Abstract
Objectives
Head and neck squamous cell carcinoma (HNSCC) cells are resistant to cell death induced by Tumor Necrosis Factor ligands such as Tumor Necrosis Factor α (TNF) or TNF-Related Apoptosis-Inducing Ligand (TRAIL) and cytotoxic chemotherapies. Recently, genetic alterations in cell death pathways including inhibitor of apoptosis proteins have been demonstrated in HNSCC. We investigated the effects of birinapant, a novel SMAC-mimetic that targets inhibitor of apoptosis proteins, alone and in combination with TNFα, TRAIL or chemotherapy docetaxel.
Study Design
Experimental study using human HNSCC cell lines in vitro and xenograft mouse model in vivo.
Methods
A panel of HNSCC cell lines with varying genetic alterations in cell death pathway components were treated with birinapant ± TNFα, TRAIL and docetaxel and were assessed for effects on cell density, cell cycle and death. Synergism was determined at varying concentrations of treatments using the Chou-Talalay method. Combination studies using birinapant ± docetaxel were performed in a xenograft mouse model.
Results
Birinapant, alone or in combination with TNFα or TRAIL, decreased cell density in cell lines, with IC50s ranging from 0.5 nM to >1 μM. Birinapant alone or with TNF significantly increased subG0 cell death in different lines. Docetaxel showed synergism with birinapant ± TNFα in vitro. Birinapant monotherapy inhibited growth in a tumor xenograft model resistant to docetaxel, and combination treatment further delayed growth.
Conclusions
Birinapant alone or in combination with TNFα or TRAIL and docetaxel decreased cell density, increased cell death, and displayed anti-tumor activity in a preclinical HNSCC xenograft exhibiting aberrations in cell death pathway components and docetaxel resistance.
Level of Evidence
NA, Animal Studies and Basic Research
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with over 600,000 new cases diagnosed each year1. Despite advances in treatment options including surgery, chemotherapy, and radiation therapy, the 5 year survival remains approximately 50%1. With the development of novel targeted molecular therapies and the large scale efforts to characterize and catalogue genomic changes in tumors by projects such as The Cancer Genome Atlas (TCGA), there is renewed hope for improvement of HNSCC treatment and outcomes.
Resistance of tumor cells to cell death signals, chemotherapy, or radiation therapy-induced cell death is a significant problem promoting development and progression of HNSCC. Cell death may be induced by ligands and receptors of the Tumor Necrosis Factor/Fas Receptor family2. These receptors induce cytoplasmic complexes and a signal cascade involving Fas-Associated Death Domain (FADD) protein, which can promote Caspase 8- and 3-mediated apoptosis2,3. However, this signaling and cell death may be blocked by Inhibitors of Apoptosis proteins (IAPs), which are encoded by BIRC family genes2 (Supp. Fig. 1).
Interestingly, recent findings from TCGA and other studies reveal that more than 40% of HNSCC harbor genomic alterations in these cell death pathways, with ~30% displaying amplifications of FADD with or without co-amplification of IAP genes BIRC2/3, while 10% form a mutually exclusive subset exhibiting mutations in Caspase 8 (CASP8)4. Expression of IAPs BIRC2 and 3 are associated with chemoresistance and poor outcomes in a variety of cancer types5. In head and neck squamous cell carcinomas, cIAP1/BIRC2 expression was also correlated with nodal metastasis and with advanced disease6,7. Moreover, binding of TNFα to its receptor in the presence of cIAP1/BIRC2 and cIAP2/BIRC3 leads to activation of Nuclear Factor-kappaB (NF-κB) transcription factors, which can promote expression of genes that further enhance cell survival, therapeutic drug resistance, and malignant progression8. However, when BIRC2/3 are inhibited, TNFα or cytotoxic damage signaling instead leads to FADD-mediated cell death8,9.
Birinapant is a potent inhibitor of cIAPs that structurally mimics the second mitochondria-derived activator of caspases (SMAC). SMAC is an endogenous protein released by mitochondria at the onset of apoptosis, which controls IAPs by binding to them and leading to their degradation, thereby enhancing apoptosis8. A growing area of interest in cancer research involves targeting IAPs with small molecule mimetics of SMAC as a method of inducing cell death in cancer cells. Birinapant has demonstrated activity in preclinical models of melanoma, colorectal, ovarian10, and breast cancer11, and is currently in phase I/II clinical trials in these tumor types12.
