Abstract
Staphylococcus aureus is the most commonly isolated gram-positive bacterium after lung transplantation and has been associated with poor post-transplant outcomes, but its effect on BOS and death in the context of the allograft inflammatory environment has not been studied. A three-state Cox semi-Markovian model was used to determine the influence of allograft S. aureus and the ELR+ CXC chemokines on the survival rates and cause-specific hazards for movement from lung transplant (State 1) to BOS (State 2), from transplant (State 1) to death (State 3), and from BOS (State 2) to death (State 3). Acute rejection, pseudomonas pneumonia, BALF CXCL5 and its interaction with S. aureus all increased the likelihood of transition from transplant to BOS. Transition to death from transplant was facilitated by pseudomonas infection and single lung transplant. Movement from BOS to death was affected by the interaction between aspergillus, pseudomonas and CXCL5, but not S. aureus. S. aureus isolation had state specific effects after lung transplantation and only in concert with elevated BALF CXCL5 concentrations did it augment the risk of BOS. Pseudomonas and elevated BALF concentrations of CXCL5 continued as significant risk factors for BOS and death after BOS in lung transplantation.
Introduction
The high incidence of chronic lung allograft dysfunction, predominantly due to bronchiolitis obliterans syndrome (BOS), limits long-term lung transplantation (LT) success. Acute cellular rejection (ACR) is the most well accepted risk factor for the development of chronic lung allograft dysfunction, but there is an growing list of infectious agents known to augment the hazard for development of BOS (1–4). Despite this infectious diversity, both gram-negative and gram-positive bacteria remain the most commonly isolated pathogens after LT (5–7). Staphylococcus aureus is the most frequently isolated gram-positive organism at many transplant centers (8–11). Some studies have found that gram-positive pulmonary infections, largely composed of S. aureus, are associated with the development of BOS (1,12,13). Moreover, gram-positive infections have been associated with increased bronchoalveolar lavage fluid (BALF) neutrophilia (14), which may indicate the presence of glutamic-acid-leucine-arginine-positive (ELR+) CXC chemokine production and presage BOS.
We recently demonstrated that infection after transplantation by the gram-negative bacterium Pseudomonas aeruginosa is a risk factor for subsequent BOS, as are high BALF concentrations of the ELR+ CXC chemokine epithelial-derived neutrophil attractant-78 (ENA-78 or CXCL5) (15). Based on these prior findings, we hypothesized that S. aureus pulmonary isolation interacts with the allograft inflammatory milieu and affects the recipient’s post-transplant “state”. As patients move between the states of Post-Transplantation, BOS and Death, different post-transplant events, such as S. aureus isolation, may have varying impacts. Such impacts can be investigated via a Markovian analysis that measures the intensity of moving from one state to another. The advantage of the Markovian analysis is that it allows us to compare the covariate effects on each transition state, or outcome, without imposing censoring on competing outcomes. The semi-Markovian model allows for the time in the current state, as well as the entry time into the state, to be factored into the risk of transitioning to the next state. This provides a flexible framework for studying and comparing the effects of prognostic factors on transitions between multiple health states. Our objectives of this study were to employ a multi-state Cox Semi-Markov model to determine the impact of S. aureus isolation and BALF CXCL5 levels on BOS, death and death after BOS.
Materials and Methods
Patient Population
Three-hundred fifty-five recipients of lung transplants at the University of California, Los Angeles (UCLA) who had BALF specimens collected between January 1, 2000 and December 31, 2008 were evaluated. This cohort has been investigated previously (15). All samples had adequate microbiologic results, 281 had adequate PFT results and 209 had complete chemokine data and are included for analysis. The evaluable population are those 209 for whom all data was available. Follow-up data through December 31, 2009 are included. Each participant provided written, informed consent under a UCLA Institutional Review Board-approved protocol (#13-000462).
Clinical Definitions and Sample Processing
Post-transplant immunosuppression, prophylaxis, pulmonary function testing and bronchoscopy was carried out according to protocols as previously reported (16). Acute rejection ≥1 and BOS ≥ Stage 1 were determined according to standard criteria (17–19). All chemokine data was obtained from BALF specimens. Donor cuff cultures were not considered in this analysis.
“Staphylococcus infection” required isolation of Staphylococcus aureus and documentation of new shortness of breath, hypoxia, altered sputum production or radiographic infiltrate. Isolations of Staphylococcus aureus lacking any of these findings are considered “colonization.” BALF preparation and determination of chemokine concentration was carried out as previously reported (16, 20). Patients were given induction and placed on immunosuppression and prophylactic antibiotics as previously described (16). Rapamycin and maintanence azithromycin was not initiated prior to the diagnosis of BOS in any study patient. Rejection episodes were treated as previously described (16).
