Abstract
The current study investigated the effects of disrupting the septohippocampal theta system on the developmental emergence of delay eyeblink conditioning. Theta oscillations are defined as electroencephalographic (EEG) waveforms with a frequency between 3–8 Hz. Hippocampal theta oscillations are generated by inputs from the entorhinal cortex and the medial septum. Theta activity has been shown to facilitate learning in a variety of paradigms, including delay eyeblink conditioning. Lesions of the medial septum disrupt theta activity and slow the rate at which delay eyeblink conditioning is learned (Berry & Thompson, 1979). The role of the septohippocampal theta system in the ontogeny of eyeblink conditioning has not been examined. In the current study, infant rats received an electrolytic lesion of the medial septum on postnatal day (P)12. Rats were later given eyeblink conditioning for 6 sessions with an auditory conditioned stimulus on P17–P19, P21–23, or P24–P26. Lesions impaired eyeblink conditioning on P21–23 and P24–26 but not on P17–19. The results suggest that the septohippocampal system comes online to facilitate acquisition of eyeblink conditioning between P19 and P21. Developmental changes in septohippocampal modulation of the cerebellum may play a significant role in the ontogeny of eyeblink conditioning.
Keywords: development, rat pup, associative learning, eyelid conditioning, septum, theta, cerebellum
INTRODUCTION
Eyeblink conditioning is a type of associative motor learning that depends on the cerebellum (Freeman & Steinmetz, 2011). In the typical delay conditioning procedure, a conditioned stimulus (CS) that does not elicit eyelid closure before training is followed by an unconditioned stimulus (US) that elicits an eyelid closure unconditioned response before training. Repeated paired presentations of the CS and US result in the development of an eyelid closure conditioned response (CR) that precedes the onset of the US. Delay eyeblink conditioning depends on the cerebellum (McCormick & Thompson, 1984), which receives input related to the CS through the pontine mossy fiber projection and input related to the US through the climbing fibers of the inferior olive (Freeman & Steinmetz, 2011). Paired activation of the mossy and climbing fiber pathways during CS-US trials causes the induction of synaptic plasticity within the cerebellum, which constitutes the memory for eyeblink conditioning (Freeman & Steinmetz, 2011).
Evidence from decerebration experiments demonstrates that most of the forebrain is not necessary for delay eyeblink conditioning (Kotani, Kawahara, & Kirino, 2002; Lovick & Zebrozyna, 1975; Norman, Buchwald, & Villablanca, 1977). Nevertheless, physiological signals from forebrain structures such as the hippocampus modulate the rate of acquisition. One such signal is a 3–8 Hz theta oscillation in the local field potential in the hippocampus, which facilitates acquisition of delay eyeblink conditioning (Berry & Seager, 2001; Berry & Thompson, 1978, 1979; Seager, Johnson, Chabot, Asaka, & Berry, 2002). Berry and Thompson (1978) found that rabbits with greater pre-training levels of theta show faster eyeblink conditioning. They subsequently found that lesions of the medial septum disrupt theta oscillations and slow the rate of delay eyeblink conditioning (Berry & Thompson, 1979). Furthermore, coupling the delivery of the CS-US pairings with the presence of theta enhances conditioning relative to uncoupled controls (Seager et al., 2002). While it is unknown precisely how theta promotes conditioning, the mechanism seems to relate to coherent activity between the hippocampus and the cerebellum. During eyeblink conditioning, hippocampal theta oscillations become synchronized with oscillations in the cerebellar cortex and interpositus nucleus (Hoffmann & Berry, 2009; Wikgren, Nokia, & Penttonen, 2010), suggesting that theta oscillations may facilitate learning by promoting plasticity within the cerebellum.
Eyeblink conditioning with an auditory or visual CS becomes progressively stronger between postnatal day (P) 17 and P24 (Goldsberry, Elkin, & Freeman, 2014; Stanton, Freeman, & Skelton, 1992). Previous studies found that the ontogeny of delay eyeblink conditioning is influenced by the development of subcortical sensory inputs to the pontine nuclei and the development of inhibitory feedback from the cerebellum to the inferior olive (Campolattaro & Freeman, 2008; Freeman & Campolattaro, 2008; Freeman, Rabinak, & Campolattaro, 2005; Ng & Freeman, 2012; Nicholson & Freeman, 2003). Developmental changes in forebrain modulation of delay eyeblink conditioning might emerge in parallel with developmental changes in the CS or US pathways.
The development of septohippocampal modulation of delay eyeblink conditioning has not been investigated. In the current study, the medial septum (mSep) was lesioned on P12 in rats. The rats were subsequently given eyeblink conditioning on P17–19, P21–23, or P24–26. We hypothesized that the developmental emergence of septohippocampal modulation would parallel the ontogeny of eyeblink conditioning. Lesions of the medial septum should therefore cause an impairment in acquisition of eyeblink conditioning that increases in severity with increasing postnatal age.