Because HNSCC exhibit genomic alterations and increased expression of FADD and IAPs/BIRCs, we explored the hypothesis that birinapant may induce cell death alone, or in combination with TNFα, TRAIL, or docetaxel, a chemotherapy approved and commonly used in patients with HNSCC. Strategies that identify new targets for cytotoxic therapy and address resistance to standard chemotherapy agents such as docetaxel are important areas of pursuit for improvement of treatment in patients with HNSCC.
MATERIALS AND METHODS
Cell lines and cell culture
HNSCC cell lines UMSCC1, 11B, and 46 were obtained from Dr. T.E. Carey at the University of Michigan (Ann Arbor, MI)13,14. These lines have been characterized for genomic alterations and expression of FADD, BIRC2/3 and CASP8 (Supp. Fig. 2). Cell lines were maintained in MEM (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco), 1% penicillin/streptomycin (Gibco), 1% L-glutamine (Gibco), and used for experiments within 3 months or 20 passages. Cultures were incubated at 37° C with 5% CO2.
XTT cell proliferation assays
To determine single agent and combination drug effects in vitro, XTT Cell Proliferation Kits (Roche, Indianapolis, IN) were used. Cells were plated in 96-well plates at predetermined densities for each cell line based on respective doubling times and log-phase growth patterns. Cells were treated with 0.01% DMSO control or one of nine concentrations of birinapant ± 20 ng/mL TNFα or 50ng/ml TRAIL (R&D systems, Minneapolis, MN) ± docetaxel (LC Laboratories, Woburn, MA), and cell density measured by XTT Cell Proliferation Kits (Roche) on a microplate reader on days 1, 3, and 5. Each cell line and dilution was assayed in six replicates. Inhibitory concentration 50% (IC50) was determined on Day 3 using the nonlinear four-parameter regression function in GraphPad Prism (La Jolla, CA). For drug combination studies, fixed ratios of each drug’s respective IC50 in each cell line was used to determine additivity, synergism, or antagonism.
Flow cytometric analysis of cell cycle and death
Flow cytometry was used to elucidate the effect of drug on cell cycle and cell death in UMSCC-46 and -11B using Cycletest Plus DNA Reagent Kit (BD BioSciences, San Jose, CA). Cells were plated 48 hours before treatment, and treated over 24 hours with 0.01% DMSO control, 20 ng/mL TNFα, 1 μM birinapant, or combinations of each. Cells were harvested, counted, and stained with propidium iodide prior to analysis on a FACS Canto flow cytometer (BD Biosciences). Data from 10,000 cells per treatment group and time point were analyzed using BD FACSDiva (BD Biosciences) software. Each experiment was performed in triplicate.
HNSCC xenograft studies
All animal experiments were carried out under protocol #1322 approved by the Animal Care and Use Committee of the NIDCD, and were in compliance with the Guide for the Care and Use of Laboratory Animal Resource (1996) National Research Council. Four- to 6-week-old female SCID/NCr-Balb/c mice were obtained from Frederick Cancer Research and Development Center (National Cancer Institute) and housed in a specific pathogen-free animal facility. Mice were inoculated with 5 × 106 UMSCC-46 cells injected subcutaneously in 0.2 mL blank MEM media into the right hind flanks. Fourteen days later, once tumors became palpable, mice were randomized into treatment arms (vehicle control n=20, 15 mg/kg Birinapant n=20, 6 mg/kg docetaxel n=20, combination n=20). Birinapant was formulated at 1.5 mg/mL in a citrate buffer solution on each treatment day, and administered by intraperitoneal injections (i.p.) for the 3 week treatment period on Wednesdays and Thursdays, while docetaxel was administered i.p. on Wednesdays, based on previously published studies10,15. Control mice received citrate buffer solution as vehicle on the same schedule. Tumor diameters were measured in two dimensions using electronic calipers, and volume was estimated using the formula V= 0.5 (L x W x W), where L equals the longer of the two measurements. Mice were euthanized when tumors reached 2 cm in diameter, were ulcerated and bleeding, or if mice suffered from pain or weight loss.