Statistical Methods
The movement of lung transplant recipients between states of transplantation, BOS and death can be characterized by a multi-state process (Figure 1). In this process, individuals start as recipients of a lung transplant (denoted by State 1). After transplantation, patients may (a) develop BOS (from State 1 to State 2), (b) die (from State 1 to State 3) or (c) die after developing BOS (from State 2 to State 3). Cox-type models were used to investigate the effects of transplant type, ACR, aspergillus, S. aureus, pseudomonas, and BALF ELR+ CXC chemokines (growth related protein-α (CXCL1), epithelial-derived neutrophil attractant-78 (CXCL5), neutrophil activating peptide-2 (CXCL7) and IL-8 (CXCL8)) on each transition (21,22). Episodes of ACR, aspergillus, S. aureus and pseudomonas are treated as time-dependent covariates to assess the effects of zero to multiple episodes. Due to the low number of S. aureus positive cultures in our cohort that were associated with clinical infection (pneumonia), we were unable to separate the effects of S. aureus pneumonia/infection from colonization within the above the models and covariates and thus use any isolation as the S. aureus variable. The multistate process can be fully characterized through transition intensities between states. The transition intensities can be modeled using Cox-like models and they represent the instantaneous hazard of progression to the next state conditionally on the current occupying state. However, the main focus of this paper is not to derive transition probabilities; rather it is to estimate transition-specific covariate effects. For transition hazards λ12 and λ13, we have and . For the transition from BOS to death, transition hazard λ23 is modeled as a function of the time spent in State 1 through a Cox semi-Markov model: . In the above formulae, λij,0 (t) are the baseline hazards from state i to j, Zij,k (t) are vectors of covariates of interest at time t for the k th patient, βij are the corresponding vectors of regression coefficients, T2 is the time of BOS, i,j∈{1,2,3}. Tests for significance were two-tailed with a statistically significant p-value threshold of 0.05. The median was used and the interquartile range is given in parenthesis immediately after, while the lower and upper limits of the hazard ratio (HR) are given in parenthesis following the HR. Analyses are performed in SAS version 9.2 (SAS Institute Inc., Cary, NC) (23) and R version 3.0.1 (24).
Figure 1.
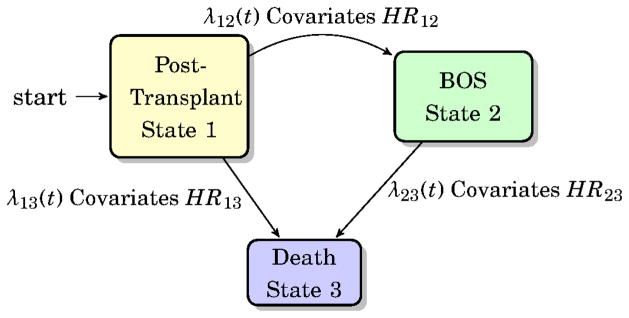
Transition model schema demonstrating the transition states.
Results
Lung Transplant Patient Population and Clinical Outcomes
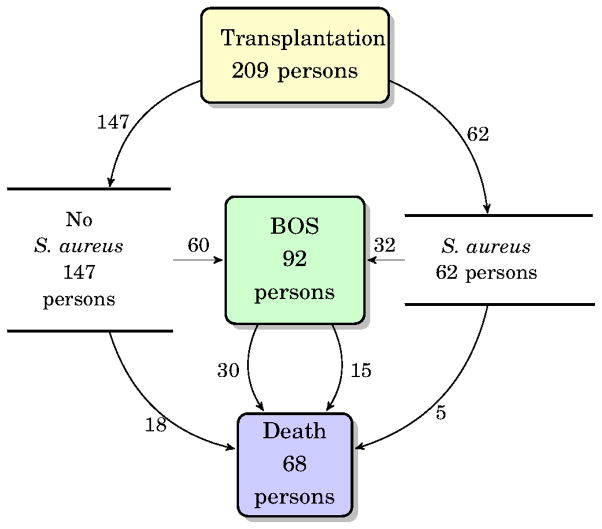
We analyzed data from 209 LT recipients for whom microbiologic, pathologic, BALF for chemokine analysis and BOS outcomes were available (Table 1). Ninety-two (44%) of these individuals developed BOS of grade ≥1 at a median time post transplantation of 693 (640) days (Figure 2). There were 253 episodes of ACR at 372 (756) days post-transplant in 131 individuals (63%). Sixty-eight LT recipients died during the follow-up (1049 (945) days) and 45 of those had developed BOS prior to death.
