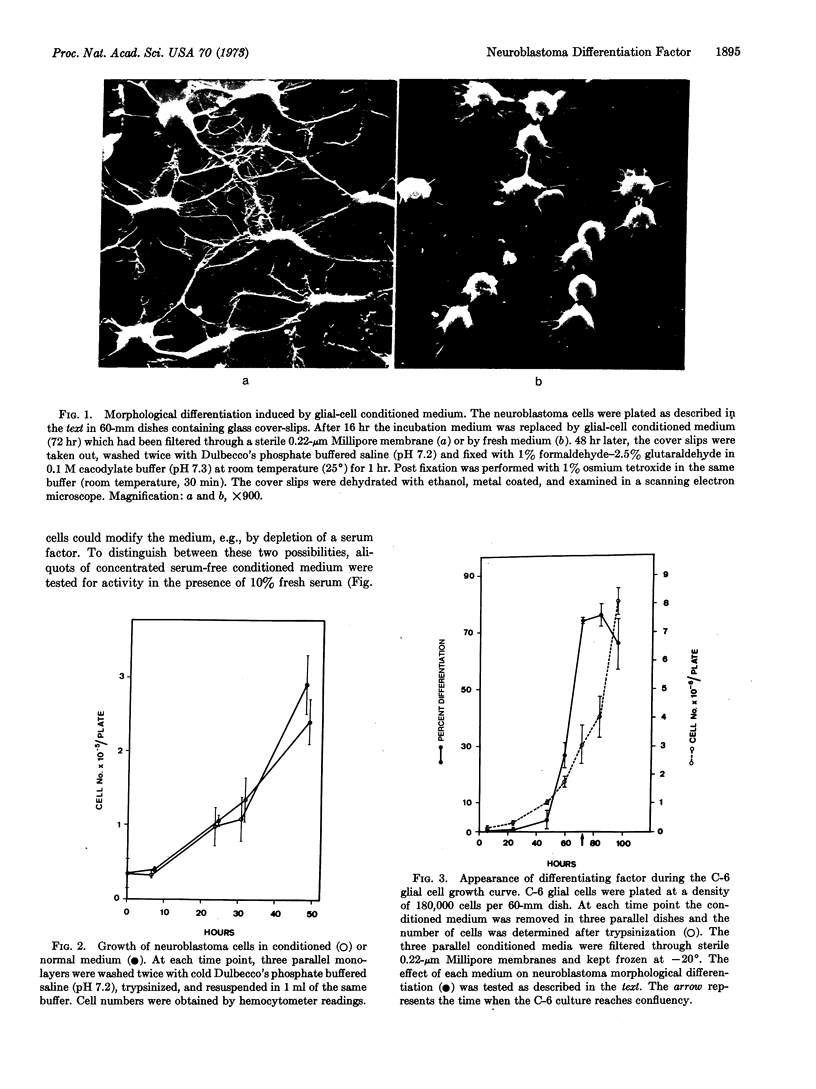
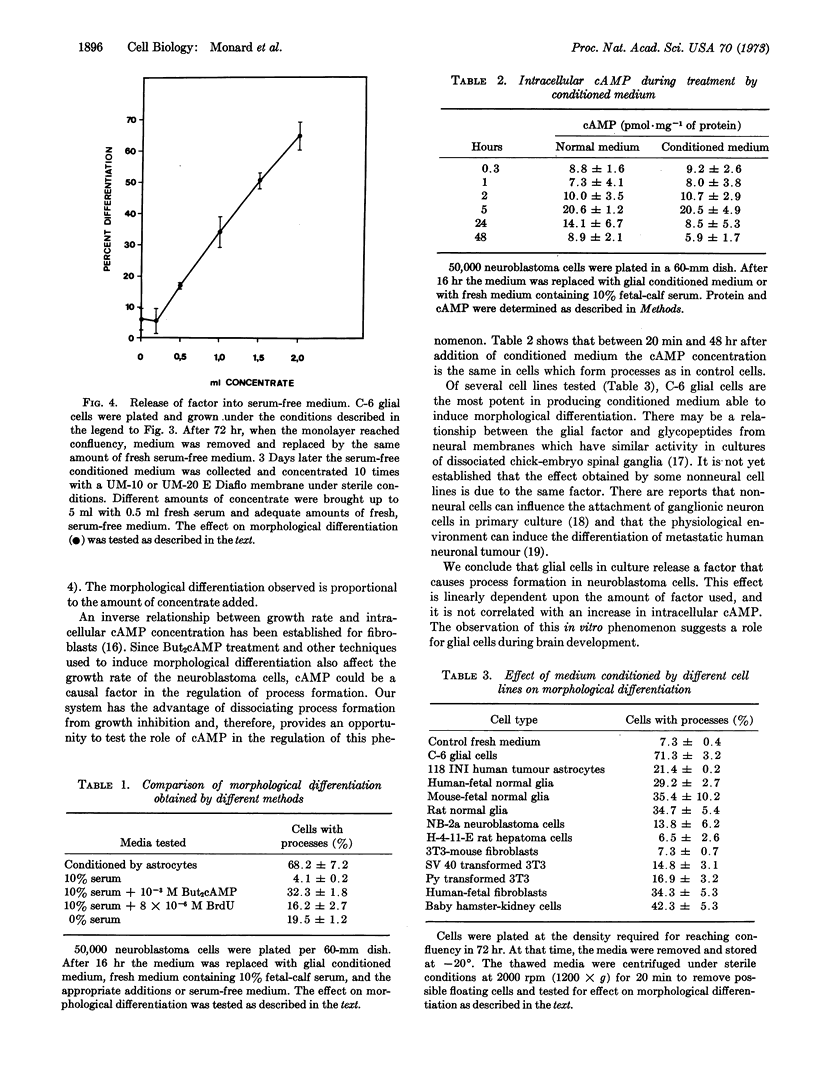
Abstract
Glial cells release a factor into their culture medium that induces a high degree of morphological differentiation in neuroblastoma cells under normal growth conditions. This phenomenon is not correlated with a change in intracellular adenosine 3′:5′-cyclic monophosphate or in the rate of cell growth. Media from other cell lines tested induce less morphological differentiation or have no effect.
Keywords: glial-neuronal interactions
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Burnham P., Raiborn C., Varon S. Replacement of nerve-growth factor by ganglionic non-neuronal cells for the survival in vitro of dissociated ganglionic neurons. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3556–3560. doi: 10.1073/pnas.69.12.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H., Wolbach S. B. The Transformation of a Malignant Paravertebral Sympathicoblastoma into a Benign Ganglioneuroma. Am J Pathol. 