METHODS
Subjects
Subjects were 51 infant Long Evans rats (female and male), counterbalanced for sex and condition, and trained on P17–19 (n=16), P21–23 (n=17), or P24–26 (n=18). All rats were given ad libitum access to food and water, and maintained on a 12 h day/night cycle. Pups trained on P21–23 or P24–26 were weaned on P19 and housed with littermates. Pups trained on P17–19 were returned to the dam and littermates following surgery and between training sessions. Experimental groups included no more than two pups from the same litter (one male and one female). All training occurred from 7 am to 7 pm. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Surgery
On P12, pups received either an electrolytic lesion or a control surgery of the medial septum. Pups were anesthetized with isoflurane (1–4%), and a craniotomy was performed with a 25G needle (+1.7 AP, +0.7 ML from bregma). Prior to surgery, lesioning electrodes were prepared by insulating stainless-steel insect pins (size 00, Austerlitz Insect Pins) with Epoxylite (E 6001, Elantas PDG, INC.), then removing insulation at the tip (~0.1 mm) of the electrode. The electrode was chemically sterilized (Cidex OPA, Advanced Sterilzation Products), secured to a stereotaxic arm, and lowered into the brain −5.3 mm at an angle 8.0° from the vertical. The electrode was angled to avoid damaging the blood vessels that run along the midline of the brain. A current of 1.0 mA (anodal, DC) was delivered with a stimulus isolator (World Precision Instruments, Sarasota FL) for 10 s for rats in the lesion condition. The duration of the surgery was approximately 45 min. Following the surgery, the incision was sutured, and the pups were returned to their home cage after recovery from anesthesia. We did not observe substantial behavioral changes in the pups given lesions after recovery from surgery/anesthesia in the group given lesions. At either P15, 19, or 22, these rats received a second surgery to implant differential electromyography (EMG) electrodes into the upper left orbicularis oculi muscle and a bipolar stimulating electrode for delivering the US caudal to the left eye (for details see Goldsberry et al., 2014).
Apparatus
A detailed description of the apparatus can be found in Goldsberry et al. (2014). Briefly, pups were trained in a conditioning chamber that was contained within a sound-attenuation chamber. Lightweight cables with connectors for both the recording EMG and the bipolar stimulating electrode were attached to a commutator. Computer software controlled the delivery of both CS and US while simultaneously recording differential eyelid EMG activity.
Conditioning Procedure
Eyeblink conditioning began two days following the second surgery. All rats received six 100-trial sessions of delay eyeblink conditioning in which a 400 ms tone CS (2.0 kHz, 85 dB) was followed by a 25 ms periorbital stimulation (2.5 mA) US. Sessions consisted of 90 paired CS-US trials and 10 CS-alone trials. The CS-alone trials were used to evaluate parameters of the CR in the absence of the UR. The inter-trial interval averaged 30 s. Each rat was trained for two sessions per day for 3 days, each session separated by a minimum of 3 hr.
Data Analysis
Behavioral data were examined offline. CRs were defined as any EMG activity during the CS that crossed a .4 unit threshold above the pre-CS baseline activity. Responses that occurred within 80 ms of CS onset were considered startle responses. Trials with EMG activity that crossed the threshold prior to the CS onset were omitted from the analysis. Repeated measures ANOVAs were performed on session data for CR percentage, amplitude, onset latency, and peak latency. Greenhouse-Geisser corrections were used when the assumption of sphericity was violated. CR amplitude, onset latency, and peak latency measures were examined on CS-alone trials in which a CR occurred. Significant group effects were further analyzed with the Tukey HSD Test. An alpha level of 0.05 was used for all statistical tests.
Histology
Following training, rats were perfused transcardially with saline and 10% formalin. Brains were then removed from the skull, blocked, and placed in a 30% sucrose formalin solution for cryoprotection. The brain was then sectioned at 50 µm using a sliding microtome. Sections were mounted on gelled slides and stained with thionin. The lesion placement was then assessed using light microscopy.
RESULTS
Lesion Placement
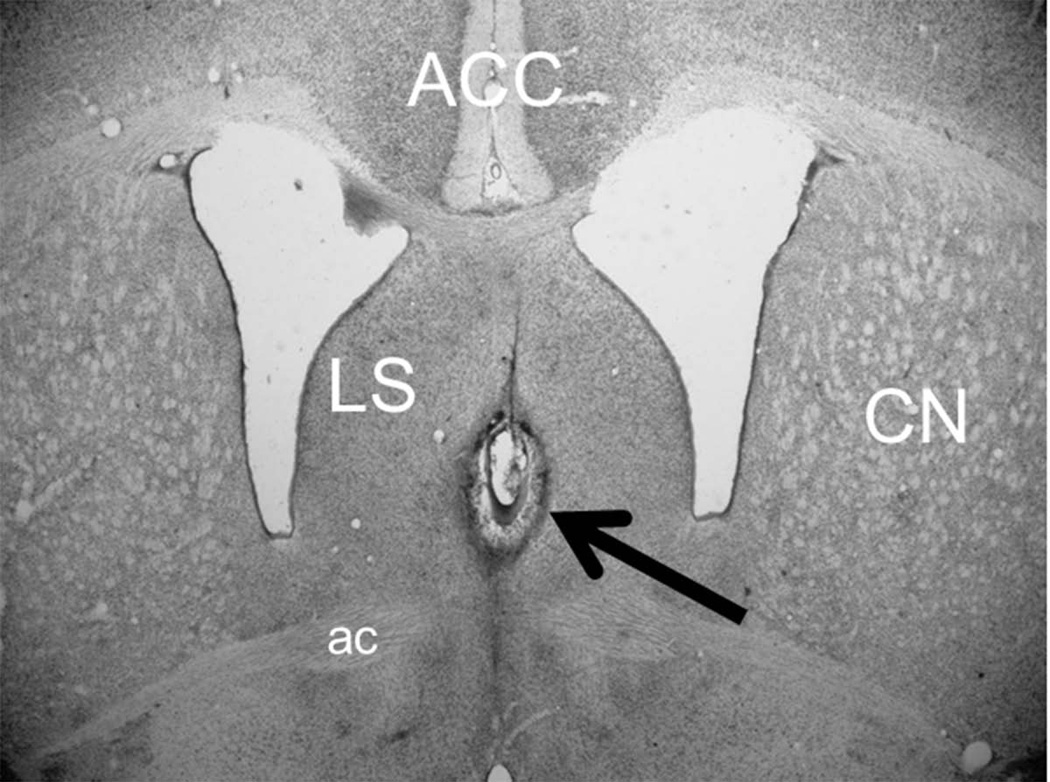
All pups in the lesion groups had lesions centered on the medial septum (Figure 1). Lesions were discrete, bilateral, and did not include significant portions of the lateral septum or fornix. Lesion extent was not quantified because previous studies found that partial lesions of the medial septum are sufficient to fully suppress theta in the hippocampus and impair delay eyeblink conditioning (Allen, Padilla, & Gluck, 2002; Berry & Thompson, 1979).