Table 1.
Demographics table overall frequencies and outcomes.
| No BOS (N=117) | BOS (N=92) | Combined (N=209) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Single Transplant | 43 | 37% | 36 | 39% | 79 | 38% | 0.73 |
| Diagnosis | 0.59 | ||||||
| Cystic fibrosis/bronchiectasis | 8 | 7% | 4 | 4% | 12 | 6% | |
| Obstructive | 41 | 35% | 38 | 41% | 79 | 38% | |
| Restrictive | 64 | 55% | 45 | 49% | 109 | 52% | |
| Vascular | 4 | 35% | 5 | 5% | 9 | 4% | |
| Aspergillus Episodes | 0.008 | ||||||
| 0 | 76 | 65% | 37 | 40% | 113 | 54% | |
| 1 | 22 | 19% | 34 | 37% | 56 | 27% | |
| 2 | 14 | 11% | 11 | 12% | 25 | 12% | |
| 3 | 2 | 2% | 2 | 2% | 4 | 2% | |
| 4 | 2 | 2% | 2 | 2% | 4 | 2% | |
| 6 | 1 | 1% | 4 | 5% | 5 | 2% | |
| 8 | 0 | 0% | 2 | 2% | 2 | 1% | |
| Pseudomonas Episodes | 0.002 | ||||||
| 0 | 90 | 76% | 46 | 50% | 136 | 65% | |
| 1 | 15 | 13% | 20 | 22% | 35 | 17% | |
| 2 | 5 | 4% | 12 | 13% | 17 | 8% | |
| 3 | 2 | 2% | 0 | 0% | 2 | 1% | |
| 4 | 2 | 2% | 7 | 8% | 9 | 4% | |
| 5 | 2 | 2% | 5 | 5% | 7 | 3% | |
| 7 | 1 | 1% | 1 | 1% | 2 | 1% | |
| 8 | 0 | 0% | 1 | 1% | 1 | 0% | |
| Pseudomonas Colonization | 0.016 | ||||||
| 0 | 92 | 78% | 51 | 56% | 143 | 69% | |
| 1 | 13 | 11% | 20 | 22% | 33 | 16% | |
| 2 | 3 | 6% | 10 | 11% | 17 | 8% | |
| 3 | 0 | 0% | 4 | 4% | 4 | 2% | |
| 4 | 7 | 3% | 4 | 4% | 7 | 3% | |
| 5 | 1 | 1% | 2 | 2% | 3 | 1% | |
| 7 | 1 | 1% | 1 | 1% | 2 | 1% | |
| Pseudomonas Infection | 0.003 | ||||||
| 0 | 112 | 96% | 74 | 81% | 186 | 89% | |
| 1 | 5 | 4% | 12 | 13% | 17 | 9% | |
| 2 | 0 | 0% | 3 | 3% | 3 | 1% | |
| 3 | 0 | 0% | 3 | 3% | 3 | 1% | |
| Acute Rejection Episodes | 0.06 | ||||||
| 0 | 51 | 43% | 27 | 29% | 78 | 37% | |
| 1 | 36 | 31% | 29 | 32% | 65 | 31% | |
| 2 | 18 | 15% | 17 | 18% | 35 | 16% | |
| 3 | 10 | 9% | 7 | 8% | 17 | 8% | |
| 4 | 2 | 2% | 7 | 8% | 9 | 4% | |
| 5 | 0 | 0% | 3 | 3% | 3 | 2% | |
| 7 | 0 | 0% | 1 | 1% | 1 | 1% | |
| 9 | 0 | 0% | 1 | 1% | 1 | 1% | |
| Staph Episodes | 0.60 | ||||||
| 0 | 87 | 74% | 60 | 65% | 147 | 71% | |
| 1 | 19 | 16% | 21 | 23% | 40 | 19% | |
| 2 | 8 | 7% | 8 | 9% | 16 | 8% | |
| 3 | 1 | 1% | 2 | 2% | 3 | 1% | |
| 4 | 2 | 2% | 1 | 1% | 3 | 1% | |
| Time to BOS/Censored | <0.001 | ||||||
| Lower quartile | 693 | 405 | 531 | ||||
| Median | 1028 | 651 | 828 | ||||
| Upper quartile | 1533 | 945 | 1354 | ||||
| Median SD | (1153+/−689) | (739+/−449) | (971 +/− 629) | ||||
| Time from Transplant to Death/Censored | |||||||
| Lower quartile | 693 | ||||||
| Median | 1028 | ||||||
| Upper quartile | 1533 | ||||||
| Median SD | (1153+/−689) | ||||||
| Time from BOS to Death/Censored | |||||||
| Lower quartile | 155 | ||||||
| Median | 333 | ||||||
| Upper quartile | 741 | ||||||
| Median SD | (476+/−430) | ||||||
Figure 2.