Statistical design for data analysis
Data from XTT assays and flow cytometric experiments were presented as mean ± standard deviation of at least 3 replicates. For in vitro drug combination studies, interactions were analyzed by the Chou-Talalay method using CompuSyn software (Combination Index (CI) <1: synergy, CI=1: additivity, CI>1: antagonism)16. For XTT assays, student’s t-tests were performed at each concentration tested for TNF vs birinapant alone and TRAIL vs birinapant alone. Curves were considered significantly different if the majority of points assessed were statistically significant, with p values of less than 0.05. For flow cytometric experiments and tumor growth analysis, significance was determined using the student’s t-test with p values of less than 0.05 considered statistically significant. For survival analysis, the Gehan-Breslow-Wilcoxon test was used and significance set to 0.05 using the Bonferroni method.
RESULTS
Birinapant differentially affects HNSCC proliferation in vitro and is enhanced by TNF and TRAIL
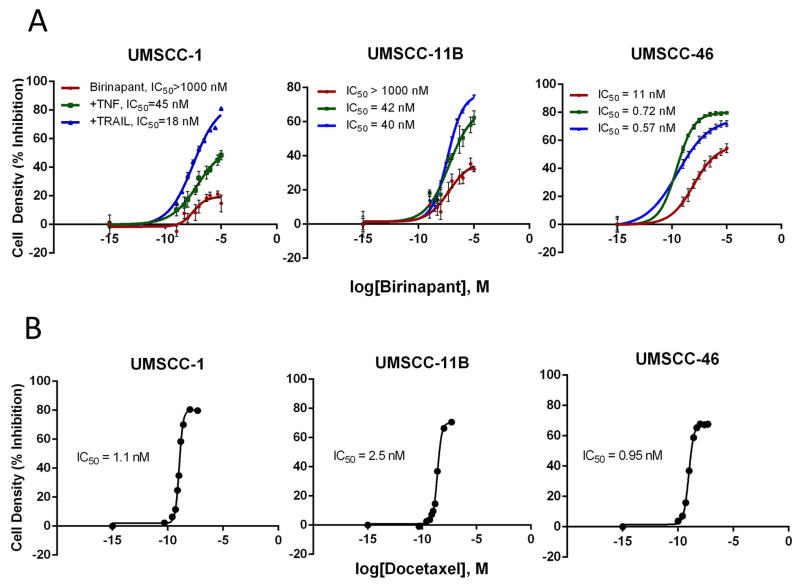
We selected 3 human HNSCC cell lines with varying alterations in cell death pathway components for examination of sensitivity to birinapant or docetaxel. Compared with normal keratinocytes, UMSCC-1 is a cell line with similar low levels of FADD and BIRC2 expression, UMSCC-11B has both increased FADD and BIRC2 expression, while UMSCC-46 has increased FADD expression and normal BIRC2 (Supp. Fig. 2). All cell lines treated with increasing birinapant concentrations between 0.5 nmol/L and 10 μmol/L over a 5-day XTT assay exhibited a dose-dependent decrease in cell density (Figure 1A). The IC50 values for birinapant for UMSCC-1 and -11B exceeded 1 μmol/L, while UMSCC-46 was sensitive to birinapant alone with an IC50 ~9.5 nmol/L (Figure 1A). The addition of 20 ng/mL of TNFα or 50 ng/mL of TRAIL potently inhibited cell density, and significantly enhanced the effects of birinapant in all three cell lines (p<0.0001). While UMSCC-1 and -11B were both relatively resistant to single agent birinapant, with <50% inhibition even at maximal concentrations of 1μM, sensitization with TNFα or TRAIL reduced IC50 values to 45 and 18 nmol/L for birinapant for UMSCC-1, and to 42 and 40 nmol/L for UMSCC-11B, respectively (Figure 1A). For UMSCC-46, co-stimulation with either TNFα or TRAIL further reduced the IC50 from 9.5 nmol/L to 0.72 and 0.57 nmol/L, respectively (Figure 1A). We then established sensitivity of our cell lines to conventional chemotherapy docetaxel prior to use in combinations. When treated with increasing concentrations, all three cell lines demonstrated sensitivity to docetaxel in vitro, with IC50 values under 3 nmol/L (Figure 1B).
Figure 1. Sensitivity of three human HNSCC cell lines to birinapant or docetaxel in vitro.
Percent inhibition of cell density with birinapant ± TNF or TRAIL, A, and docetaxel, B, over a range of concentrations was determined during log growth phase on day 3 by XTT assay. Data points from 6 replicates per concentration were used to compare to controls to calculate percentages of inhibition. The mean and SD were plotted and shown, with IC50 determined by non-linear four parametric regression. TNF or TRAIL enhanced curves were significantly different from birinapant alone (p<0.01).
Birinapant ± TNF alters cell cycle distribution and increases cell death
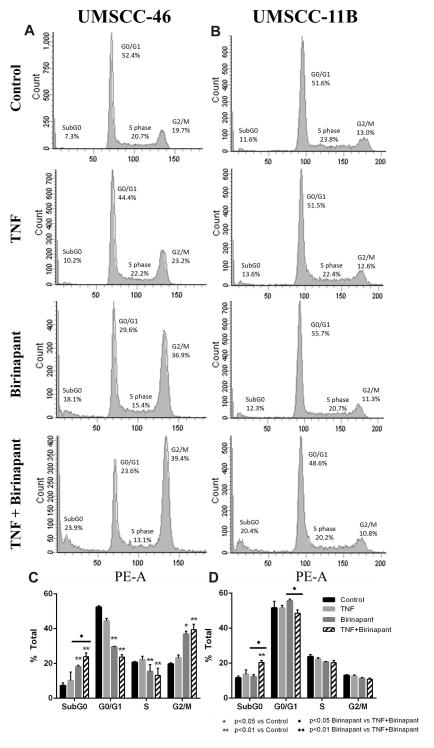
To further evaluate the effects of birinapant on cell cycle or death in UMSCC-46 and -11B, DNA cytofluorometric analyses were performed (Figure 2). In UMSCC-46, cells treated with birinapant or birinapant and TNF exhibited significantly increased sub-G0 DNA fragmentation at 24 hours, indicating a marked effect on cell death (Figure 2A,C). The combined effect of TNF and birinapant was significantly more effective at inducing cell death than birinapant alone (p<0.05). Additionally, a significant increase in G2/M phase population was observed with birinapant alone, and enhanced with the addition of TNF (p<0.01). In UMSCC-11B, the combination of TNF with birinapant also significantly increased cell death (p<0.01), while birinapant alone did not have an effect (Figure 2C,D). In this cell line, there were no other changes in cell cycle distribution in response to treatment. The greater effects on subG0 DNA and G2/M are consistent with the relatively higher sensitivity of UMSCC-46 observed with birinapant alone or in combination with TNF.
Figure 2. Effects of birinapant and TNF on cell cycle and cell death.
UMSCC-46 or -11B cells were plated in monolayer for 48 hours, and treated with birinapant and TNF alone or in combination. Cells were harvested for cell cycle analysis at 24 hours post-treatment and analyzed by DNA cytofluorometry. Representative histograms (A, B) and summary data of three independent experiments shown (C, D). Summary data were calculated and presented as mean ± SD, and statistical significance was calculated by t-test as indicated.
Birinapant demonstrates synergistic activity with docetaxel in HNSCC cell lines in vitro
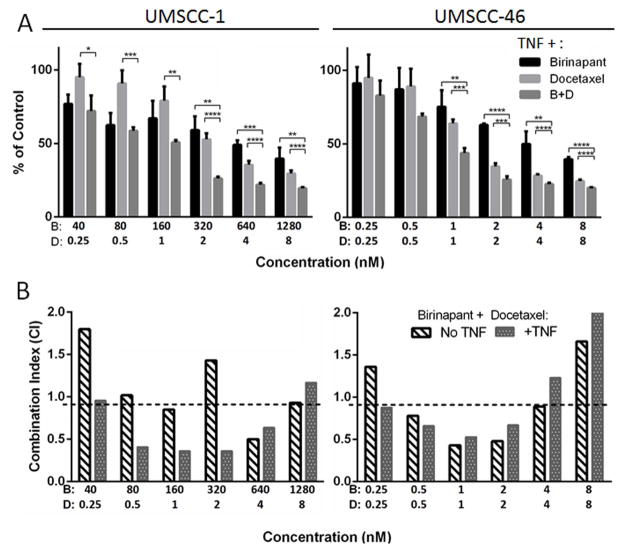
To determine the effect of birinapant in combination with the conventional chemotherapy docetaxel widely used in HNSCC, XTT assays were performed in birinapant resistant UMSCC-1 or sensitive UM-SCC-46 cells, for combinations of birinapant with docetaxel with or without TNFα. Combinations were performed at fixed ratios spanning previously estimated IC50 values for each drug, using ~0.25 to 8 times the IC50. Figure 3A confirms that compared to UMSCC-1, UMSCC-46 was relatively more sensitive to lower concentrations of birinapant alone. However, both lines showed evidence of significantly increased response to combination treatment compared to either drug alone at higher concentrations (p<0.05). The combination index of each experiment was determined by the Chou-Talalay method using CompuSyn software, which defines synergism as a combination index <0.9, and antagonism >1.1 (Figure 3B). For UMSCC-46, birinapant and docetaxel were synergistic between ~0.5–4 nM, and a similar result was seen without or with the addition of TNFα. In UMSCC-1, evidence for synergism at lower concentrations was observed only with the addition of TNFα (Figure 3B). These results suggested that birinapant alone or in combination with docetaxel may be more active in HNSCC cell lines such as UMSCC-46 that are sensitive to birinapant alone.
Figure 3. Combination effects of birinapant and docetaxel.
Percent growth inhibition was assessed by XTT on day 3 post-treatment with varying doses of birinapant ± TNF, docetaxel, or birinapant ± TNF + docetaxel in fixed ratios. A, Cell density relative to untreated control cells after treatment with birinapant (B), docetaxel (D), or combination (B+D) (*p<0.05, **p<0.01, ***p<0.001; t-test). B, Results were analyzed for synergism using CompuSyn software, in which Combination Index (CI) <0.9 denotes synergism (below dashed line); CI 0.9–1.1, additive; CI >1.1, antagonism.
Combination effects of birinapant and docetaxel in a UMSCC-46 xenograft model
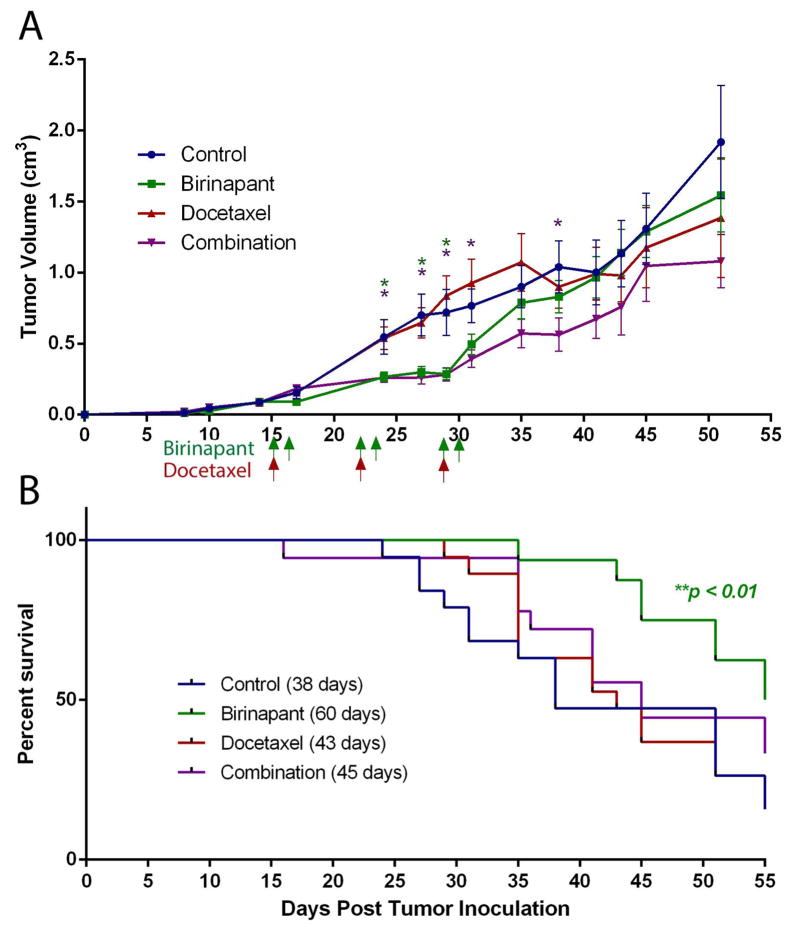
Based on the studies above providing evidence for sensitivity of UMSCC-46 to birinapant alone and in combination with docetaxel, we evaluated the activity of these treatments in HNSCC in vivo. Nine days into the treatment period, birinapant alone demonstrated a significant difference in tumor volume compared to control (p<0.05) (Figure 4A). Surprisingly, docetaxel showed no single agent activity in vivo, despite sensitivity of the cell line in vitro. The combination treated group exhibited significantly decreased tumor volumes compared to control (p<0.05), with prolonged activity compared to the birinapant monotherapy group. Birinapant as a single agent significantly improved survival to a median of 60 days compared to 38 days in control animals (p<0.01) (Figure 4B). Docetaxel alone and the combination treatment did not reach significance in the survival analysis.
Figure 4. Effects of birinapant and docetaxel treatment on tumorigenesis and survival in a human HNSCC xenograft model.
A human HNSCC xenograft model was established utilizing FADD overexpressing UMSCC-46 cells implanted subcutaneously into the flanks of SCID mice (n = 20 mice per treatment group). Treatment was initiated when tumors were palpable, 15 days after implantation. Mice were treated with 15 mg/kg of birinapant i.p. two days a week, 6 mg/kg docetaxel i.p. once weekly, or both for 3 weeks. Arrows represent treatment days. A, Tumor volume was reduced by birinapant and combination treatment compared to control (*p<0.05, t-test). Error bars, SEM. B, Mice bearing UMSCC-46 xenografts treated with birinapant alone showed a median survival advantage of 22 days compared to control (*p<0.01, Gehan-Breslow-Wilcoxon test).
DISCUSSION
In this study, we demonstrate that human HNSCC cell lines with varying alterations in cell death pathway molecules exhibit differential sensitivity to birinapant alone or when co-stimulated with either TNFα or TRAIL in vitro. Interestingly, UMSCC-46, a mutant TP53 cell line with FADD overexpression but cIAP1 expression similar to keratinocytes, was highly sensitive to birinapant monotherapy. Meanwhile, UMSCC-1 and -11B lines exhibiting relatively low or high levels of both FADD and BIRC2, respectively, were relatively resistant to birinapant alone, but sensitized by combination with TNFα or TRAIL. We also found that the combination of TNF and birinapant induced increased cell death and cell cycle changes compared to either treatment alone in UMSCC-46 and -11B. One possible explanation for the differential sensitivity of the cell lines to birinapant is that the balance between cell death protein FADD and its antagonist cIAP1 (BIRC2) may affect intrinsic baseline sensitivity to IAP inhibitors such as birinapant. However, both of the other lines exhibited sensitization to the combination of TNFα/TRAIL with birinapant, consistent with previously established effects of birinapant in degrading cIAP1. In future studies it will be interesting to examine if high levels of FADD are predictive of response when inhibiting cIAP1 using a SMAC mimetic, or modified by the presence of relatively higher levels of cIAP1, as in UMSCC-11B.
Based on publically available TCGA data, ~30% of human HNSCC exhibit amplification of FADD, and 8% co-amplification with BIRC2/3 (cIAP1/2), rendering HNSCC among cancers with the highest prevalence of these death pathway alterations potentially actionable for investigation of SMAC mimetics. Because many patients exhibit intrinsic or acquired resistance to standard chemotherapy agents, which may be in part due to evasion of apoptosis, we also explored the potential of birinapant to overcome this resistance and sensitize HNSCC to the conventional chemotherapy drug docetaxel. In cell density assays, we demonstrated potential synergistic activity between birinapant and docetaxel at a range of doses without TNFα in UMSCC-46 and with TNFα in UMSCC-1.
Because of the sensitivity of UMSCC-46 cells to birinapant mono- and combined docetaxel therapy observed in vitro, we selected this cell line to further examine the effects of treatment in UMSCC-46 xenografts in vivo. Birinapant alone significantly inhibited tumor growth and prolonged survival >20 days compared to control, consistent with activity observed in vitro. However, this cell line exhibited surprising resistance to docetaxel alone in vivo, despite being docetaxel sensitive in vitro. Both birinapant and combination treated animals showed a similar significant delay in tumor growth during the treatment period, although there was a trend towards separation of their tumor growth curves afterwards, with the combination group maintaining tumor growth delay for an additional week compared to birinapant alone. In the survival analysis, however, only birinapant monotherapy demonstrated a significantly improved overall survival compared to control animals.
There are a number of reasons why the synergistic response between birinapant and docetaxel that was observed in vitro may not have been clearly seen in vivo. The UMSCC-46 xenograft model was surprisingly resistant to docetaxel monotherapy in vivo, which may have masked a combination effect when given with birinapant. The separation between the birinapant and combination groups’ tumor growth curves that emerged following the conclusion of the treatment period supports the idea that there is some differential effect of dual treatment. Because all treatments were given in a 48 hour span, an alternate schedule with a longer period of treatment could be explored for effects on enhancing the therapeutic window and activity. Alternatively, the limited activity could be due to other defects determining resistance to docetaxel. While taxanes such as docetaxel may inhibit cell division and proliferation via their effects on microtubule assembly at similar concentrations in cell lines in short term assays, cytotoxic effects of taxanes have been shown to be enhanced by wild type TP5317 whereas TP53 is mutated in UMSCC-4618 and most HNSCC19. It would be of interest in future studies to determine if HNSCC with wtTP53 and greater sensitivity to cytotoxic effects of docetaxel alone would also demonstrate a greater combination effect when given with birinapant.
In conclusion, we have seen that TNF and TRAIL potently sensitize HNSCC lines to birinapant treatment. Several TRAIL compounds have been studied in clinical trials, and although they have been well tolerated, their anticancer responses have been poor20–22. Instead of using TRAIL or TNF itself, there is interest in finding ways to induce innate secretion of these cytokines. Recently, Beug et al showed that certain viruses and adjuvants could stimulate a potent cytokine storm, inducing a release of TNF and TRAIL that lead to synergism with SMAC mimetics23. In head and neck cancer patients undergoing radiotherapy, there is evidence for induction of TNF24. Thus, patients undergoing radiotherapy may be the ideal candidates for investigational use of birinapant, particularly in those who have persistent or recurrent disease following definitive treatment.
CONCLUSION
SMAC mimetic birinapant as a monotherapy was able to significantly delay tumor growth and improve host survival in a human HNSCC xenograft model. Importantly, this effect was observed in a model that proved to be resistant to conventional chemotherapy with docetaxel in vivo, indicating potential for further investigation in patients with docetaxel chemotherapy resistant HNSCC. Provocatively, the UMSCC-46 model exhibits alterations similar to ~30% of HNSCC with FADD amplifications by TCGA data. This subset exhibiting co-amplification of FADD with oncogene cyclin D1 (CCND1) at the same locus is known to have an especially poor prognosis. Based on the results we observed in this project, this subset may be ideal candidates for investigational studies with a SMAC mimetic. Further studies with alternate dosing schedules, combinations, and additional animal models may help to establish optimal combinations of SMAC mimetic with chemotherapy or other cytotoxic therapies in HNSCC.
Supplementary Material
The extrinsic cell death pathway, induced by DNA damage, and the intrinsic pathway, triggered by ligands of the TNF family, are both crucial pathways that promote activation of caspase-mediated apoptosis. However, this signaling and cell death may be blocked by Inhibitor of Apoptosis Proteins (IAPs), the protein products of BIRC family genes, shown by the black lines (negative regulation). Cytotoxic agents like docetaxel cause DNA damage via the extrinsic cell death pathway. This promotes release of SMAC from the mitochondria, which inhibits and degrades IAPs, thereby allowing apoptosis to proceed. Alternatively, the intrinsic pathway works through the induction of a cytoplasmic complex and signaling cascade involving FADD and RIP1, demonstrated by the arrows (positive regulation). Ultimately, both pathways converge on caspase-3 to initiate apoptosis. Birinapant is a SMAC mimetic that inhibits IAPs, with greatest effect on cIAP1 shown by the bolded line, to enhance both of these pathways and potentiate apoptosis.
RNA was isolated from UMSCC cells, and the differential gene expression was examined by an Illumina bead-based microarray, using Illumina HumanWG-6-v3-BeadChip. Differentially regulated gene expression in UMSCC cells was compared with normal human primary mucosa cells (HOK). Y axis represents the bead counts reflecting relative gene expression.
Acknowledgments
Supported by NIDCD intramural projects ZIA-DC-000016, 73, 74 and the NIH-Medical Research Scholars Program (DFE, GS).
Footnotes
Birinapant was provided to NIDCD and authors (DFE, GES, SGC, SS, ZC and CVW) for research under a Materials Transfer Agreement with the National Cancer Institute and TetraLogic Pharmaceuticals.
Presented at: Combined Otolaryngological Spring Meetings- Triological Society Las Vegas, NV, USA, 5/16/14
Financial Disclosures: Supported by NIDCD intramural projects ZIA-DC-000016,73,74 and the NIH-Medical Research Scholars Program (DFE, GES).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012 Dec 6;31(49):5045–5060. doi: 10.1038/onc.2012.7. [DOI] [PubMed] [Google Scholar]
- 3.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nature reviews. Molecular cell biology. 2011 Nov;12(11):757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katherine A, Hoadley CF, Matthew D, Wilkerson, et al. Multi-tumor analysis of TCGA data identifies expression commonalities across tumor types. [abstract]. Paper presented at : Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013; Washington, DC. [Google Scholar]
- 5.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nature reviews. Cancer. 2010 Aug;10(8):561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 6.Tanimoto T, Tsuda H, Imazeki N, et al. Nuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomas. Cancer letters. 2005 Jun 16;224(1):141–151. doi: 10.1016/j.canlet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Qi S, Mogi S, Tsuda H, et al. Expression of cIAP-1 correlates with nodal metastasis in squamous cell carcinoma of the tongue. International journal of oral and maxillofacial surgery. 2008 Nov;37(11):1047–1053. doi: 10.1016/j.ijom.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nature reviews. Drug discovery. 2012 Feb;11(2):109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 9.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007 Nov 16;131(4):669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Benetatos CA, Mitsuuchi Y, Burns JM, et al. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Molecular cancer therapeutics. 2014 Apr;13(4):867–879. doi: 10.1158/1535-7163.MCT-13-0798. [DOI] [PubMed] [Google Scholar]
- 11.Allensworth JL, Sauer SJ, Lyerly HK, Morse MA, Devi GR. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-alpha-independent mechanism. Breast cancer research and treatment. 2013 Jan;137(2):359–371. doi: 10.1007/s10549-012-2352-6. [DOI] [PubMed] [Google Scholar]
- 12.Condon SM, Mitsuuchi Y, Deng Y, et al. Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. Journal of medicinal chemistry. 2014 May 8;57(9):3666–3677. doi: 10.1021/jm500176w. [DOI] [PubMed] [Google Scholar]
- 13.Krause CJ, Carey TE, Ott RW, Hurbis C, McClatchey KD, Regezi JA. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Archives of otolaryngology (Chicago, Ill: 1960) 1981 Nov;107(11):703–710. doi: 10.1001/archotol.1981.00790470051012. [DOI] [PubMed] [Google Scholar]
- 14.Carey TE. Establishment of epidermoid carcinoma cell lines. In: REW, editor. Head and Neck Cancer. New York: John Wiley & Sons Inc; 1985. pp. 289–316. [Google Scholar]
- 15.Bradshaw-Pierce EL, Steinhauer CA, Raben D, Gustafson DL. Pharmacokinetic-directed dosing of vandetanib and docetaxel in a mouse model of human squamous cell carcinoma. Molecular cancer therapeutics. 2008 Sep;7(9):3006–3017. doi: 10.1158/1535-7163.MCT-08-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010 Jan 15;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny MV, Schulte TW, Nguyen P, Mimnaugh EG, Trepel J, Neckers L. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer research. 1995 Oct 15;55(20):4623–4626. [PubMed] [Google Scholar]
- 18.Bradford CR, Zhu S, Ogawa H, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head & neck. 2003 Aug;25(8):654–661. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 19.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2007 Dec 20;357(25):2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakelee HA, Patnaik A, Sikic BI, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010 Feb;21(2):376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forero-Torres A, Shah J, Wood T, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer biotherapy & radiopharmaceuticals. 2010 Feb;25(1):13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camidge DR. Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use in the treatment of solid tumors. Expert opinion on biological therapy. 2008 Aug;8(8):1167–1176. doi: 10.1517/14712598.8.8.1167. [DOI] [PubMed] [Google Scholar]
- 23.Beug ST, Tang VA, LaCasse EC, et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nature biotechnology. 2014 Feb;32(2):182–190. doi: 10.1038/nbt.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xanthinaki A, