Abstract
Somatosensory feedback is important for the modulation of normal locomotion in adult animals, but we do not have a good understanding of when somatosensory information is first used to modulate motility during embryogenesis or how somatosensation is first used to regulate motor output. We used pyridoxine administration (vitamin B6), which is known to mostly kill proprioceptive neurons in adult mammals and embryonic chicks, to explore the role of proprioceptive feedback during early embryonic motility in the chick. Injection of pyridoxine on embryonic day 7 (E7) and E8 reduced the amplitude of leg movements recorded on E9 and the number of large, healthy neurons in the ventral-lateral portion of the DRGs. We conclude that proprioception is initially used during embryogenesis to modulate the strength of motor output, but that it is not incorporated into other aspects of pattern generation until later in development as poly-synaptic pathways develop.
Keywords: chick, embryo, proprioception, pyridoxine, kinematics, motility, somatosensation, central pattern generator
The requirement for sensory feedback to coordinate motor activity in adults is unequivocal. However, the role that somatosensory information plays during early spontaneous embryonic motility is not clear. In order for somatosensation to play a role in motility, it stands to reason that functional sensory circuitry must first be established. In chicks, spontaneous movement begins 4 days after an egg is laid (embryonic day 4 - E4). It take roughly one day for a fertilized egg to be laid by the hen and roughly 21 more days for the embryo to hatch. Monosynaptic connections between proprioceptive neurons and motor neurons are first formed at the end of E7 (Davis, Frank, Johnson, & Scott, 1989; Lee, Koebbe, & O’Donovan, 1988). It is possible that proprioceptive information could be incorporated into motor patterns at this time. In fact, Oppenheim (1972) reported that reflex responses to flipping the limb are elicited as early as E7.5 and that responses to limb stroking appear by E8.5. However, the ability to evoke a reflexive behavior does not mean that sensory information is able to modulate spontaneous/intentional motility.
In the chick, observations of changes in electromyographic (Bekoff, 1976) and kinematic (Bradley, 1999; Sharp, Ma, & Bekoff, 1999) recordings between E9 and E13 have been suggested to result from sensory modulation of spinal/motor circuitry during this time. Likewise, results from experiments which manipulate physical constraints on the embryo are most consistent with the idea that sensory feedback may alter motility at this time. Bradley (1997) showed that acute reduction of amniotic fluid altered motility in E9 chicks. However, it is not clear from this study whether alterations were responsive, due to changes in sensory feedback, or passive, due to movement restriction. On the other hand, Bradley and Sibelski (2000) showed that although both intra- and inter-limb joint coordination could be altered by near immobilization of the ankle on E12, this manipulation did not cause changes on E9. Likewise, it has been shown in rat fetuses that limb yoking on E19–21 alters inter-limb coordination (Robinson, 2005; Robinson, Kleven, & Brumley, 2008). This time frame in rats (Kucera, Walro, & Reichler, 1988; 1989; Milburn, 1973) is similar to the E9-E13 period in chick with respect to sensorimotor development. Taken as a whole, these data strongly indicate that embryonic motility can be modulated by somatosensation shortly after synaptic incorporation, but these experiments do not explicitly determine what sensory modalities are responsible for these changes.
To more directly determine the role of somatosensation in modulating embryonic motility, it would be desirable to selectively remove a specific sensory modality and to then assay for changes in behavior. This is not an easy task, but pyridoxine toxicity is a reasonable approach. High doses of pyridoxine (vitamin B6) have been shown to mostly kill large proprioceptive neurons with somata in dorsal root ganglia (DRGs) and to cause severely ataxic locomotion in many adult mammals (human - Schaumburg, Kaplan, Windebank, Vick, Rasmus, Pleasure, & Brown, 1983; rat and dog - Antropol and Tarlov, 1942; rat and guinea pigs - Xu, Sladky, & Brown, 1989; rat - Helgren, Cliffer, Torrento, Cavnor, Curtis, DiStefano, Wiegand, & Lindsay, 1997; Krinke, Naylor, & Skorpil, 1985; cat - Pearson, Misiaszek, & Hulliger, 2003). Unfortunately, the mechanism of pyridoxine toxicity is unknown and damage to large diameter cutaneous neurons has been reported (e.g. Pearson et al., 2003). None the less, pyridoxine toxicity in cats has been used to gain an understanding of how proprioception is involved in balance (Stapley, Ting, Hulliger, & Macpherson, 2002) and recovery from peripheral nerve injury (Pearson et al., 2003).