The overall outcomes for study subjects. The number of individuals reaching each state is shown within each state box. Sixty-two of the subjects had an isolation of S. aureus, while 147 had no isolation of S. aureus.
Episodes of Staphylococcus
In the 355 LT recipients for whom microbiologic data was available, there were 248 isolations of S. aureus from respiratory secretions in 109 persons at a median of 1 (55) day post-transplantation over the study period, supporting the fact that S. aureus is a common isolate in this population (11,13). In the evaluable population, those with adequate PFT and chemokine results, there were 92 isolations considered not to be infection at a median of 43 (162) days post-transplantation in 61 persons. There were 11 isolations classified as pneumonia in 9 persons. The median days to S. aureus pneumonia was 518 (620) (Figure 2).
Movement from Post-Transplantation to BOS (State 1 to State 2)
Acute cellular rejection, overall pseudomonas, BALF CXCL5 and S. aureus, via an interaction with CXCL5, were significantly associated with an increased risk of BOS development after LT (Table 2). BALF CXCL5 concentrations did augment the risk of BOS whether S. aureus was present or not, but its impact was greater when S. aureus was present (HR, 1.09 per 100 pg/mL increase [1.01–1.17]; P=0.03) than when it was absent (HR, 1.01 per 100 pg/mL [1.00–1.02]; P=0.02). That is, there was an 8.8% increase in the hazard for BOS when S. aureus was present. The interaction between CXCL5 and staphylococcus was such that the effect of S. aureus on BOS was only present as BALF concentrations of CXCL5 increased (S. aureus present and CXCL5 50 pg/mL, HR 1.03 [0.64–1.65]; P=0.92, versus S. aureus present and CXCL5 1200 pg/mL HR, 2.45 [1.00–5.96]; P=0.05). Pseudomonas overall (HR, 1.89 [1.18–3.01]; P=0.01) and acute rejection (HR, 1.92 [1.22–3.00]; P=0.005) episodes also increased the hazard for progression to BOS after lung transplantation (Table 2). In this cohort, there was no statistically significant effect from aspergillus.
Table 2.
Transition from State 1 to State 2 (Post-transplantation to BOS). This model includes CXCL5 and the interaction between staphylococcus and CXCL5.
| Significance of Covariates | P-value | |||
|---|---|---|---|---|
| Transplant Type | 0.167 | |||
| Aspergillus Status | 0.181 | |||
| Acute Rejection Status | 0.005 | |||
| Pseudomonas Status | 0.008 | |||
| Staph Status | 0.962 | |||
| CXCL5 level | 0.021 | |||
| Staph Status * CXCL5 level | 0.047 | |||
| Covariate Effects Testing | HR | Lower Limit | Upper Limit | P-value |
| Transplant:Single vs Double | 1.35 | 0.88 | 2.08 | 0.167 |
| Aspergillus: Yes vs None | 1.37 | 0.86 | 2.16 | 0.181 |
| Acute Rejection: Yes vs None | 1.92 | 1.22 | 3.00 | 0.005 |
| Pseudumonas Overall: Yes vs None | 1.89 | 1.18 | 3.01 | 0.008 |
| Staph: Yes vs None, at CXCL5 50 pg/mL | 1.03 | 0.64 | 1.65 | 0.915 |
| Staph: Yes vs None, at CXCL5 100 pg/mL | 1.07 | 0.67 | 1.71 | 0.790 |
| Staph: Yes vs None, at CXCL5 200 pg/mL | 1.15 | 0.72 | 1.83 | 0.558 |
| Staph: Yes vs None, at CXCL5 600 pg/mL | 1.56 | 0.89 | 2.72 | 0.122 |
| Staph: Yes vs None, at CXCL5 1200 pg/mL | 2.45 | 1.00 | 5.96 | 0.049 |
| Staph: Yes vs None, at CXCL5 1600 pg/mL | 3.31 | 1.04 | 10.51 | 0.042 |
| Staph: Yes vs None, at CXCL5 2000 pg/mL | 4.48 | 1.07 | 18.79 | 0.040 |
| Difference in the effect of having Staph present, at CXCL5=200 vs at CXCL5=25 | 1.16 | 1.02 | 1.32 | 0.026 |
| Difference in the effect of having Staph present, at CXCL5=400 vs at CXCL5=25 | 1.37 | 1.04 | 1.81 | 0.026 |
| Effect of CXCL5 per 100 pg/mL increase, when Staph not present (Staph = No) | 1.01 | 1.00 | 1.02 | 0.021 |
| Effect of CXCL5 per 100 pg/mL increase, when Staph is present (Staph = Yes) | 1.09 | 1.01 | 1.17 | 0.026 |
| Difference in the effect of CXCL5 per 100 pg/mL increase, when Staph=Yes vs when Staph=No | 1.08 | 1.00 | 1.16 | 0.047 |
Movement from Post-Transplantation to Death (State 1 to State 3)
Death without BOS was associated with pseudomonas infection (HR, 4.17 [1.56–11.17]; P=0.004), not colonization, and type of transplant (HR, 2.94 for single lung transplant [1.39–6.22]; P=0.005) (Table 3), with single LT being detrimental. The ELR+ CXC chemokines, including CXCL5, were not associated with death before BOS. Aspergillus, ACR and S. aureus isolation were also not significant co-variates in this transition.