Figure 1.
Image of a coronal section of the infant rat brain showing a representative lesion of the medial septum (arrow). The lesion was centered on the medial septum and did not include damage to the lateral septum (LS) or anterior commissure (ac). The anterior cingulate cortex (ACC) and caudate nucleus (CN) are also labeled to provide landmarks for spatial reference.
Eyeblink Conditioning
There were no significant statistical effects related to sex and that variable will therefore not be discussed further.
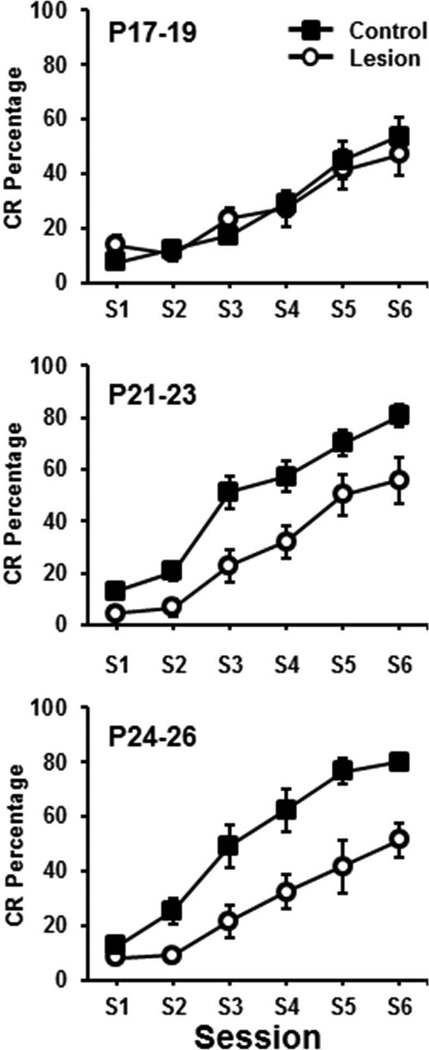
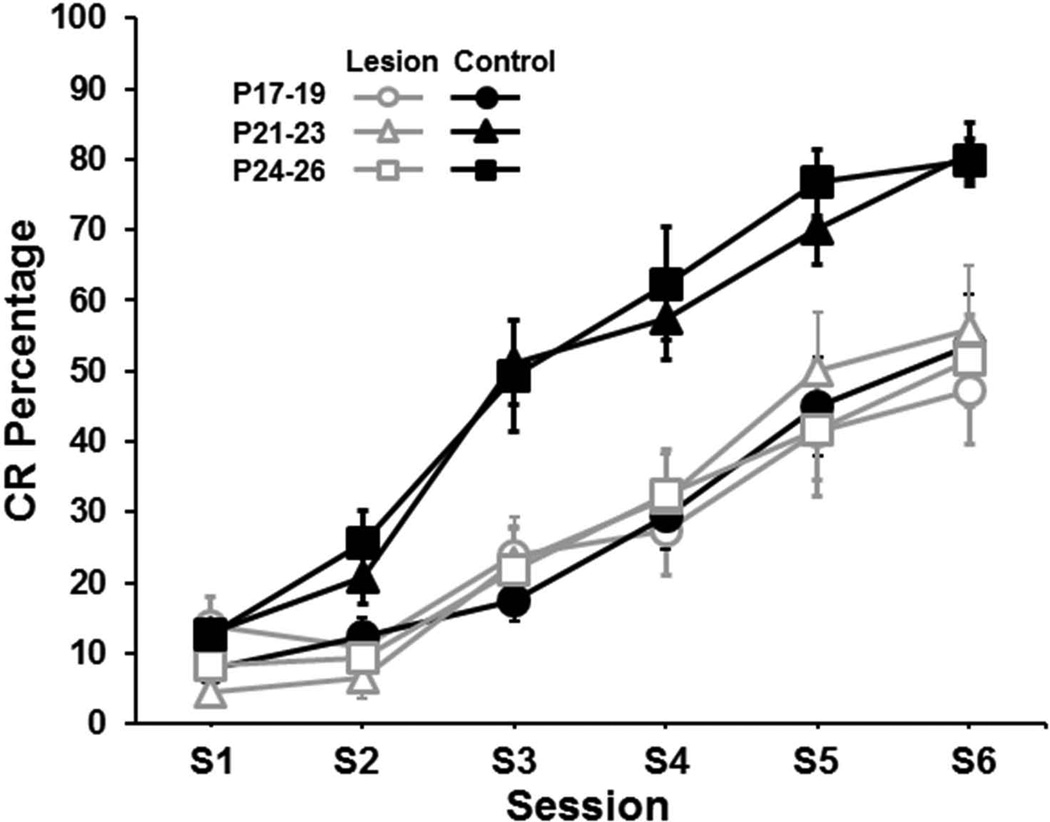
The percentage of CRs increased across training sessions in all groups and there was an increase in CRs as a function of age (Figure 2). Pups in the control groups trained on P21–23 and P24–26 showed a greater increase in CRs as sessions progressed relative to the P17–19 group, which is consistent with the age-related increase in eyeblink conditioning demonstrated in previous studies (Goldsberry et al., 2014). Rat pups given lesions of the medial septum showed an impairment on P21–23 and P24–26 but not on P17–19. Importantly, the acquisition curves of all of the lesion groups were nearly identical and did not differ from the control group trained on P17–19 (Figure 3). These observations were supported by an Age (P17–19, P21–23, P24–25) × Condition (control vs. lesion) × Session (6) ANOVA, which yielded significant main effects of Age, F(2,45) = 4.994, p < 0.02 and Condition, F(1,45) = 18.474, p < 0.01, and an Age × Condition interaction, F(2,45) = 4.507, p < 0.02. Post hoc tests of the Age×Condition interaction indicated that the control and lesion groups differed significantly for the P24–26 age group (p < 0.05), but not for the other age groups. However, the P24–26 and P21–23 control groups differed from the P17–19 control group (p < 0.05) and the lesion groups did not differ from each other or from the P17–19 control group. There was also an Age × Session interaction, F(5.94, 45) = 2.826, p < 0.02 and a Condition × Session interaction, F(2.97,45) = 4.585, p < 0.01. However, there was no Age × Condition × Session interaction. The Age × Session interaction was due to an increase in CR percentage in the older groups relative to the P17–19 groups in sessions 3–6. The Condition × Session interaction was due to increased CRs in the control groups relative to the lesion groups in sessions 3–6.
Figure 2.
Mean (± SE) conditioned response (CR) percentage for rat pups given lesions of the medial septum (circles) or control surgery (squares) and trained on delay eyeblink conditioning on postnatal days (P)17–19 (upper), P21–23 (middle), or P24–26 (lower) across 100-trial sessions.
Figure 3.
Mean (± SE) conditioned response (CR) percentage for rat pups given lesions of the medial septum (gray open symbols) or control surgery (black closed symbols) and trained on delay eyeblink conditioning on postnatal days (P)17–19 (circles), P21–23 (triangles), or P24–26 (squares) across 100-trial sessions.
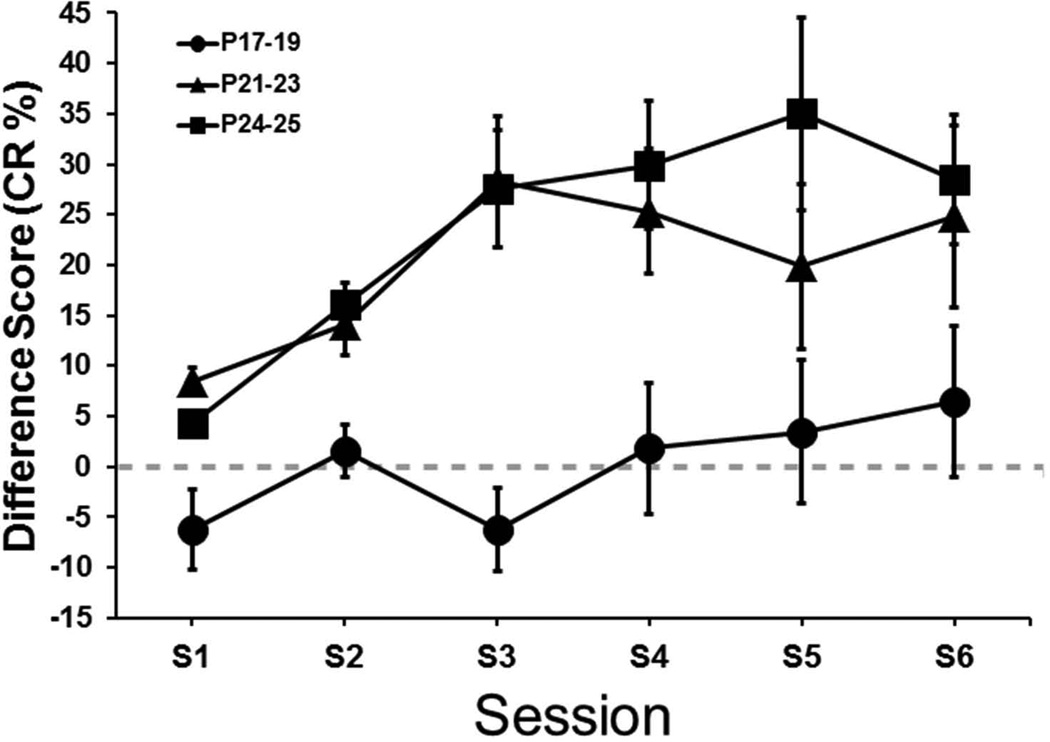
The CR percentage data were further examined by calculating difference scores between the CR percentage for each pup, for each session, in the three lesion groups and the session means of their respective age-matched control group (Figure 4). This measure was used as a more direct way of examining the magnitude of the difference between the control and lesion groups. An Age (P17–19, P21–23, P24–26) × Session (6) ANOVA was conducted and yielded a main effect of Age, F(2,21) = 7.91, p < 0.01. The main effect of Age was due to significantly higher difference scores for the P21–23 and P24–26 groups relative to the P17–19 group (p < 0.05). The P21–23 and P24–26 groups did not differ significantly. Thus, on the basis of the difference scores, the P17–19 group was not impaired by the lesion, whereas the P21–23 and P24–26 groups were impaired to an equivalent extent.
