open thoracic aortic repair, especially because major open thoracic aortic surgery is considered to have the highest risk for cardiac morbidity and mortality.15
Although no studies to date have examined the incidence of cardiac events and the extent of preoperative cardiac workup required before TEVAR, there are several studies in the literature concerning cardiac evaluation in patients undergoing abdominal aortic aneurysm repair that provide useful data for comparison. Troisi et al3 described an experience similar to the current study in which 531 open or endovascular elective abdominal aortic aneurysm repair patients underwent preoperative screening with ECG, TTE, and cardiology consultation. Nearly two thirds of the patients underwent a stress test, with only 25% having a positive test result. Of those with a positive stress test result, nearly 40% underwent subsequent coronary angiography, with 38% having subsequent coronary revascularization. The incidence of cardiac morbidity and mortality was 6.8% and 0.6%, respectively. Thus, even though the majority of patients in the Troisi study underwent stress testing, only 3.7% had a subsequent revascularization procedure, similar to the low incidence of preoperative revascularization performed in the current study before TEVAR. Bub et al17 reported their experience with endovascular repair of thoracoabdominal and juxtarenal aneurysms with branched/fenestrated endografts. They observed MIs in 7.7% of patients and a cardiac-related mortality of 2%. Notably, all patients had serial postoperative troponin measurements, and MI was defined by consensus criteria. A preoperative stress test was performed in 73% of patients, of which 85% had normal findings. Thus, the results of the Bub study support the findings of the current study in that the use of stress testing would appear to be without merit in most patients. Finally, specific to TEVAR, Chung et al18 found that cardiac mortality was 1.8% and 4.8% at <30 days and >30 days, respectively; however, they did not describe their method of risk stratification or cardiac workup. As a whole, these prior studies demonstrate cardiac event rates and mortality risks similar to those presented in this study and also support the use of a more limited cardiac workup with ECG and TTE before TEVAR with the avoidance of stress testing or coronary angiography in most patients.
The role of coronary revascularization before major vascular surgery has been examined as a method to limit cardiac morbidity and mortality. Initially, it was believed that coronary revascularization should be done before major vascular surgery19,20; however, this paradigm was called into question by the Coronary Artery Revascularization Prophylaxis (CARP) trial and other studies.11,21 Whereas some of these studies were criticized because of revascularization of low-risk cardiac patients, Poldermans et al12 conducted a randomized controlled trial of only high-risk cardiovascular patients and demonstrated that coronary revascularization before major vascular surgery did not provide a short- or long-term survival advantage. In addition, should a coronary stent be placed for revascularization, the issues of dual antiplatelet therapy and potential delay of surgery must be considered.2,22 These data suggesting preoperative revascularization to be of questionable benefit would also appear to support the currently proposed algorithm of limited cardiac evaluation before TEVAR, which led to preoperative coronary angiography in only 1.3% of patients.
By starting with a limited cardiac workup before TEVAR, unnecessary tests are avoided in the majority of patients. However, this approach emphasizes the importance of a careful history and physical examination, which is essential to determine whether further workup is necessary, and findings such as congestive heart failure symptoms or angina should prompt further evaluation. In addition, the preoperative ECG and TTE can provide valuable information about a patient’s cardiac status, with Q waves or bundle branch block on ECG or focal wall motion abnormalities, reduced ventricular function, or significant valvular disease on TTE, for example, indicating patients in whom additional workup may be indicated. Whereas other groups have incorporated cardiology evaluation as part of the standard preoperative cardiac workup, our practice is to refer patients for cardiology evaluation only in the case of an abnormality detected on initial limited workup. On the basis of the low incidence of cardiac events and mortality observed with this approach, we think this practice is safe and saves health system resources and time.
Another issue that was not addressed in this paper but is important to the prevention of perioperative cardiovascular events is optimization of medical therapy in the perioperative period. Before elective surgery, elements of lifestyle modification as well as addressing patient comorbidities have been demonstrated to reduce cardiac events.9 Modifiable factors include smoking cessation, strict glycemic control for diabetics, control of hypertension, and, perhaps most important, the use of statins. Randomized data from Durazzo et al23 revealed a threefold decrease in cardiac events for patients who took 20 mg of atorvastatin, regardless of lipid status, for at least 30 days before major vascular surgery compared with placebo. O’Neill et al24 also demonstrated a protective effect of statins in the perioperative phase. Moreover, others have shown that abrupt perioperative withdrawal of statins can lead to an increase in the incidence of adverse cardiac events.25,26 The use of β-blockers can also help decrease perioperative cardiac risk, particularly if a long-acting agent is initiated before surgery.27 However, recent studies have established that β-blockers should be employed only in high-risk patients or as continuation for patients already receiving a β-blocker.28,29 Specifically, they should not be used in intermediate- or low-risk vascular surgery patients.9
Also warranting discussion are the three cardiac deaths that occurred. Two of the patients who suffered a cardiac death were urgent cases performed for impending aortic rupture. In both cases, there was a significant cardiac history; one patient had an ejection fraction of 30% as well as a history of MI, whereas the other patient had a history of CAD with coronary artery bypass grafting more than 30 years before her TEVAR procedure. The workup for the first patient was ECG and TTE; the second patient had only ECG. However, the urgent nature of their cases precluded further workup as surgical delay was thought to be risk prohibitive. These two deaths highlight the fact that close attention should be paid to patients undergoing nonelective TEVAR, particularly those with cardiac risk factors, in the perioperative period. The third cardiac death occurred in a patient with an extensive cardiac history (two previous MIs, history of coronary artery bypass grafting on two separate occasions, congestive heart failure symptoms at presentation) who underwent an elective hybrid thoracoabdominal aortic aneurysm repair. His workup also consisted of only ECG and TTE; he had no unstable symptoms by history, and his ejection fraction was 50% by TTE. However, he suffered a perioperative MI with fatal cardiac arrest. It is unknown if he would have benefited from a more extensive workup, but on the basis of the size of his aneurysm, the risk of rupture without repair was considered very high.
Limitations
The current study has several notable limitations. First, given the observational, nonrandomized nature of the study, true head-to-head comparison of the various approaches to preoperative cardiac evaluation was not performed. In addition, the overall study cohort was relatively small, and there were only a few patients who had no workup, a stress test, or coronary angiography, thus limiting the sample size of each of these groups. Similarly, there were few cardiac events, which prevented adjustment for other patient risk factors that may have influenced the results presented. Moreover, as this was a single-institution study, there is the possibility of a selection bias in the patient cohort selection that may have influenced both the outcomes presented and subsequent analyses.
CONCLUSIONS
The risk of a postoperative cardiac event after TEVAR is low (2.4%), and initial screening with either resting TTE or ECG, in addition to assessment of cardiac symptom status and physical examination, appears adequate for most TEVAR patients. As such, we recommend resting TTE or ECG as the initial cardiovascular screening mechanism in patients undergoing TEVAR, with subsequent more invasive studies if initial screening reveals significant cardiovascular abnormalities.
Acknowledgments
A.M.G. is supported by National Institutes of Health grantL30HL115769-01.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented as an oral presentation at the Thirty-eighth Annual Meeting of the Southern Association for Vascular Surgery, Palm Beach, Fla, January 15–18, 2014.
AUTHOR CONTRIBUTIONS
Conception and design: AG, RM, GH
Analysis and interpretation: AG, BE
Data collection: AG
Writing the article: AG, RM, GH
Critical revision of the article: AG, BE, MS, JV, JH, RM, GH
Final approval of the article: AG, BE, MS, JV, JH, RM, GH
Statistical analysis: AG, BE
Obtained funding: Not applicable
Overall responsibility: GH
REFERENCES
- 1.L’Italien GJ, Cambria RP, Cutler BS, Leppo JA, Paul SD, Brewster DC, et al. Comparative early and late cardiac morbidity among patients requiring different vascular surgery procedures. J Vasc Surg. 1995;21:935–944. doi: 10.1016/s0741-5214(95)70221-0. [DOI] [PubMed] [Google Scholar]
- 2.Omar HR, Mangar D, Camporesi EM. Preoperative cardiac evaluation of the vascular surgery patient—an anesthesia perspective. Vasc Endovascular Surg. 2012;46:201–211. doi: 10.1177/1538574412438950. [DOI] [PubMed] [Google Scholar]
- 3.Troisi N, Dorigo W, Lo Sapio P, Pratesi G, Pulli R, Gensini GF, et al. Preoperative cardiac assessment in patients undergoing aortic surgery: analysis of factors affecting the cardiac outcomes. Ann Vasc Surg. 2010;24:733–740. doi: 10.1016/j.avsg.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Back MR, Schmacht DC, Bowser AN, Stordahl N, Cuthbertson D, Johnson BL, et al. Critical appraisal of cardiac risk stratification before elective vascular surgery. Vasc Endovascular Surg. 2003;37:387–397. doi: 10.1177/153857440303700602. [DOI] [PubMed] [Google Scholar]
- 5.Ferro CR, de Oliveira DC, Guerra Fde F, de Lucena AJ, Nunes FP, Ortiz ST, et al. Prevalence and risk factors for combined coronary artery disease and aortic aneurysm. Arq Bras Cardiol. 2007;88:40–44. doi: 10.1590/s0066-782x2007000100007. [DOI] [PubMed] [Google Scholar]
- 6.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. Circulation. 2006;113:2662–2674. doi: 10.1161/CIRCULATIONAHA.106.176009. [DOI] [PubMed] [Google Scholar]
- 7.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52:674–683. 683, e1–e3. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Parmar CD, Torella F. Prediction of major adverse cardiac events in vascular surgery: are cardiac risk scores of any practical value? Vasc Endovascular Surg. 2010;44:14–19. doi: 10.1177/1538574409349320. [DOI] [PubMed] [Google Scholar]
- 9.Bauer SM, Cayne NS, Veith FJ. New developments in the preoperative evaluation and perioperative management of coronary artery disease in patients undergoing vascular surgery. J Vasc Surg. 2010;51:242–251. doi: 10.1016/j.jvs.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 10.Hartley RA, Pichel AC, Grant SW, Hickey GL, Lancaster PS, Wisely NA, et al. Preoperative cardiopulmonary exercise testing and risk of early mortality following abdominal aortic aneurysm repair. Br J Surg. 2012;99:1539–1546. doi: 10.1002/bjs.8896. [DOI] [PubMed] [Google Scholar]
- 11.McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 12.Poldermans D, Schouten O, Vidakovic R, Bax JJ, Thomson IR, Hoeks SE, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49:1763–1769. doi: 10.1016/j.jacc.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 13.Adult cardiac surgery database training manual v2.73. [Accessed June 14, 2013];2012 Available at: http://www.sts.org/sites/default/files/documents/Training Manual Update 8 12.pdf. [Google Scholar]
- 14.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 15.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 16.Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg. 2010;52:860–866. doi: 10.1016/j.jvs.2010.05.008. discussion: 866. [DOI] [PubMed] [Google Scholar]
- 17.Bub GL, Greenberg RK, Mastracci TM, Eagleton MJ, Panuccio G, Hernandez AV, et al. Perioperative cardiac events in endovascular repair of complex aortic aneurysms and association with preoperative studies. J Vasc Surg. 2011;53:21–27. e1–e2. doi: 10.1016/j.jvs.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Chung J, Corriere MA, Veeraswamy RK, Kasirajan K, Milner R, Dodson TF, et al. Risk factors for late mortality after endovascular repair of the thoracic aorta. J Vasc Surg. 2010;52:549–554. doi: 10.1016/j.jvs.2010.04.059. discussion: 555. [DOI] [PubMed] [Google Scholar]
- 19.Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) J Am Coll Cardiol. 2002;39:542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines for assessing and managing the perioperative risk from coronary artery disease associated with major noncardiac surgery. American College of Physicians. Ann Intern Med. 1997;127:309–312. doi: 10.7326/0003-4819-127-4-199708150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Mesh CL, Cmolik BL, Van Heekeren DW, Lee JH, Whittlesey D, Graham LM, et al. Coronary bypass in vascular patients: a relatively high-risk procedure. Ann Vasc Surg. 1997;11:612–619. doi: 10.1007/s100169900099. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SH, Fasseas P, Orford JL, Lennon RJ, Horlocker T, Charnoff NE, et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42:234–240. doi: 10.1016/s0735-1097(03)00622-3. [DOI] [PubMed] [Google Scholar]
- 23.Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leao P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967–975. doi: 10.1016/j.jvs.2004.01.004. discussion: 975–6. [DOI] [PubMed] [Google Scholar]
- 24.O’Neil-Callahan K, Katsimaglis G, Tepper MR, Ryan J, Mosby C, Ioannidis JP, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45:336–342. doi: 10.1016/j.jacc.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 25.Schouten O, Hoeks SE, Welten GM, Davignon J, Kastelein JJ, Vidakovic R, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100:316–320. doi: 10.1016/j.amjcard.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 26.Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg. 2008;35:139–144. doi: 10.1016/j.ejvs.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Poldermans D, Boersma E, Bax JJ, Thomson IR, van de Ven LL, Blankensteijn JD, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341:1789–1794. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- 28.Group PS, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–990. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]