Table 3.
Transition from State 1 to State 3 (Post-transplantation to Death). Only Ps. aeruginosa infections and single lung transplant were associated with death.
| Significance of Covariates | P-value | |||
|---|---|---|---|---|
| Transplant Type | 0.005 | |||
| Acute Rejection Status | 0.629 | |||
| Aspergillus Status | 0.277 | |||
| Pseudomonas Colonization Status | 0.539 | |||
| Pseudomonas Infection Status | 0.004 | |||
| Staph Status | 0.611 | |||
| Covariate Effects Testing | HR | Lower Limit | Upper Limit | P-value |
| Transplant:Single vs Double | 2.94 | 1.39 | 6.22 | 0.005 |
| Acute Rejection: Yes vs None | 0.83 | 0.40 | 1.74 | 0.629 |
| Aspergillus: Yes vs None | 1.53 | 0.71 | 3.30 | 0.277 |
| Pseudomonas Colonization: Yes vs None | 1.29 | 0.57 | 2.88 | 0.539 |
| Pseudomonas Infection: Yes vs None | 4.17 | 1.56 | 11.17 | 0.004 |
| Staph: Yes vs None | 0.80 | 0.34 | 1.88 | 0.611 |
Movement from BOS to Death (State 2 to State 3)
The likelihood of transitioning to death after BOS was increased by the interactions between aspergillus, pseudomonas and CXCL5 (Table 4), but not S. aureus. The interaction between aspergillus and any pseudomonas (infection or colonization) was an inclusive disjunction, such that once isolation of either organism had occurred any further isolations of either organism did not further increase the risk of death (Table 4). Pseudomonas and CXCL5 also interacted, thus consideration of the impact of pseudomonas must account for the presence or absence of aspergillus as well as the concentration of CXCL5, as shown in Table 4. As the concentration of CXCL5 increased, the contributory effect on death from pseudomonas decreased, such that once the levels of CXCL5 were extremely high the impact of pseudomonas was of borderline significance (P=0.06 when CXCL5 concentration is 600 pg/mL), likely because individuals with such elevated levels of CXCL5 were at high risk of death regardless of pseudomonas status. Transitioning to death after BOS was not influenced by S. aureus nor other ELR+ CXC chemokines.
Table 4.
Transition from State 2 to State 3 (BOS to Death). Model demonstrates the effect of aspergillus, CXCL5 and their interactions with Pseudomonas.