Figure 4.
Mean (± SE) conditioned response (CR) percentage difference from the respective control groups for rat pups given lesions of the medial septum and trained on delay eyeblink conditioning on postnatal days (P)17–19 (circles), P21–23 (triangles), or P24–26 (squares) across 100-trial sessions.
Conditioned response amplitude was measured from CS-alone trials. The younger control group and the lesion groups had several pups with no CR amplitude data for at least one session (no CRs on any of the 10 CS-alone trials) and therefore could not be included in the repeated measures ANOVA. To avoid losing too much power in the CR amplitude analysis we averaged the CR amplitude measure for the first half (sessions 1–3) and second half (sessions 4–6) of training. As a result, data from only 1 pup was excluded (P21–23 lesion group) from the analysis. An Age (P17–19, P21–23, P24–25) × Condition (control vs. lesion) × Half (sessions 1–3 vs. sessions 4–6) ANOVA on the CR amplitude data yielded a main effect of Age, F(2, 44) = 5.853, p < 0.01 and an Age × Condition × Half interaction, F(2,44) = 4.259, P < 0.01. Post hoc tests of the interaction indicated that CR amplitude for the second half of training was significantly higher in the control groups trained on P21–23 and P24–26 relative to the control group trained on P17–19 (p < 0.05). The CR amplitude of the lesion groups did not differ from each other or from the P17–19 control group. These results indicate a developmental increase in CR amplitude in the controls that was not evident in the lesion groups.
No significant effects of the lesion were found for the CR onset latency or CR peak latency measures, suggesting that CR timing was not affected by medial septum lesions.
DISCUSSION
Acquisition rate increased with postnatal age, replicating previous findings (Goldsberry et al., 2014; Stanton et al., 1992). Rat pups given lesions of the medial septum on P12 were not significantly impaired when given eyeblink conditioning on P17–19, but pups trained on P21–23 or P24–26 were impaired relative to their age-matched control groups (Figures 2 and 3). The learning curves for all of the lesion groups and the control group trained on P17–19 were nearly identical (Figure 3). These findings suggest that the septohippocampal system starts to modulate acquisition of delay eyeblink conditioning between P19 and P21.
The nearly identical learning curves of the lesion groups and the P17–19 control group may raise concerns about a floor effect that obscured developmental differences between these groups. However, the CR percentage in these groups was substantially higher than typically found with unpaired training at these ages, suggesting that there was a moderate level of associative learning (Goldsberry et al., 2014). This moderate level of associative learning would presumably be susceptible to disruption. Indeed, we previously found that the modest level of eyeblink conditioning in pups trained on P17–19 can be reduced substantially (< 10% CRs) by amygdala inactivation (Ng & Freeman, 2013). The amygdala inactivation data suggest that the floor for CR percentage in infant eyeblink conditioning is substantially lower than the CR percentage in the lesion groups or the P17–19 control group in the current study. It is therefore unlikely that the similarity in learning curves in these groups was due to a floor effect.
The pups in the older groups were weaned on P19, which is 2 days earlier than the standard weaning day. This early weaning was necessary to avoid the effects of acute maternal separation during eyeblink conditioning in the P21–23 group. Acute maternal separation and the associated nutritional/fluid deprivation has been shown to facilitate eyeblink conditioning in rat pups (Stanton et al., 1992). It is possible that the P21–23 group was still experiencing some of these deprivation effects and had facilitated acquisition. We expected the control group trained on P21–23 to have an acquisition rate that was faster than the P17–19 group but slower than the P24–26 group (Stanton, Fox, & Carter, 1998). Contrary to our expected results, the P21–23 group acquired eyeblink conditioning at the same rate as the P24–26 group, suggesting that early weaning might have had an enduring effect that carried over to training on P21–23. The mechanism by which early weaning accelerates acquisition on P21–23 is not known, but it might involve facilitation of septohippocampal theta (Berry & Swain, 1989).
Medial septal lesions produced an impairment in acquisition of delay eyeblink conditioning in pups trained on P21–23 or P24–26, presumably by suppressing hippocampal theta (Berry & Thompson, 1979). The behavioral impairment in the current experiment replicates the effects of electrolytic lesions of the medial septum in adult rabbits (Berry & Thompson, 1979). Electrolytic lesions were also used in the current study and there might be concern about whether damage to fibers of passage could have produced the conditioning deficit in older pups. This is unlikely, however, since fiber-sparing excitotoxic lesions or cholinergic blockade within the medial septum impair the rate of acquisition to the same degree as the impairment in the older groups in the current study (Allen et al., 2002; Solomon & Gottfried, 1981). Each of these manipulations of the medial septum results in suppression of hippocampal theta, which causes impaired acquisition of eyeblink conditioning (Solomon, Solomon, Schaaf, & Perry, 1983). The absence of impaired acquisition in pups given medial septal lesions and trained on P17–19 therefore suggests that the septohippocampal theta modulation of eyeblink conditioning does not emerge ontogenetically until at least P21. Hippocampal theta itself is found on P10 (Vanderwolf, Kramis, Gillespie, & Bland, 1975), but continues to increase in frequency and amplitude until P23 (Leblanc & Bland, 1979). Thus, the development of septohippocampal theta modulation of cerebellar learning may depend on developmental changes in theta properties. The quantitative relationships between theta amplitude and frequency and modulation of cerebellar function have not been investigated.
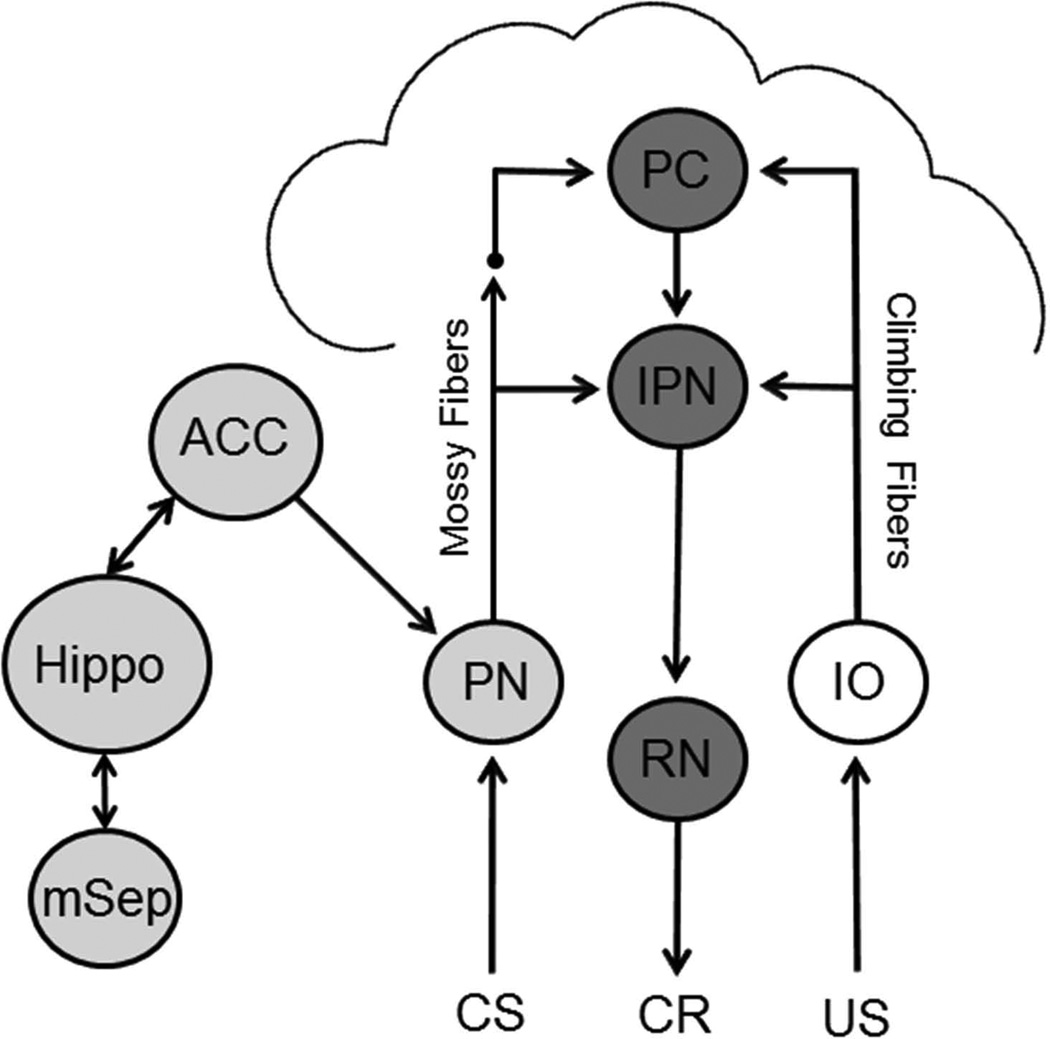
Medial septal input to the hippocampus is thought to play a role in acquisition of delay eyeblink conditioning by helping to generate theta in the hippocampus (Berry & Hoffmann, 2011; Berry & Thompson, 1979). Models of interactions between the septum and hippocampus postulate that medial septum-induced theta helps encode a representation of the CS and distinguish the CS from other stimuli such as elements of the context (Hasselmo, 2005; Rokers, Mercado, Allen, Myers, & Gluck, 2002). This enhancement of encoding may facilitate eyeblink conditioning by sending coherent input to the cerebellum (Rokers et al., 2002). Feedback from the hippocampus to the septum may also play a role in CS processing during eyeblink conditioning by providing an error signal when the CS is relatively novel or poorly predicted which increases medial septal activity and thereby facilitates hippocampal encoding of the CS (Rokers et al., 2002). It is not clear how enhanced CS processing in the hippocampus influences CS processing in the cerebellum but a possible pathway is through the anterior cingulate cortex (Berry & Hoffmann, 2011) and its projection to the pontine nuclei (Siegel, Kalmbach, Chitwood, & Mauk, 2012; Weible, Weiss, & Disterhoft, 2007). It is also possible that hippocampal interactions with other cerebral cortical areas influences input to the cerebellum since most of the cerebral cortex sends direct input to the pontine nuclei (Glickstein, Stein, & King, 1972; Legg, Mercier, & Glickstein, 1989; Mihailoff, Lee, Watt, & Yates, 1985). The ontogeny of septohippocampal modulation is therefore likely to cause an ontogenetic change in delay eyeblink conditioning by facilitating CS processing and thereby facilitating CS input to the cerebellum. Developmental changes in the septohippocampal facilitation of eyeblink conditioning might be related to developmental changes in sensory input to the septum, feedback from the hippocampus to the septum, or hippocampal interactions with downstream circuitry such as the anterior cingulate (Figure 5).
Figure 5.
Schematic diagram of the neural circuitry underlying acquisition of delay eyeblink conditioning. Conditioned stimulus (CS) input is projected to the cerebellum via the pontine nucleus (PN) mossy fiber projection to the anterior interpositus nucleus (IPN) and Purkinje cells (PC) in the cortex. Unconditioned stimulus (US) input is projected to the cerebellum via the climbing fiber projection from the inferior olive (IO). Conditioned response (CR) is generated through projections from the IPN to the red nucleus (RN), and then the facial motor nucleus (not shown). The medial septum (mSep) projects to the hippocampus (Hippo) and contributes to the generation of theta. The hippocampus sends a feedback projection to the septum. The hippocampus influences the cerebellum through the anterior cingulate cortex (ACC) and/or other cortical areas.
The developmental time course of the septohippocampal theta modulation in eyeblink conditioning is consistent with other measures of hippocampal function. Hippocampus-dependent spatial memory develops between P16 and P21 in rats as measured by delayed alternation and water maze memory (Freeman & Stanton, 1991; Green & Stanton, 1989; Rudy, Stadler-Morris, & Albert, 1987). Contextual fear conditioning and the context preexposure facilitation effect also depend on the hippocampus and emerges ontogenetically at around P23 in rats (Jablonski, Schiffino, & Stanton, 2012; Rudy, 1993; Rudy & Morledge, 1994; Schiffino, Murawski, Rosen, & Stanton, 2011). Moreover, disrupting hippocampal development with early ethanol exposure impairs the context preexposure facilitation effect in developing rats (Jablonski & Stanton, 2014). The findings from spatial memory, fear conditioning, and eyeblink conditioning studies all indicate that hippocampal processes emerge ontogenetically around the time of weaning in rats. The emergence of the septohippocampal theta system may provide a common mechanism, playing a critical role in the ontogeny of memory within the amygdala, cerebellum, and cerebral cortex.
In addition to developmental changes in hippocampal contributions to memory, ontogenetic changes have been found in cerebellar, amygdala, and cerebral cortical mechanisms underlying learning and memory. Developmental changes in the CS and US neural pathways into the cerebellum combine to influence the development of eyeblink conditioning (Campolattaro & Freeman, 2008; Freeman & Campolattaro, 2008; Freeman, Rabinak, & Campolattaro, 2005; Ng & Freeman, 2012; Nicholson & Freeman, 2003). There is a change in amygdala function early in development whereby the amygdala does not process aversive stimuli to establish fear conditioning until rat pups are older than P10 (Landers & Sullivan, 2012; Sullivan, Landers, Yeaman, & Wilson, 2000). Amygdala processing of aversive stimuli is suppressed in the presence of the dam in young pups and is unmasked by administering corticosterone into the amygdala (Moriceau & Sullivan, 2006; Moriceau, Wilson, Levine, & Sullivan, 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007). Developmental changes in the mechanisms underlying retention of fear conditioning are seen in older rat pups. Prefrontal cortical engagement in the retention/retrieval of fear conditioning becomes evident between P17 and P24 (Kim, Li, Hamlin, McNally, & Richardson, 2012; Li, Kim, & Richardson, 2012a, 2012b). Prefrontal cortical development also plays a role in the development of spatial memory during this period (Freeman & Stanton, 1992; Jablonski, Watson, & Stanton, 2010; Watson & Stanton, 2009). Ontogenetic changes in learning and memory mechanisms in hippocampal, cerebellar, amygdala, and cerebral cortical systems indicate that the developing brain has substantial constraints on early learning despite the ubiquity of neural plasticity mechanisms.
The current findings indicate that the septohippocampal theta system starts modulating acquisition of eyeblink conditioning between P19 and P21. The ontogenetic emergence of septohippocampal modulation parallels the behavioral emergence of eyeblink conditioning, suggesting that the development of septohippocampal modulation plays a role in the development of cerebellar learning. Thus, developmental changes in this forebrain modulatory system combine with developmental changes in the brainstem CS and US pathways to subserve the ontogenetic emergence of eyeblink conditioning (Figure 5).
Acknowledgments
This work was supported by National Institutes of Health grant NS038890 to J.H.F.
REFERENCES
- Allen MT, Padilla Y, Gluck MA. Ibotenic acid lesions of the medial septum retard delay eyeblink conditioning in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2002;116:733–738. doi: 10.1037//0735-7044.116.4.733. [DOI] [PubMed] [Google Scholar]
- Berry SD, Hoffmann LC. Hippocampal theta-dependent eyeblink classical conditioning: coordination of a distributed learning system. Neurobiology of Learning and Memory. 2011;95:185–189. doi: 10.1016/j.nlm.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Berry SD, Seager MA. Hippocampal theta oscillations and classical conditioning. Neurobiology of Learning & Memory. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Berry SD, Swain RA. Water deprivation optimizes hippocampal activity and facilitates nictitating membrane conditioning. Behavioral Neuroscience. 1989;103:71–76. doi: 10.1037//0735-7044.103.1.71. [DOI] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Medial septal lesions retard classical conditioning of the nicitating membrane response in rabbits. Science. 1979;205:209–211. doi: 10.1126/science.451592. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proceedings of the National Academy of Sciences (USA) 2008;105:8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Campolattaro MM. Ontogenetic change in the auditory conditioned stimulus pathway for eyeblink conditioning. Learning & Memory. 2008;15:823–828. doi: 10.1101/lm.1131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning & Memory. 2005;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Stanton ME. Fimbria-fornix transections disrupt the ontogeny of delayed alternation but not position discrimination in the rat. Behavioral Neuroscience. 1991;105:386–395. doi: 10.1037//0735-7044.105.3.386. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Stanton ME. Medial prefrontal cortex lesions and spatial delayed alternation in the developing rat: recovery or sparing? Behavioral Neuroscience. 1992;106:924. doi: 10.1037//0735-7044.106.6.924. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning & Memory. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Stein J, King RA. Visual input to the pontine nuclei. Science. 1972;178:1110–1111. doi: 10.1126/science.178.4065.1110. [DOI] [PubMed] [Google Scholar]
- Goldsberry ME, Elkin ME, Freeman JH. Sensory system development influences the ontogeny of eyeblink conditioning. Developmental Psychobiology. 2014;56:1244–1251. doi: 10.1002/dev.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behavioral Neuroscience. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?--Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hoffmann LC, Berry SD. Cerebellar theta oscillations are synchronized during hippocampal theta-contingent trace conditioning. Proceedings of the National Academy of Sciences (USA) 2009;106:21371–21376. doi: 10.1073/pnas.0908403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Developmental Psychobiology. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Stanton ME. Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: dose-response and post-training consolidation effects. Alcohol. 2014;48:35–42. doi: 10.1016/j.alcohol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Watson DJ, Stanton ME. Role of medial prefrontal NMDA receptors in spatial delayed alternation in 19-, 26-, and 33-day-old rats. Developmental Psychobiology. 2010;52:583. doi: 10.1002/dev.20465. [DOI] [PubMed] [Google Scholar]
- Kim JH, Li S, Hamlin AS, McNally GP, Richardson R. Phosphorylation of mitogen-activated protein kinase in the medial prefrontal cortex and the amygdala following memory retrieval or forgetting in developing rats. Neurobiology of Learning and Memory. 2012;97:59–68. doi: 10.1016/j.nlm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kotani S, Kawahara S, Kirino Y. Classical eyeblink conditioning in decerebrate guinea pigs. European Journal of Neuroscience. 2002;15:1267–1270. doi: 10.1046/j.1460-9568.2002.01963.x. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34:101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc MO, Bland BH. Developmental aspects of hippocampal electrical activity and motor behavior in the rat. Experimental Neurology. 1979;66:220–237. doi: 10.1016/0014-4886(79)90076-1. [DOI] [PubMed] [Google Scholar]
- Legg CR, Mercier B, Glickstein M. Corticopontine projection in the rat: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. Journal of Comparative Neurology. 1989;286:427–441. doi: 10.1002/cne.902860403. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behavioral Neuroscience. 2012a;126:217–225. doi: 10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Updating memories: changing the involvement of the prelimbic cortex in the expression of an infant fear memory. Neuroscience. 2012b;222:316–325. doi: 10.1016/j.neuroscience.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Zebrozyna AW. Classical conditioning of the corneal reflex in the chronic decerebrate rat. Brain Research. 1975;89:337–340. doi: 10.1016/0006-8993(75)90724-6. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Mihailoff GA, Lee H, Watt CB, Yates R. Projections to the basilar pontine nuclei from face sensory and motor regions of the cerebral cortex in the rat. Journal of Comparative Neurology. 1985;237:251–263. doi: 10.1002/cne.902370209. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KH, Freeman JH. Developmental changes in medial auditory thalamic contributions to associative motor learning. Journal of Neuroscience. 2012;32:6841–6850. doi: 10.1523/JNEUROSCI.0284-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KH, Freeman JH. Amygdala inactivation impairs eyeblink conditioning in developing rats. Developmental Psychobiology. 2013;56:999–1007. doi: 10.1002/dev.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nature Neuroscience. 2003;6:532–537. doi: 10.1038/nn1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Buchwald JS, Villablanca JR. Classical conditioning with auditory discrimination of the eye blink in decerebrate cats. Science. 1977;196:551–553. doi: 10.1126/science.850800. [DOI] [PubMed] [Google Scholar]
- Rokers B, Mercado E, 3rd, Allen MT, Myers CE, Gluck MA. A connectionist model of septohippocampal dynamics during conditioning: closing the loop. Behavioral Neuroscience. 2002;116:48–62. [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: dissociation of "proximal"- and "distal"-cue-based behaviors. Behavioral Neuroscience. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of Learning and Memory. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proceedings of the National Academy of Sciences (USA) 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. Journal of Neurophysiology. 2012;107:50–64. doi: 10.1152/jn.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Gottfried KE. The septohippocampal cholinergic system and classical conditioning of the rabbit's nictitating membrane response. Journal of Comparative and Physiological Psychology. 1981;95:322–330. doi: 10.1037/h0077779. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Solomon SD, Schaaf EV, Perry HE. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science. 1983;220:329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression? Neuropharmacology. 1998;37:623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH, Kramis R, Gillespie LA, Bland BH. Hippocampal Rhythmic Slow Activity and Neocortical Low-Voltage Fast Activity: Relations to Behavior. In: Isaacson RL, Pribram KH, editors. The Hippocampus. Vol. 2. New York, NY: Plenum Press; 1975. pp. 101–128. [Google Scholar]
- Watson DJ, Stanton ME. Medial prefrontal administration of MK-801 impairs T-maze discrimination reversal learning in weanling rats. Behavioural Brain Research. 2009;205:57. doi: 10.1016/j.bbr.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145:288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Nokia MS, Penttonen M. Hippocampo-cerebellar theta band phase synchrony in rabbits. Neuroscience. 2010;165:1538–1545. doi: 10.1016/j.neuroscience.2009.11.044. [DOI] [PubMed] [Google Scholar]