It is now known that pyridoxine administration in the chick embryo on E7 and E8 results in the loss of most large diameter, TrkC positive, sensory neurons when assayed on E13 (Sharp and Fedorovich, submitted). Additionally, this treatment spares motor neurons and small diameter sensory neurons expressing substance P and CGRP. As greater than 90% of TrkC positive neurons are proprioceptive in chick embryos (Oakley, Garner, Large, & Frank, 1995; Oakley, Lefcort, Plouffe, Ritter, & Frank, 2000), this suggests that pyridoxine is more selective in chicks than in mammals or that it is more selective during embryogenesis. Therefore, we used injections of pyridoxine to explore the ability of one somatosensory modality, proprioception, to modulate spontaneous motility of E9 chick embryos
METHODS
Animals
All procedures on chick embryos were in compliance with the guidelines of the University of Colorado and Southern Illinois University Institutional Animal Care and Use Committees. A total of 30 animals was used in this study.
Embryonated White Leghorn eggs (Gallus gallus domesticus) were obtained from a local supplier and incubated under standard conditions. Eggs were collected the day they were laid and held at 55°F which suspends development until they are placed in the incubator. E0 was defined as the day eggs were incubated. Eggs were removed from the incubator on E7 and E8 and candled to reveal the location of blood vessels in the chorioallentoic membrane. A position free of blood vessels was selected and 100 µl of physiological saline (PBS), or PBS containing 3.25, 7.5 or 15 mg of pyridoxine hydrochloride (Sigma), was injected into the egg just under the chorioallentois. Pyridoxine was then absorbed into the blood supply via the extra-embryonic blood vessels.
Establishment of pyridoxine dosage
A quick screen for an appropriate pyridoxine dosage was conducted on four embryos. Each of the animals was treated with saline on E7 and E8 containing one of the following amounts of pyridoxine: 0, 3.25, 7.5 or 15 mg. Dosages higher than this were not tested as 20 mg of pyridoxine will not dissolve directly in 100 µl of PBS.
Kinematic recordings
All embryos used for behavioral analysis were injected with PBS alone (n=14) or PBS containing 7.5 mg of pyridoxine (n=12). Kinematic recording of the right leg were made on E9 as previously described (Sharp et al., 1999). Briefly, the shell was opened and the extra-embryonic membranes were carefully incised and deflected. Embryos were then adhered to two paddles on the back with tissue adhesive and the position of leg joints were indicated with fingernail polish. Ten to twenty minutes of motility were recorded at 60 frames/s via a stereo microscope equipped with a color video camera. Embryos were then euthanized with CO2 gas (AVMA 1993). Approximately 10 minutes of motility were analyzed for each animal (6 animals/treatment group). Each period of analysis began with the onset of the first movement in the recording. Only complete motility episodes or inter-episode intervals were tabulated during the 10 minute period. Five episodes of motility from each embryo with durations that most closely bracketing the mean duration of untreated E9 embryos (29 s; Sharp et al., 1999) were digitized for joint angle measurements using Motus software (Peak Performance Technologies Inc., Englewood, CO).
Statistical analysis
Measurements of episode duration, inter-episode interval, joint (ankle, knee and hip) angles (maximum, minimum and rest) and joint excursion were made as previously described (Sharp et al., 1999). To normalize for slight differences in marker placement on the embryos, the magnitude of joint extension and flexion from rest were calculated as the absolute value of the joint maximum or minimum angle minus the resting angle. The data were grouped into five categories 1) episode duration, period and inter-episode interval, 2) joint excursions, 3), 4) and 5) ankle, knee and hip angles (extension, flexion and rest). Mean values from each animal were compared for each category using MANOVA. P<0.01 was required for statistical significance for each category thus yielding a total significance level of 0.05. The Tukey-Kramer post hoc test (P<0.05) was used for determining significance within each category.