| Significance of Covariates | P-value | |||
|---|---|---|---|---|
| Transplant Type | 0.357 | |||
| Acute Rejection Status | 0.572 | |||
| Aspergillus Status | 0.002 | |||
| Pseudumonas Overall Status | <.0001 | |||
| Aspergillus Status * Pseudumonas Overall Status | 0.004 | |||
| Staph Status | 0.275 | |||
| CXCL5 level | 0.003 | |||
| Pseudumonas Overall Status * CXCL5 level | 0.002 | |||
| Covariate Effects Testing | HR | Lower Limit | Upper Limit | P-value |
| Transplant:Single vs Double | 1.34 | 0.72 | 2.52 | 0.357 |
| Acute Rejection: Yes vs None | 0.74 | 0.26 | 2.09 | 0.572 |
| Staph: Yes vs None | 1.86 | 0.61 | 5.65 | 0.275 |
| Effect of Pseudomonas Present versus None, when aspergilllus=None and CXCL5=50 pg/mL | 7.23 | 3.27 | 15.99 | <.0001 |
| Effect of Pseudomonas Present versus None, when aspergilllus=None and CXCL5=100 pg/mL | 6.38 | 2.95 | 13.82 | <.0001 |
| Effect of Pseudomonas Present versus None, when aspergilllus=None and CXCL5=200 pg/mL | 4.97 | 2.34 | 10.59 | <.0001 |
| Effect of Pseudomonas Present versus None, when aspergilllus=Present and CXCL5=200 pg/mL | 0.64 | 0.18 | 2.24 | 0.482 |
| Effect of Aspergillus Present vs None, when Pseudomonas=None | 3.87 | 1.63 | 9.16 | 0.002 |
| Effect of Aspergillus Present vs None, when Pseudomonas=Present | 0.49 | 0.15 | 1.65 | 0.252 |
| Effect of per CXCL5 100 pg/mL increase, when Pseudomonas=None | 1.27 | 1.08 | 1.48 | 0.003 |
| Effect of per CXCL5 100 pg/mL increase, when Pseudomonas=Present | 0.99 | 0.95 | 1.03 | 0.543 |
Discussion
Based on our clinical approach to patients in different states post lung transplantation (e.g., before or after BOS), we leveraged the advantages of a Cox Semi-Markov multistate model to allow the assessment of specific hazard ratios for each covariate at different stages post-transplantation, which we have previously shown to be an important consideration in interpretation of covariate effect. S. aureus, via an interaction with the ELR+ CXC chemokine CXCL5, did increase the risk of BOS development after lung transplantation. Additionally, pseudomonas infection and ACR episodes increased the risk of developing BOS, while pseudomonas infection and single LT increased the risk of death before BOS. We were unable to show an effect for S. aureus in the transition to death after transplantation (State 1 to State 3), but this may have been due either to small numbers of persons with S. aureus pneumonia, or to surprisingly good outcomes for the general transplant population despite the presence of S. aureus, as discussed below. S. aureus was also not a statistically significant covariate in the transition to death after BOS (State 2 to State 3) possibly because of few episodes of S. aureus after BOS, but as we have shown previously, pseudomonas is via an interaction with aspergillus and CXCL5 (15). These data suggest that pseudomonas and S. aureus interact with the ELR+ CXC chemokine CXCL5 to negatively impact LT outcomes in a state dependent manner.
Transition to BOS is Affected by S. aureus, CXCL5, Overall Pseudomonas and Acute Rejection
S. aureus isolation was not a risk factor for the development of BOS without an interaction with CXCL5. In this model, pseudomonas pneumonia was alone sufficient to increase the risk of BOS, as was ACR, both of which are known risk factors (15,25–28). It is possible that had we been able to compare S. aureus pneumonia with colonizations we may have found a significant effect of S. aureus pneumonia on its own as others have (1,12). A recent evaluation of S. aureus infection in a large cohort of LT recipients found an association of S. aureus with the development of BOS, but the BALF milieu was not considered and neither were known competing risks for BOS such as aspergillus, ACR or pseudomonas (13). In fact, that study found an association of S. aureus with subsequent development of acute cellular rejection, which suggests that S. aureus itself may not be responsible for the increased risk of BOS per se, but rather the increased frequency of ACR. In our model, the presence of S. aureus demonstrated an increased effect on BOS development given concurrent BALF CXCL5 concentration increases. Indeed, BALF CXCL5 concentrations were strongly supportive of the S. aureus effect, raising the possibility that the BALF inflammatory environment including CXCL5 is an important driver in the progression to BOS, as evidenced by the fact that CXCL5 was alone a risk for BOS as it was in our previous investigation (15). Nevertheless, “multiple hits” via CXCL5 interaction with S. aureus appear to augment the risk.
Pseudomonas Infection and Type of Transplant Affect Death Before BOS
Consistent with our prior findings, only pseudomonas infection and single lung transplantation were risks in our model for death before BOS. We would not necessarily anticipate that BALF chemokine levels should predict overall death. Although death from S. aureus pneumonia is certainly possible, as it is from pseudomonas pneumonia, it did not increase the hazard for death. It is possible that this is due to smaller numbers of S. aureus pneumonia episodes as compared to pseudomonas pneumonia which precluded us from analyzing S. aureus pneumonia separately from non-pneumonia. However, others have shown a lower than expected incidence of death from S. aureus in transplant populations. One study looked at S. aureus bacteremia univariate outcomes in 70 solid organ transplants, 26 of whom were lung transplants who had the highest rates of S. aureus bacteremia, and found that although the risk of death was greater for those with pneumonia as the primary source, organ transplant recipients had lower 30-day mortality rates than non-transplanted controls (29). Shields, et al also found a much lower than expected 30-day mortality in a large cohort of lung transplant patients with S. aureus (13).
Transition to Death after BOS
As in our prior investigations, any pseudomonas or aspergillus isolation was a risk for death via their interaction, and via an interaction with CXCL5 for pseudomonas. This interaction involves pseudomonas and CXCL5, and pseudomonas and aspergillus. Because we cannot consider pseudomonas in this transition without considering CXCL5, this becomes a three-way interaction. Both pseudomonas and aspergillus are risk factors for death. However, there is an interaction between them. This interaction is such that once either pseudomonas or aspergillus have been isolated, subsequent isolation of either organism does not further increase the risk of death. The established risk of death is however not negated nor reduced. Thus, the risk increases once either pseudomonas or aspergillus are isolated, but isolating either again does not further increase the risk of death. We hypothesize that this is due to a persistently elevated inflammatory environment within the graft including elevated CXCL5 levels, such that a further increase in the hazard for death cannot, in our model, be detected. This does suggest that once a patient is in State 2 (BOS) an added insult such as aspergillus or pseudomonas easily places the patient at risk for death.
Perhaps surprisingly S. aureus isolation was not a risk factor for death after BOS. High concentrations of CXCL5 increased the risk of transitioning to death after BOS, hence when the allograft milieu is highly inflamed, as evidenced by markedly elevated CXCL5 concentrations, there is less influence from the presence of pseudomonas or aspergillus. We had previously hypothesized that the allograft milieu is at a “heightened” state of inflammation once BOS develops, which would allow more minor insults, such as pseudomonas or aspergillus colonization, to hasten death. It seems however, that S. aureus isolation was not a sufficient insult in our cohort, or levels of CXCL5 were alone enough to allow for progression of death via its inflammatory effect independent of S. aureus. Both pseudomonas and aspergillus are often chronic colonizers of lung transplant recipients and could therefore be realistically expected to produce a more meaningful, subacute to chronic alteration of the allograft milieu affecting progression of underlying allograft dysfunction. Chronic colonization of the lower airways with S. aureus was rare in our cohort and in the lung transplant population in general, especially as compared with the cystic fibrosis population in whom, at least one study has found an association with decline in lung function (30). Nevertheless, the clinical implication is that efforts should be put toward eradication of aspergillus and pseudomonas in BOS, rather than S. aureus, if our findings are validated in future, larger multi-center studies.
Limitations of the present study include the number of staphylococcal pneumonia episodes precluding us from separating S. aureus pneumonia from non-pneumonia, which may hinder our ability to detect a significant effect from pneumonia due to this organism. Furthermore, the single-center design limits generalization of these findings to other centers. We were also unable to include many more BOS risk factors in the model due to sample size and lack of uniform data collection for such variables as primary graft dysfunction, lymphocytic bronchitis, GERD and BALF neutrophilia. The exclusion of such variables is unlikely to alter our main finding that S. aureus is by itself not a significant risk factor for the subsequent development of BOS.
In conclusion, we have shown that S. aureus isolation has state specific effects after lung transplantation and that only in concert with elevated BALF CXCL5 concentrations does it augment the risk of BOS. We have also found that pseudomonas and elevated BALF concentrations of the ELR+ CXC chemokine CXCL5 continue as significant risk factors for BOS and death after BOS in lung transplantation.
Acknowledgments
ALG received grant support from K23 HL102220 and a Cystic Fibrosis Foundation Pilot and Feasibility Grant, JAB from R01 HL112990 and CTOT 11532596, SSW from K23 HL094746 and the Joyce & Saul Brandman Foundation Fund, and GL from NIH CA016042, U54 RR031268 and P01 AT003960. The project was also supported by the National Center for Advancing Translational Sciences through UCLA CTSI grant UL1 TR000124.
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- BALF
bronchoalveolar lavage fluid
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Valentine VG, Gupta MR, Walker JE, Seoane L, Bonvillain RW, Lombard GA, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transpl. 2009;28:163–9. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, et al. Aspergillus Colonization of the Lung Allograft Is a Risk Factor for Bronchiolitis Obliterans Syndrome. Am J Transplant. 2009;9:1903–11. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigt SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, et al. Altered Levels of CC Chemokines During Pulmonary CMV Predict BOS and Mortality Post-Lung Transplantation. Am J Transplant. 2008;8:1512–22. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigt SS, Derhovanessian A, Liao E, Hu S, Gregson AL, Kubak BM, et al. CXCR3 Chemokine Ligands During Respiratory Viral Infections Predict Lung Allograft Dysfunction. Am J Transplant. 2012;12:477–84. doi: 10.1111/j.1600-6143.2011.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parada MT, Alba A, Sepulveda C. Early and late infections in lung transplantation patients. Transplant Proc. 2010;42:333–5. doi: 10.1016/j.transproceed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Guisado M, Givalda J, Ussetti P, Ramos A, Morales P, Blanes M, et al. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007;7:1989–96. doi: 10.1111/j.1600-6143.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 7.Horvath J, Dummer S, Loyd J, Walker B, Merrill WH, Frist WH. Infection in the transplanted and native lung after single lung transplantation. Chest. 1993;104:681–5. doi: 10.1378/chest.104.3.681. [DOI] [PubMed] [Google Scholar]
- 8.Valentine VG, Bonvillain RW, Gupta MR, Lombard GA, LaPlace SG, Dhillon GS, et al. Infections in lung allograft recipients: ganciclovir era. J Heart Lung Transpl. 2008;27:528–35. doi: 10.1016/j.healun.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Zeglen S, Wojarski J, Wozniak-Grygiel E, Siola M, Jastrzebski D, Kucewicz-Czech E, et al. Frequency of Pseudomonas aeruginosa colonizations/infections in lung transplant recipients. Transplant Proc. 2009;41:3222–4. doi: 10.1016/j.transproceed.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 10.Campos S, Caramori M, Teixeira R, Afonso J, Carraro R, Strabelli T, et al. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc. 2008;40:822–4. doi: 10.1016/j.transproceed.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Deusch E, End A, Grimm M, Graninger W, Klepetko W, Wolner E. Early bacterial infections in lung transplant recipients. Chest. 1993;104:1412–6. doi: 10.1378/chest.104.5.1412. [DOI] [PubMed] [Google Scholar]
- 12.Gupta MR, Valentine VG, Walker JE, Lombard GA, LaPlace SG, Seoane L, et al. Clinical spectrum of gram-positive infections in lung transplantation. Transpl Infect Dis. 2009;11:424–31. doi: 10.1111/j.1399-3062.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 13.Shields RK, Clancy CJ, Minces LR, Kwak EJ, Silveira FP, Abdel Massih RC, et al. Staphylococcus aureus infections in the early period after lung transplantation: epidemiology, risk factors, and outcomes. J Heart Lung Transplant. 2012;31:1199–206. doi: 10.1016/j.healun.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Vos R, Vanaudenaerde BM, Dupont LJ, Raemdonck DEV, Verleden GM. Transient airway colonization is associated with airway inflammation after lung transplantation. Am J Transplant. 2007;7:1278–87. doi: 10.1111/j.1600-6143.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 15.Gregson AL, Wang X, Weigt SS, Palchevskiy V, Lynch JP, 3rd, Ross DJ, et al. Interaction Between Pseudomonas and CXC Chemokines Increases Risk of BOS and Death in Lung Transplantation. Am J Respir Crit Care Med. 2013;187:518–26. doi: 10.1164/rccm.201207-1228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregson AL, Hoji A, Palchevskiy V, Hu S, Weigt SS, Liao E, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA- T regulatory cells. PloS ONE. 2010;5:e11354. doi: 10.1371/journal.pone.0011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transpl. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transpl. 1993;12:713–6. [PubMed] [Google Scholar]
- 19.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transpl. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 20.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–62. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen PK, Gill RD. Cox’s regression model for counting process: a large sample study. Ann Stat. 1982;10:1100–20. [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J Roy Stat Soc B Met. 1972;34:187–220. [Google Scholar]
- 23.Institute SAS. SAS/STAT user’s guide. SAS Institute; 2005. Version 9.1. [Google Scholar]
- 24.R Development Core Team. R: A Language and Environment for Statistical Computing. 2009 http://www.R-project.org.
- 25.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85:771–4. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 26.Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Van Raemdonck DE, Dupont LJ, Verleden GM. De novo or persistent pseudomonal airway colonization after lung transplantation: importance for bronchiolitis obliterans syndrome? Transplantation. 2008;86:624–5. doi: 10.1097/TP.0b013e318182295d. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb J, Mattner F, Weissbrodt H, Dierich M, Fuehner T, Strueber M, et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Resp Med. 2009;103:743–9. doi: 10.1016/j.rmed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care. 2004;170:1022–6. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 29.Malinis MF, Mawhorter SD, Jain A, Shrestha NK, Avery RK, van Duin D. Staphylococcus aureus bacteremia in solid organ transplant recipients: evidence for improved survival when compared with nontransplant patients. Transplantation. 2012;93:1045–50. doi: 10.1097/TP.0b013e31824bf219. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest. 2004;126:412–9. doi: 10.1378/chest.126.2.412. [DOI] [PubMed] [Google Scholar]