Abstract
This study was undertaken to investigate changes in RNA expression in previously healthy adult human skin following thermal injury induced by contact with hot metal that was undertaken as part of aesthetic scarification, a body modification practice. Subjects were recruited to have pre-injury skin and serial wound biopsies performed. 4 mm punch biopsies were taken prior to branding and 1 hour, 1 week, and 1, 2 and 3 months post injury. RNA was extracted and quality assured prior to the use of a whole-genome based bead array platform to describe expression changes in the samples using the pre-injury skin as a comparator. Analysis of the array data was performed using k-means clustering and a hypergeometric probability distribution without replacement and corrections for multiple comparisons were done. Confirmatory q-PCR was performed. Using a k of 10, several clusters of genes were shown to co-cluster together based on Gene Ontology classification with probabilities unlikely to occur by chance alone. OF particular interest were clusters relating to cell cycle, proteinaceous extracellular matrix and keratinization. Given the consistent expression changes at one week following injury in the cell cycle cluster, there is an opportunity to intervene early following burn injury to influence scar development.
Introduction
The development of thick, erythematous, pruritic and painful contractile scars known as hypertrophic scars are common following skin injury and are the most common complication following burn injury.[1] Progress has been made in developing porcine models of scarring that share some molecular characteristics with human scars. However, these models do not match the gross phenotype of the thick scars seen in humans.[2] Additionally, the cost and expertise required to manage these large animals may preclude many investigators from incorporating these models in to their experiments.
Following a consensus conference of burn researchers and clinicians, several research priorities were proposed. Particularly, it was recommended that future burn research be performed using early and serial biopsies of human skin from the same individual. As well, recommendations were made that future studies in burn research include samples from small partial thickness and full thickness burn wounds that heal spontaneously without tangential excision and split thickness skin grafting.[3]
These recommendations pose challenges to the clinical burn researcher. Although serial samples of burn wounds may be collected in a clinical setting, burn patients may present at various times following their injury. As well, the treatments used to manage burn injuries should influence the wound environment and potential to develop scars. Furthermore, collecting normal, pre-injury skin is not possible from patients presenting after they all ready have a burn.
The purpose of this study was to devise a method to collect pre-injury, early and serial samples of untreated human burn wounds. Secondly, to use an analysis technique of bead array data based on gene ontology classification to describe RNA expression changes following burn injury.
Methods
This study was approved by the institutional review board of The University of Texas Southwestern Medical Centre at Dallas. All subjects provided informed, written consent to participate in the study. Subjects were recruited through self-presentation to a body modification studio where an advertisement describing the study was posted. Potential subjects requesting aesthetic scarification contacted the principal investigator for screening. Written informed consent was obtained. Subjects over 18 years old without pre-existing skin disorders at the proposed scarification site that could participate in three months of follow up were included. Subjects were excluded for allergies to any study materials or if the proposed anatomic site for branding was in an area unsafe to perform a punch biopsy in the opinion of the investigator.
The body modification artist and subject determined the design of the branding and resultant scar. The investigator then determined two locations in the area to be branded. These locations were cleaned with alcohol swabs, and each site was injected with 1cc of 2% lidocaine without epinephrine. Pre-injury skin was then sampled with a standard 4mm punch biopsy.
Then, the artist heated the metal brand using a propylene torch until it was glowing red. The brand was applied to the skin for a sufficient duration in the artist's opinion to induce a deep partial to full thickness burn injury sufficient to create a scar. Subjects were instructed by the artist to wash the brand with soap and water but were advised to apply no other treatments.
One hour after branding, the investigator identified the second anaesthetized area within the burned region and biopsied it. The samples were transported immediately in RNAlater to storage at 4°C.
Using the same protocol, follow up 4mm punch biopsies were collected one week (4-8 days), 1 month (22-28 days), 2 months (55-65 days) and 3 months (85-95 days) post branding. At each follow up visit, the subjects rated the severity of pain at the branding site and the intensity of itch at the branding site using a 0 to 10 visual analog scale. One investigator (VG) scored the scars using the Vancouver Scar Scale.
Samples were treated with Dnase1 and extraction was performed using the QIAGEN RNEasy kit as described by the manufacturer. RNA quality was confirmed using an Agilent bioanalyzer.
Samples were labeled using a standard protocol. Briefly, a Reverse Transcription Master Mix was prepared using the Ambion Master Mix Calculator for the Illumina TotalPrep kit. This master mix was applied to each sample and centrifuged. The samples were then inclubated for 2 hours at 42C. Following incubation, a second strand master mix was prepared and placed on ice. This was added to each sample and centrifuged before being incubated at 16C for 2 hours. cDNA was purified by adding binding buffer to each sample and passing them through a cDNA filter cartridge. This was washed with a washing buffer that included ETOH. Following this, an in vitro transcription mix was prepared using the same calculator and was added to each cDNA sample. This was then incubated at 37C for 14 hours. Nuclease free water was added to stop the in vitro transcription reaction and the samples centrifuged briefly. cRNA was purified by adding cRNA binding buffer and 100% ethanol to each sample. The samples were then passed through a cRNA filter cartridge and washed with the wash buffer. The cRNA was then eluted with nuclease free water that was pre-heated to 58C.
The samples were analyzed using standard protocols for Illumina Human WG-6 v3.0 expression beadchip arrays as described in Illumina Catalog #BD-901-1002, Part #11322355 Rev A. Each subject's six samples were analyzed on the same array. Briefly, this array allows for six samples to be analyzed within a single array, thereby reducing any array to array variability between subjects. The Illumina bead array technology is based on silica beads that self assemble in microwells that are evenly spaced on the array. Each bead is covered with specific oligonucleotides that are the capture sequences for the array. There are between 20 and 30 beads of each type. The labeled cRNA and bead oligonucleotide interaction is scanned using a proprietary system of lasers and software to produce the final output and image files.
Follow up confirmatory PCR (Taqman ABI 7900HT RT-PCR) tests were performed on selected genes in triplicate using an 18S ribosomal control. Full methodology and data details are available online at ArrayExpress (Accession EMTAB-1323).
A log base 2 transformation was performed on the raw probe intensities and the results were normalized within each array using the Excel “standardize” function. T-tests were performed for each time point's probe intensity compared with the pre-injury intensity to most significantly differentially expressed top 1% of genes, which were then grouped using k-means clustering with k=10 using R.
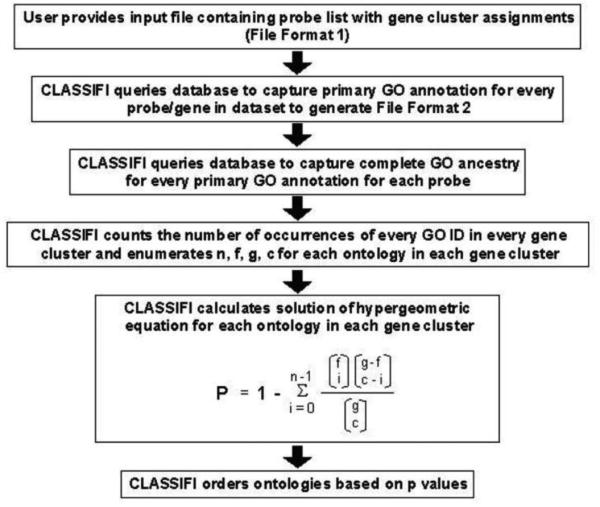
Each cluster was analyzed using a hypergeometric probability distribution without replacement based on Gene Ontology (GO) classification (Cluster Assignment for Biological Inference or CLASSIFI) to identify significant co-clustering of genes with similar GO annotations.[4] The CLASSIFI algorithm is outlined in figure 1. A Bonferroni correction of 14,000 for multiple comparisons was preformed.
Figure 1.
CLASSIFI algorithm
One way ANOVA was performed on the confirmatory PCR results, with significance defined as p-value <0.05 using Dunnett's post hoc test for each biopsy sample compared with the within subject pre-injury control.
Results
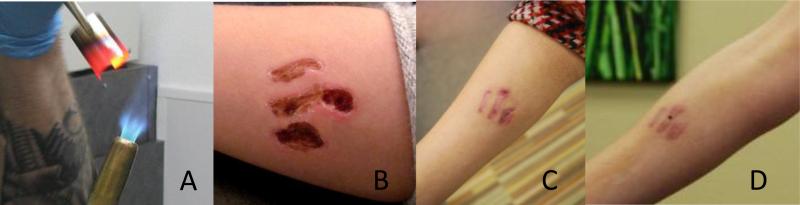
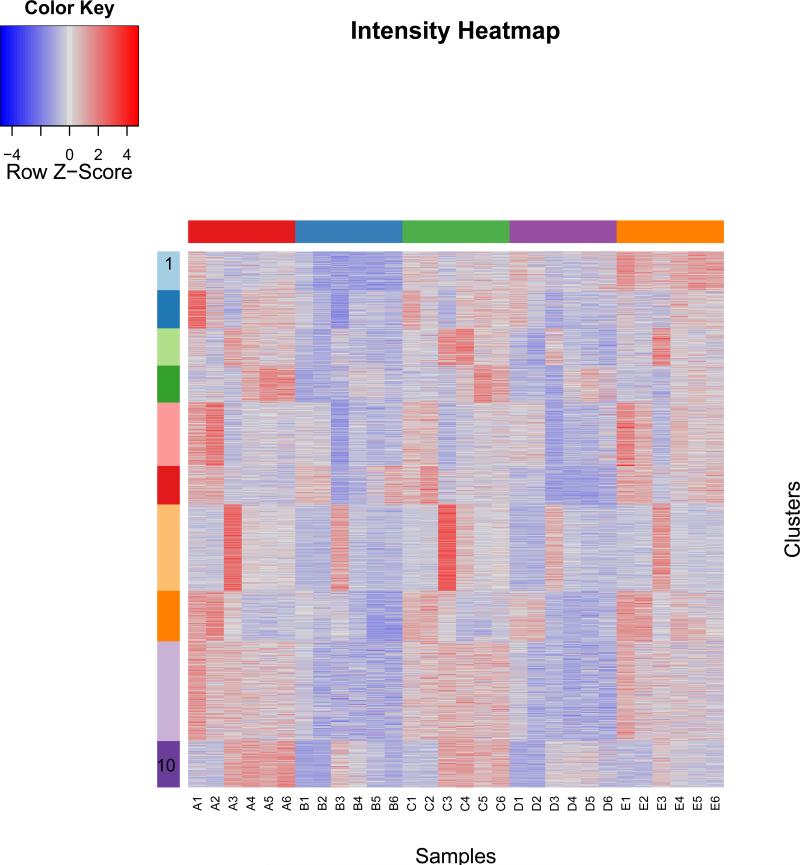
Four female and one male subjects were recruited. The subjects completed all study visits. No adverse events were reported through the course of the study. The characteristics of the subjects are reported in table 1. Pain scores and Vancouver Scar Scale scores are presented in table 2. The total combined estimated body surface area burned through branding was less than 1% for each subject. The branding procedure and resultant wounds are demonstrated in Figure 2A-D. Figure 3 illustrates a heat map of the expression values of the most significantly differentially expressed genes arranged by gene cluster using k = 10. Seven clusters showed statistically significant co-clustering by Gene Ontology classification using CLASSIFI including clusters 2 (keratinization), 4 (proteinaceous extracellular matrix), 6 (mitochondrion) and 7 (cell cycle). The CLASSIFI results are presented in table 3 and selected gene names are presented in table 4.
Table 1.
Subject characteristics
| Subject ID | Age | Gender | Injury Site | Instrument temperature (Celsius) |
|---|---|---|---|---|
| A | 27 | F | Right volar forearm | 149.6 |
| B | 26 | M | Left scapula | 140.3 |
| C | 29 | F | Right lateral shoulder | 178.9 |
| D | 37 | F | Left posterior leg | 164.3 |
| E | 22 | F | Back | 141.6 |
Table 2.
Clinical outcomes from branding
| Subject | Pain 1 hour | Pain 1 week | Pain 1 month | Pain 2 months | Pain 3 months | Total VSS 3 months |
|---|---|---|---|---|---|---|
| A | 0 | 1 | 3 | 0 | 1 | 7 |
| B | 0 | 1 | 0 | 0 | 1 | 4 |
| C | 0 | 1 | 1 | 0 | 0 | 7 |
| D | 0 | 2 | 1 | 0 | 0 | 8 |
| E | 0 | 3 | 0 | 0 | 0 | 5 |
| Subject | Itch 1 hour | Itch 1 week | Itch 1 month | Itch 2 months | Itch 3 months | Total VSS 3 months |
|---|---|---|---|---|---|---|
| A | 0 | 1 | 6 | 7 | 4 | 7 |
| B | 0 | 0 | 0 | 3 | 4 | 4 |
| C | 0 | 3 | 10 | 6 | 4 | 7 |
| D | 0 | 3 | 1 | 2 | 0 | 8 |
| E | 0 | 0 | 4 | 0 | 0 | 5 |
Table 2: Visual analog scale scores (0-10) for severity of pain and intensity of itch in burned area. Total Vancouver Scar Scale (VSS) scores at three months post branding.
Figure 2.
A) heating the metal brand B) wound one week post branding C) one month branding D) scar 3 months post branding
Figure 3.
heat map of expression values arranged by gene cluster using k = 10
Table 3.
CLASSIFI Results
| Cluster ID | GO ID | g | f | c | n | adjusted p-value | GO name |
|---|---|---|---|---|---|---|---|
| 1 | GO:0000004 | 1826 | 322 | 132 | 39 | 4.804683506 | biological_process unknown |
| 2 | GO:0031424 | 1826 | 9 | 130 | 9 | 5.3152E-07 | keratinization |
| 3 | GO:0005730 | 1826 | 85 | 127 | 17 | 0.591972345 | nucleolus |
| 4 | GO:0005578 | 1826 | 42 | 126 | 14 | 0.004042409 | proteinaceous extracellular matrix |
| 5 | GO:0043492 | 1826 | 12 | 216 | 8 | 0.162121589 | ATPase activity, coupled to movement of substances |
| 6 | GO:0005739 | 1826 | 111 | 131 | 36 | 2.18337E-12 | mitochondrion |
| 7 | GO:0007049 | 1826 | 110 | 294 | 53 | 4.53879E-12 | cell cycle |
| 8 | GO:0000004 | 1826 | 322 | 172 | 58 | 0.000846032 | biological_process unknown |
| 9 | GO:0005554 | 1826 | 322 | 340 | 100 | 2.14525E-05 | molecular_function unknown |
| 10 | GO:0005783 | 1826 | 82 | 158 | 21 | 0.034984589 | endoplasmic reticulum |
Table 3: CLASSIFI Results: Column heads: Cluster ID: k means cluster number, GO ID: Gene Ontology identifier, g: total number of probes in data set, f: total number of probes within given Gene Ontology identifier in the data set, c: the number of probes within each cluster, n: the number of probes with a given Gene Ontology identifier within each cluster, adjusted p-value: probability that genes with a given Gene Ontology identifier that would co cluster by chance alone given the proportion of genes annotated with the given term in the data set, GO name: Gene Ontology name
Table 4.
Selected Gene Names By Cluster
| Cluster_2 | Cluster_4 | Cluster_7 | ||
|---|---|---|---|---|
| KRT2A | ACHE | ACVR1B | FLJ25416 | PSME1 |
| LCE1A | AMBN | AKAP8 | FOXC1 | PSME2 |
| LCE1B | BGN | ANXA1 | GPR132 | PTPRC |
| LCE1E | COL16A1 | APPBP1 | GTPBP4 | RANBP1 |
| LCE1F | COL18A1 | APRIN | GTSE1 | RCC1 |
| LCE2A | COL1A1 | BCL6 | GTSE1 | RB1CC1 |
| LCE2B | COL1A2 | BLM | HCAP-G | RUNX3 |
| LCE2C | COL1A2 | BOP1 | HORMAD1 | RUVBL1 |
| LOR | COL3A1 | BRCA2 | ID4 | SGOL1 |
| COL5A1 | BUB1 | IFNW1 | SGOL2 | |
| COL5A2 | C10CRF7 | IL8 | SKP1A | |
| COMP | C18ORF24 | ILF3 | SMARCB1 | |
| DAG1 | CCNDBP1 | INCENP | SMC2L1 | |
| DPT | CCNE1 | KHDRBS1 | SPC25 | |
| EMID1 | CDC16 | KIAA1914 | STK11 | |
| FN1 | CDC25 | KIF11 | TACC3 | |
| IMPG1 | CDC25A | KIF2 | TAF1 | |
| LAMA3 | CDCA8 | KIF23 | TBX3 | |
| LAMB3 | CDK6 | KLHDC3 | TDRD1 | |
| LAMC2 | CDKN3 | MAPRE1 | TOP3A | |
| LGALS3 | CHECK2 | MCM3 | TREX1 | |
| LUM | CHES1 | MCM6 | TTK | |
| MATN2 | CHFR | MCM7 | TTN | |
| MMP1 | CKS2 | MYH9 | TUBB | |
| MMP15 | CLIP1 | NCAPG2 | TXNIP | |
| MMP23B | CNEPE | NOTCH2 | UBE2I | |
| MMP3 | CUL4B | NUSAP1 | UHMK1 | |
| MMP7 | CYP26B1 | PBK | UPT5H | |
| MMP9 | DCTN | PDLD4 | WTAP | |
| MUC4 | DCTN1 | PDLD6IP | XRCC2 | |
| SPARC | DDX12 | PDPN | ||
| SPON1 | DYNCQH1 | PELO | ||
| TIMP1 | E2F4 | PPP1CB | ||
| TIMP3 | E2F4 | PPP2R3B | ||
| TNC | EIF4G2 | PSMB9 | ||
| WNT10A | EXO1 | PSMC5 | ||
| WNT11 | FAM33A | PSMC6 | ||
| WNT7A | FANCD2 | PSMD10 | ||
| ZP2 | FLJ22624 | PSMD12 | ||
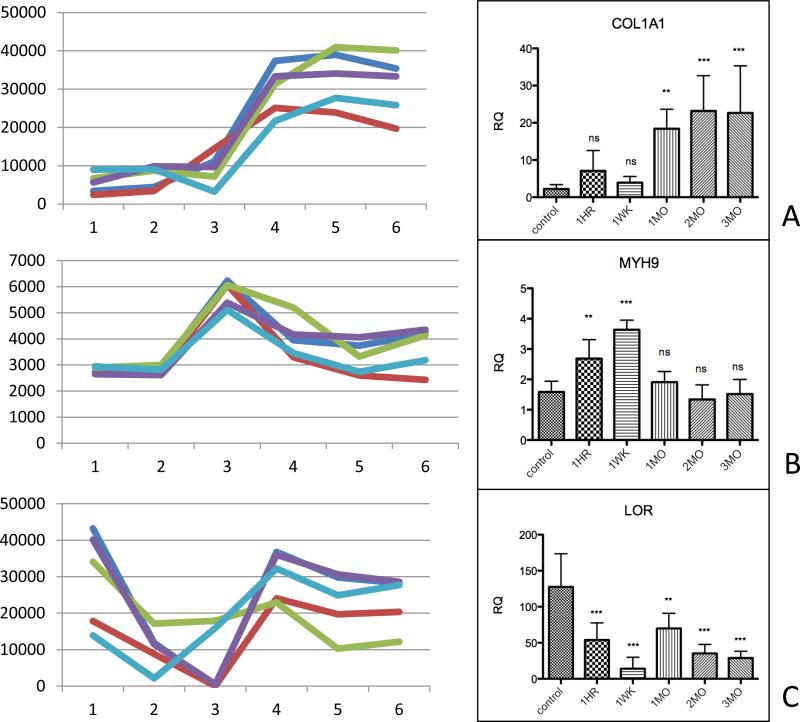
There was a similar pattern of expression between subjects. Cluster 2 contained genes whose expression were stably up-regulated within 1 week after injury, including collagen 1A1 (COL1A1). Cluster 4 contained genes whose expression were stably down-regulated within 1 week after injury, including loricrin. Cluster 7 contained genes showing transient up-regulation at 1 week post burn, including myosin heavy chain. Confirmatory qRT-PCR showed statistically significant differences in mRNA levels that matched the temporal pattern of bead array intensities (figure 4).
Figure 4.
Left column: raw intensity of bead array output for selected genes, each line represents a single subject's data. Right column: q-PCR results for corresponding genes from aggregated samples from all subjects
Discussion
To collect pre-injury, early and serial samples of untreated human burn wounds we decided to recruit subjects from individuals seeking out aesthetic scarification or ‘branding.’ Scarification has a history in many cultures and in some cases may even be used as a traditional medical treatment without supporting evidence.[5] In western cultures, aesthetic scarification has been adopted as a form of body modification. The creation of an aesthetic scar may be done by applying a heated object to the skin, frostbite, electrocautery or full thickness skin excision. As with any procedure that creates a defect in the skin, there is a risk of developing cellulitis or other complications.[6] However, individuals whom undergo aesthetic scarification are instructed not to treat the wound to advance closure, but instead to perpetuate the open wound to increase the likelihood of developing a scar.
One study of adolescents in the United Kingdom attributed an increase in self-reported substance abuse with body modifications including piercings and tattoos. However, in the body modification group, only one subject had been branded.[7] No significant evidence currently exists to link aesthetic scarification with substance abuse, psychological disorders or other abnormalities in adults. This study was not designed to address any of these considerations.
Like other body modifications such as piercing and dermal implants, aesthetic scarification is regulated differently in various jurisdictions. At the study site, aesthetic scarification is not specifically addressed in the legislation relating to body modification, however, the branding was all carried out in a facility licensed by the state to perform all other forms of body modification.
The ethics of performing this study were carefully considered prior to initiating the project. Specifically, risks to the subjects, artist and investigators were all addressed.
The subjects were all ready members of the body modification community and sought out modification independent of the investigators. Although other groups, such as some American college associations and sports groups are described in the lay literature as incorporating branding in to their activities, these actions must be considered coercive and were not considered for this study. Aesthetic scarification is practiced outside of a research study and as such we felt there was no additional risk posed to the subjects outside of having a 4mm punch biopsy performed. Considering this biopsy is a routine clinical and research procedure, the study was not considered high risk.
The artist was interviewed prior to the study to inquire whether or not repeated punch biopsies would adversely affect the outcome of his work and as such negatively impact his business in the body modification market. In his opinion, the small punch biopsies would not significantly alter his designs in the long term and he attested to this in writing to the institutional review board.
Besides standard universal precautions regarding the handling of biological samples, we considered the possibility of psychological risk to the investigator from witnessing the branding procedures and performing sampling outside of the institutional setting. Safety mechanisms such as pre-arranged check in times during the procedures and the availability of psychological debriefings were established.
Although the findings were consistent between subjects, we do not make any extrapolation of these findings to the clinical scenario of patients with large burn injuries with hypermetabolism. Typically aesthetic branding is performed without any form of anaesthesia, however, we felt it necessary and medically consistent to perform the biopsies using a local anaesthetic. To maintain our focus on the small burn, we specifically avoided anaesthetic with epinephrine as this experiment was not meant to model the situation experienced by the patient with the large total body surface area burn.[8] The same dose and type of injected local anaesthesia was used for each sample, and so any possible effect of the lidocaine should have been consistent between samples and would not have significantly affected the results as the major comparisons were between pre-injury and subsequent collection times.
There are clear limitations in this study. First, the sample is small, predominantly female, exclusively Caucasian and from disparate anatomic regions. Given the sample size, it is impossible to know what if any effect each of these factors may have on the result. However, given the exploratory nature of this study as well as the available resources expanding the study in this otherwise poorly described population was not possible. Secondly, using a small volume biopsy and bead-array technology did not allow for additional histologic descriptors of the wound and resultant scar. However, this methodology has been previously reported in skin and scar investigations.[9] Third, using k-means relies on a pre-specified number of clusters and assigning all outliers to a cluster.[10] This, in addition to choosing random centers for each cluster may result in different cluster outputs each time the k-means is applied. The use of CLASSIFI was meant to enrich the interpretation of the k-means clustering by using the functional Gene Ontology classification.
We selected the top 1% differentially expressed genes for our candidates to take a liberal approach within this small sample size. The follow up clustering and CLASSIFI analysis were undertaken to explore for genes related less by participant by more by the temporal patterns and Gene Ontology classifications.
Overall, the response to injury is strikingly consistent between subjects. This cohort provides unique insight into the skin transcriptional changes that occur after burn injury. Other studies have described transcription differences between normal skin and scar, however, there was considerable variation in sampling time and none that investigated untreated human wounds or included pre-injury samples for comparison. Some consistencies were observed, including the late and ongoing up-regulation of COL1A1 expression seen in this experiment and other descriptions of burn scarring.[9, 11] In addition, two new significant findings are described here.
First is the intense transient response of the cluster 7 genes, which includes interleukin-8 and activin A receptor type 1B. Considering the important role that prolonged inflammation and the Smad / TGF-beta signaling proteins are thought to have in scar development, this finding implies that there may be an opportunity to intervene early after injury to investigate potential scar modification treatments rather than later after the scar has been established.[12-15]
Second is the sustained down-regulation of loricrin and other products of cornification three months post injury despite wound healing (figure 2D). Although keratinocyte – dermal fibroblast communication is thought to be significant in regulating dermal collagen production in scars, less has been described regarding the function of burn scar-related keratinocytes. Burn survivors describe challenges in managing ambient temperature and humidity as barriers to return to work, suggesting that abnormal epidermal function is impactful in determining disability associated with burn injury.[16]
Acknowledgments
Funding Sources: National Institute of Health and National Center for Research Resources, The University of Texas Southwestern Medical Center at Dallas Clinical Translational Science Initiative, 5 KL2 RR024983-02 ; National Center for Medical Rehabilitation Resources at the NIH, and the Association of Academic Physiatrists K12 HD1001097-13
This work was performed in Dallas, Texas, United States. It is attributable to The University of Texas Southwestern Medical Center at Dallas and the University of Calgary
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conclusion: Uninjured human skin has consistent responses to hot contact burn injury as described by bead-array technology. The practice of aesthetic scarification may allow for further burn and scar research to be conducted without treatment confounders.
Contributor Information
Vincent A. Gabriel, Division of Physical Medicine and Rehabilitation, Departments of Clinical Neurosciences, Surgery and Pediatrics, Alberta Children's Hospital Research Institute, Firefighters’ Burn Treatment Centre, University of Calgary.
Elizabeth A. McClellan, Department of Mathematical and Computer Sciences, Metropolitan State University of Denver, Denver, CO, USA emcclel3@msudenver.edu.
Richard H. Scheuermann, Division of Informatics, J. Craig Venter Institute, San Diego, CA, USA rscheuermann@jcvi.org.
References
- 1.Esselman PC. Burn rehabilitation: an overview. Archives of physical medicine and rehabilitation. 2007;88(12 Suppl 2):S3–6. doi: 10.1016/j.apmr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Zhu KQ, et al. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15(Suppl 1):S32–9. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engrav LH, Garner WL, Tredget EE. Hypertrophic scar, wound contraction and hyperhypopigmentation. Journal of burn care & research : official publication of the American Burn Association. 2007;28(4):593–7. doi: 10.1097/BCR.0B013E318093E482. [DOI] [PubMed] [Google Scholar]
- 4.Lee JA, et al. Components of the antigen processing and presentation pathway revealed by gene expression microarray analysis following B cell antigen receptor (BCR) stimulation. BMC bioinformatics. 2006;7:237. doi: 10.1186/1471-2105-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Kumar PR. Skin branding. Journal of postgraduate medicine. 2004;50(3):204. [PubMed] [Google Scholar]
- 6.Karamanoukian R, et al. Aesthetic skin branding: a novel form of body art with adverse clinical sequela. Journal of burn care & research : official publication of the American Burn Association. 2006;27(1):108–10. doi: 10.1097/01.bcr.0000191958.51354.cd. [DOI] [PubMed] [Google Scholar]
- 7.Brooks TL, et al. Body modification and substance use in adolescents: is there a link? The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2003;32(1):44–9. doi: 10.1016/s1054-139x(02)00446-9. [DOI] [PubMed] [Google Scholar]
- 8.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 9.Paddock HN, et al. Analysis of gene expression patterns in human postburn hypertrophic scars. The Journal of burn care & rehabilitation. 2003;24(6):371–7. doi: 10.1097/01.BCR.0000095508.96754.E0. [DOI] [PubMed] [Google Scholar]
- 10.Kerr G, et al. Techniques for clustering gene expression data. Computers in biology and medicine. 2008;38(3):283–93. doi: 10.1016/j.compbiomed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, et al. Gene expression of early hypertrophic scar tissue screened by means of cDNA microarrays. The Journal of trauma. 2004;57(6):1276–86. doi: 10.1097/01.ta.0000108997.49513.dc. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Ferrer M, et al. Dermal transforming growth factorbeta responsiveness mediates wound contraction and epithelial closure. The American journal of pathology. 2010;176(1):98–107. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tredget EE, et al. Transforming growth factorbeta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFNalpha and IFNgamma in vitro and in vivo. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2000;20(2):143–51. doi: 10.1089/107999000312540. [DOI] [PubMed] [Google Scholar]
- 14.Sadick H, et al. TGFbeta1 antisense therapy modulates expression of matrix metalloproteinases in keloidderived fibroblasts. International journal of molecular medicine. 2008;22(1):55–60. doi: 10.3892/ijmm.22.1.55. [DOI] [PubMed] [Google Scholar]
- 15.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. The Journal of pathology. 2013;229(2):298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esselman PC, et al. Barriers to return to work after burn injuries. Archives of physical medicine and rehabilitation. 2007;88(12 Suppl 2):S50–6. doi: 10.1016/j.apmr.2007.09.009. [DOI] [PubMed] [Google Scholar]