Anatomy
Embryos which survived the dosage establishment experiment were collected on E9 for anatomical assessment. Spinal columns were dissected and the lumbosacral region was immersion-fixed in 4% paraformaldehyde over night. They were embedded in paraffin, sectioned at 10 µm and stained with cresyl violet. The third lumbosacral DRG on the right side was then analyzed. All large cells in the ventral-lateral region with distinct nuclear membranes, at least one nucleolus and large cytoplasmic volume were counted at 400× in every fourth section to prevent counting the same cell more than once (e.g. Clarke and Oppenheim, 1995).
RESULTS
Upon examination at E9, all animals in the pyridoxine dosage establishment group, except the one treated with 15 mg of pyridoxine, were alive and healthy. While it is not certain that 15 mg of pyridoxine is truly lethal, a dosage of 7.5 mg of pyridoxine was selected for the behavioral studies.
Of the animals used for behavioral analysis, three pyridoxine-treated animals and one saline-injected animal died prior to examination on E9. The remaining animals were all healthy and normal in appearance on E9. This indicated that a dosage of 7.5 mg of pyridoxine was not a general health problem for the embryos and those that died most likely sustained lethal damage from the injection process itself. 3 pyridoxine-treated and 7 saline-treated embryos did not survive preparation for kinematic recording.
Motility episodes
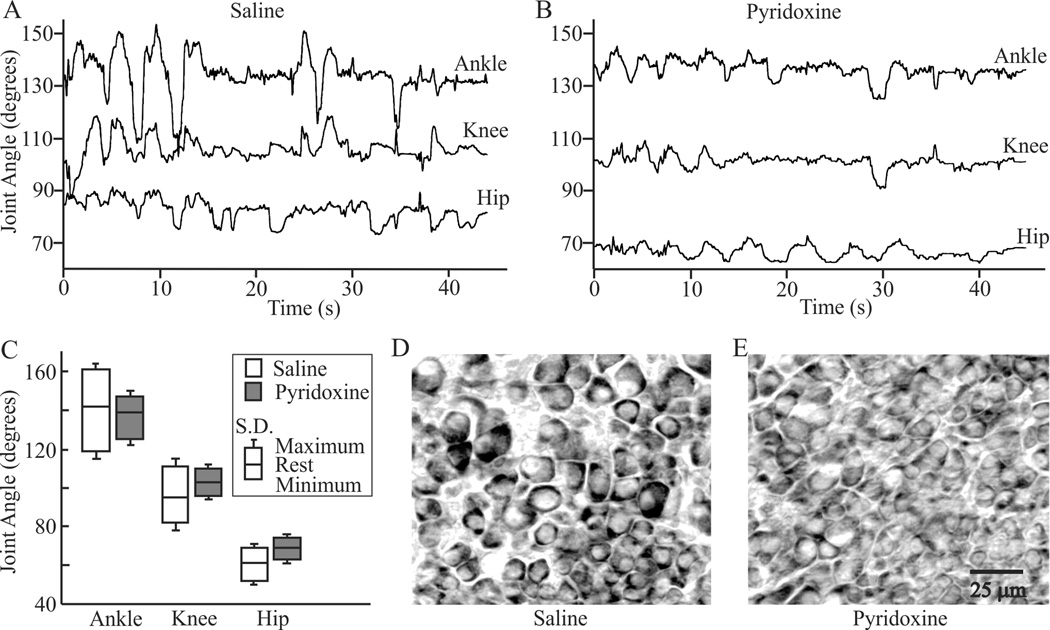
Both groups in this study displayed spontaneous, episodic motility patterns as is typical at E9 (e.g. Bradley, 1999; Hamburger and Balaban, 1963; Sharp et al., 1999; Watson and Bekoff, 1990). Selecting five motility episodes from each animal for kinematic analysis resulted in the analysis of 59% and 71% of motility episodes for saline and pyridoxine treated animals respectively. Recordings from saline treated animals (Fig. 1A) displayed characteristic alternation of extension and flexion of the joints with a strong correlation of movement among the joints as has been reported previously for normal embryos (Bradley, 1999; Sharp et al., 1999; Watson and Bekoff, 1990). Pyridoxine treatment (Fig. 1B) did not alter the patterning of extension and flexion or the appearance of coordination among the limbs, but it did reduce the amplitude of movements. This was apparent particularly for the ankle which shows the greatest range of movement in untreated animals (Sharp et al., 1999). There was a significant effect of pyridoxine treatment on excursions (Wilk’s lambda (λ) = 0.18, F(1,34) = 11, P = 0.005). Post hoc comparison of mean excursion for each joint showed a significant decrease in excursion caused by pyridoxine treatment for each joint (ankle (saline, pyridoxine; mean +/− s.d.): 41° +/− 7.0, 23° +/− 5.2; knee: 29° +/− 6.5, 15° +/− 4.6; hip: 17° +/− 3.3, 11° +/− 3.7).
Figure 1.
Effects of pyridoxine on E9 leg motility and DRG morphology. Representative joint angle vs. time plots of the ankle, knee and hip for individual motility episodes are shown from saline- (A) and pyridoxine-treated (B) embryos. Notice that the general pattern of concurrent extension and flexion of the joints is maintained after pyridoxine treatment. C) Average joint angle characteristics for the ankle knee and hip of saline- and pyridoxine-treated embryos. The middle line of each box represents the average resting angle. The average joint extension and flexion are shown relative to the resting angle as the top and bottom lines of the boxes respectively. Standard deviations from the mean extension and flexion are shown as error bars. Representative images of small and large neurons in the third lumbosacral DRG from (D) saline and (E) pyridoxine-treated embryos. Images are from the ventral lateral edge of each ganglion.
The excursion of a joint is the result of activity from extensor and flexor muscles acting across a joint to alter the relative position of limb segments from their resting positions. To understand better how pyridoxine treatment resulted in the reduction of joint excursion, the effect of pyridoxine treatment on these specific joint features (extension, flexion, resting angle, Fig. 1C) were examined for each joint. Pyridoxine had a significant effect on these features for the ankle (λ = 0.18, F(1,34) = 19, P = 0.0009) and the knee (λ = 0.30, F(1,34) = 9.5, P = 0.0077), but not the hip (λ = 0.40, F(1,34) = 6.1, P = 0.024). Post hoc comparisons for the ankle showed that pyridoxine caused a significant reduction in extension (19° +/− 3.3, 8° +/− 3) and flexion (23° +/− 3.8, 14° +/− 2.9), but not in the resting angle (142° +/− 7.1, 139° +/− 4.9). Likewise, for the knee, pyridoxine caused a significant reduction in extension (16° +/− 3.7, 7° +/− 2) and flexion (13° +/− 3.9, 7° +/− 2), but not in the resting angle (95° +/− 6.1, 103° +/− 9.4). Pyridoxine did cause non-significant decreases in hip extension (8° +/− 2, 5° +/− 2) and flexion (9° +/−1, 6° +/−2) from the resting values (61° +/− 12, 69° +/− 7.0), but it required the summation of these alterations to result in the significant reduction in hip excursion. Therefore, the effects of pyridoxine on leg movements were greatest distally and decreased moving proximally.
Periodicity of movement
The duration of leg movement episodes and inter-episode intervals was examined as previously described (Sharp et al., 1999). An episode of activity was defined as any movement of the right leg that was separated from other movements of the leg by 10 seconds or more of inactivity. The frequency distributions of episode duration and interval are summarized in Table 1. The frequency distribution of movement duration was bimodal for both treatments as has been described for normal (un-injected) embryos (Sharp et al., 1999) and both means were quite similar (saline: 30 +/− 22 s; pyridoxine - 32 +/− 17 s). Pyridoxine treatment caused a small decrease in the number of total movement episodes (51 to 42) resulting from a shift in the average number of movement episodes/10 minutes (8 +/− 2 to 7 +/− 2) that was not tested for significance. Inter-episode intervals were broadly spread between 10 and 100 s for both saline- and pyridoxine-treated animals with means showing a high degree of variability (saline: 48 +/− 26 s; pyridoxine: 64 +/− 34 s). Also, the difference in periodicity (episode duration + subsequent inter-episode interval) for saline- (77 +/− 36 s) and pyridoxine-treated animals (95 +/− 43 s) was quite variable. Pyridoxine treatment did not have a significant effect on any of these means (λ = 0.76, F(1,34) = 0.72, P = 0.59)
Table 1.
Occurrences of Episode Duration (Dur) and Interval (Int) tabulated in 5 second intervals (< or = time indicated below) from six Saline (S) and six Pyridoxine (P) treated Embryos
| Time (s) | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dur (S) | 11 | 3 | 2 | 1 | 4 | 7 | 3 | 2 | 6 | 2 | 4 | 1 | 3 | 1 | 1 | |||||
| Dur (P) | 8 | 2 | 6 | 4 | 8 | 7 | 5 | 1 | 1 | |||||||||||
| Int (S) | 3 | 8 | 3 | 2 | 2 | 3 | 4 | 3 | 1 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 3 | |||
| Int (P) | 2 | 3 | 1 | 2 | 2 | 2 | 3 | 5 | 4 | 4 | 3 | 1 | 1 |
Note: 3 additional occurrence for Int (P) at 145, 150 and 151 s.
Anatomy
We examined the lumbosacral DRGs and spinal cord of three E9 embryos: one saline treated, one treated with 7.5 mg of pyridoxine and one treated with 3.75 mg of pyridoxine. We observed no gross differences between the treated and control spinal cords. However, a significant number of large neurons in the ventral-lateral portion of the DRGs, considered to be proprioceptive (Ia) afferents (Willis and Coggeshall, 2004), in treated chicks lacked clear nuclear profiles (Fig. 1H and I). Instead, the nuclei were uniformly dark, similar to dying neurons (e.g. Antropol and Tarlov, 1942). The smaller neurons in the dorsal-medial area of the DRGs appeared normal. The number of normal appearing neurons in the ventral-lateral portion of the third lumbosacral DRG on the right side of the embryos was reduced by pyridoxine treatment in a dose dependent manner: saline - 808, 3.75 mg - 454 and 7.5 mg - 80.
DISCUSSION
The purpose of this study was to determine if the removal of proprioception via pyridoxine overdose would result in altered embryonic motility and thus indicate that proprioception is used to modulate spinal central pattern generating circuitry at this time. Our results show that pyridoxine administration on E7 and E8, as synapses from proprioceptive neurons are forming in the spinal cord of the chick (Davis et al., 1989; Lee et al., 1988), alters one component of E9 embryonic motility. Specifically, joint excursions of the ankle, knee and hip are reduced. While the reduction in extension and flexion of the ankle and knee caused by pyridoxine are all independently significant, it requires the concurrent consideration of reductions in extension and flexion of the hip to reveal a significant reduction in hip excursion. Interestingly, periodic characteristics of motility episodes as well as the apparent coordination among the joints during extension and flexion are not altered.
It has been shown in chick that administration of 3.75 mg of pyridoxine, half the dose used in this study, on E7 and E8 causes the loss of large diameter TrkC-positive neurons while sparing small diameter substance P- and CGRP-positive sensory neurons as well as motor neurons (Sharp and Fedorovich, submitted). While it has not been determined if large diameter cutaneous neurons are damaged by pyridoxine in chick, it is likely that the majority of this lesion is proprioceptive in nature. As in adult mammals shortly after pyridoxine administration (Antropol and Tarlov, 1942; Krinke et al., 1985), we observed in this study that many of the large neurons in the ventral-lateral portion of the DRGs appeared to be in a state of nuclear degeneration and that there was a very large reduction in the number of normal ventral-lateral neurons. We did not observe any other gross changes in the DRGs of spinal cords of pyridoxine-treated embryos unlike the decrease in size of these structures previously reported on E13 (Sharp and Fedorovich, submitted). It is likely that at E9 there has been insufficient time for the complete degeneration of damaged neurons. None the less, the changes in embryonic behavior observed after pyridoxine treatment suggest that these degenerating neurons are no longer functionally able to transduce or relay sensory information normally.
The reductions in joint excursion observed in this study suggest that proprioceptive feedback on E9 acts as a gain control mechanism to increase the amplitude of motor output as has been described for the Ia afferent system in adult mammals (Pearson, Misiaszek, & Fouad, 1998). Gain control though the adult pathway is bi-synaptic or poly-synaptic. It is not clear if pyridoxine-sensitive gain control on E9 is mono-synaptic or poly-synaptic. It is possible that gain control could be accomplished by a direct synaptic connection from Ia afferents onto motor neurons, but this does not rule out the possibility of a bi- or poly-synaptic pathway as well. Additionally, the reduction in movement amplitude could be the result of other spinal-motor learning mechanisms that could be occurring during the two day treatment period, though such a mechanism has not been identified to date.
Immobilization of the ankle of E12 chicks (Bradley and Sibelski, 2000) or limb yoking on E19–21 in rat (Robinson, 2005; Robinson et al., 2008) gives rise to changes in movement of the wing or contra-lateral limb, respectively. As no mono-synaptic connection can account for these changes, poly-synaptic mechanisms have been assumed to account for these changes. It is possible that the changes described in this current study represent modulation of the spinal central pattern generator prior to the establishment of the complex poly-synaptic pathways that were revealed by the immobilization and yoking studies. However, generalized loss of proprioception does not allow for the assessment of mono- vs. poly-synaptic pathways with the experimental design used in this study. Likewise, the experimental design of limb immobilization or yoking precludes a complete assessment for the possibility of mono-synaptic modulation of the obstructed limb.
Unlike its role in adult locomotion (Pearson, Misiaszek, & Fouad, 1998), proprioceptive feedback is not acting to coordinate phase transitions at E9 in the chick. Instead, alternation of extension and flexion among joints appears to be controlled by the spinal components of the motor circuits. This is consistent with the ability of the isolated embryonic spinal cord to generate fictive locomotor patterns (Landmesser and O’Donovan, 1984). It is likely that the role of proprioceptive information becomes more complex as higher order synapses develop and poly-synaptic pathways from other sensory systems become integrated. These more sophisticated modes of modulation are likely to become more necessary as the embryo begins to encounter spatial restrictions within the shell.
If proprioception is simply acting as a gain mechanism during E9 motility, why then does pyridoxine not reduce the amplitude of extension and flexion of all leg joints uniformly? More specifically, why are the decreases in hip extension and flexion not significant independently? The differences in how proprioception regulates joint excursion of each joint could arise from differential sensory innervation of flexor and extensor muscles that control each of the joints. For example, hip muscles may not have much proprioceptive innervation or high synaptic efficacy and this time. A more trivial explanation could be that changes in the hip angle are not very large and a combination of experimental noise, variability in embryonic movement and limited sensory feedback simply results in changes too small to exceed signal to noise issues. Alternatively, the biomechanics of moving the limb through the fluidic embryonic environment could generate this type of difference as the drag exerted on each limb segment could be significantly different. For example, the drag on more distal components of the leg such as the foot, which is very long relative to the rest of the leg at this stage, may require greater sensory feedback to allow for proper regulation of ankle movements. Greater sensory regulation of distal limb segments may be required additionally to assist in regulating general limb length as the embryo becomes constrained by its spatial limits. Further directed physiological studies as well as modeling studies should be conducted to resolve these issues.
In conclusion, our results indicate that proprioception is playing an important role in the regulation of spontaneous embryonic motility only 1.5 days after monosynaptic sensory projections onto motor neurons are first formed. Since the loss of proprioceptive neurons gives rise to a generalized decrease in joint excursion, it appears that proprioceptive feedback on E9 provides a direct gain control mechanism for each muscle or muscle synergy. Most likely, the role of proprioception in modulating embryonic motility then becomes more complex as higher order synapses develop. Embryonic pyridoxine lesion should be a useful tool to explore further the role of proprioception in the regulation of embryonic and adult motility as well as a means to determine the necessity of embryonic sensory experience for proper sensorimotor development.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (F32 HD-07884 to A.A.S) and the National Science Foundation (IBN9630498 to A.B.).
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Andrew A. Sharp, Email: asharp@siumed.edu.
Anne Bekoff, Email: Anne.bekoff@colorado.edu.
REFERENCES
- Antropol W, Tarlov IM. Experimental study of the effects produced by large doses of vitamin B6. Journal of Neuropathology and Experimental Neurology. 1942;1:330–336. [Google Scholar]
- AVMA. Report of the AVMA on euthanasia. Journal of the American Veterinary Medical Association. 1993;202:230–249. [PubMed] [Google Scholar]
- Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Research. 1976;106:271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Reduction in buoyancy alters parameters of motility in E9 chick embryos. Physiology and Behavior. 1997;62:591–595. doi: 10.1016/s0031-9384(97)00168-6. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Transformations in embryonic motility in chick: kinematic correlates of type I and II motility at E9 and E12. Journal of Neurophysiology. 1999;81:1486–1494. doi: 10.1152/jn.1999.81.4.1486. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Sebelski C. Ankle restraint modifies motility at E12 in chick embryos. Journal of Neurophysiology. 2000;83:431–440. doi: 10.1152/jn.2000.83.1.431. [DOI] [PubMed] [Google Scholar]
- Clark PGH, Oppenheim RW. Neuron death in vertebrate development: in vitro methods. Methods in Cell Biology. 1995;46:277–321. [PubMed] [Google Scholar]
- Davis BM, Frank E, Johnson FA, Scott SA. Development of central projections of lumbosacral sensory neurons in the chick. Journal of Comparative Neurology. 1989;279:556–566. doi: 10.1002/cne.902790405. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Balaban M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Developmental Biology. 1963;7:533–545. doi: 10.1016/0012-1606(63)90140-4. [DOI] [PubMed] [Google Scholar]
- Helgren M, Cliffer K, Torrento K, Cavnor C, Curtis R, DiStefano P, Wiegand S, Lindsay R. Neurotrophin-3 administration attenuates deficits of pyridoxine-induced large-fiber sensory neuropathy. Journal of Neuroscience. 1997;17:372–382. doi: 10.1523/JNEUROSCI.17-01-00372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke G, Naylor DC, Skorpil V. Pyridoxine megavitaminosis: an analysis of the early changes induced with massive doses of vitamin B6 in rat primary sensory neurons. Journal of Neuropathology and Experimental Neurology. 1985;44:117–129. [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Innervation of developing intrafusal muscle fibers in the rat. American Journal of Anatomy. 1988;183:344–358. doi: 10.1002/aja.1001830408. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Role of nerve and muscle factors in the development of rat muscle spindles. American Journal of Anatomy. 1989;186:144–160. doi: 10.1002/aja.1001860205. [DOI] [PubMed] [Google Scholar]
- Landmesser LT, O’Donovan MJ. Activation patterns of embryonic chick hindlimb muscles recorded in-ovo and in an isolated spinal cord preparation. Journal of Physiology. 1984;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Koebbe MJ, O’Donovan MJ. The development of sensorimotor synaptic connections in the lumbosacral spinal cord of the chick embryo. Journal of Neuroscience. 1988;8:2530–2543. doi: 10.1523/JNEUROSCI.08-07-02530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn A. Early development of muscle spindles in the rat. Journal of Cell Science. 1973;12:175–195. doi: 10.1242/jcs.12.1.175. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Garner AS, Large TH, Frank E. Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development. 1995;121:1341–1350. doi: 10.1242/dev.121.5.1341. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Plouffe P, Ritter A, Frank E. Neurotrophin-3 promotes the survival of a limited subpopulation of cutaneous sensory neurons. Developmental Biology. 2000;224:415–427. doi: 10.1006/dbio.2000.9804. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. An experimental investigation of the possible role of tactile and proprioceptive stimulation in certain aspects of embryonic behavior in the chick. Developmental Psychobiology. 1972;5:71–91. doi: 10.1002/dev.420050109. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Annals of the New York Acadamy of Sciences. 1998;16:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Hulliger M. Chemical ablation of sensory afferents in the walking system of the cat abolishes the capacity for functional recovery after peripheral nerve lesions. Experimental Brain Research. 2003;150:50–60. doi: 10.1007/s00221-003-1445-1. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: Evidence for motor learning in the rat fetus. Developmental Psychobiology. 2005;47:328–344. doi: 10.1002/dev.20103. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Kleven GA, Brumley MR. Prenatal development of interlimb motor learning in the rat fetus. Infancy. 2008;13:204–228. doi: 10.1080/15250000802004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg H, Kaplan J, Windebank A, Vick N, Rasmus S, Pleasure D, Brown MJ. Sensory neuropathy from pyridoxine abuse. The New England Journal of Medicine. 1983;309:445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- Sharp AA, Ma E, Bekoff A. Developmental changes in leg coordination of the chick at embryonic days 9, 11, 13: uncoupling of ankle movements. Journal of Neurophysiology. 1999;82:2406–2414. doi: 10.1152/jn.1999.82.5.2406. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. Journal of Neuroscience. 2002;22:5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Bekoff A. A kinematic analysis of hindlimb motility in 9- and 10-day old chick embryos. Journal of Neurobiology. 1990;21:651–660. doi: 10.1002/neu.480210412. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord: volume 1: primary afferent neurons and the spinal dorsal horn. New York: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]