1927 May;3(3):203–216.7. [PMC free article] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos G., Hermetet J. C., Reeber A., Zanetta J. -P., Treska-Ciesielski J. The composition of glycopeptides, derived from neural membranes, which affect neurite growth in vitro. FEBS Lett. 1972 Aug 15;24(3):247–250. doi: 10.1016/0014-5793(72)80365-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Macintyre E. H., Wintersgill C. J., Perkins J. P., Vatter A. E. The responses in culture of human tumour astrocytes and neuroblasts to N 6 , O 2' -dibutyryl adenosine 3',5'-monophosphoric acid. J Cell Sci. 1972 Nov;11(3):639–667. doi: 10.1242/jcs.11.3.639. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pitot H. C., Jost J. P. Control of biochemical expression in morphologically related cells in vivo and in vitro. Natl Cancer Inst Monogr. 1967 Sep;26:145–166. [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. X-ray-induced morphological differentiation of mouse neuroblastoma cells in vitro. Nature. 1971 Dec 24;234(5330):471–473. doi: 10.1038/234471a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Habel K., Green H. Antigenic and cultural properties of cells doubly transformed by polyoma virus and SV40. Virology. 1965 Oct;27(2):179–185. doi: 10.1016/0042-6822(65)90157-1. [DOI] [PubMed] [Google Scholar]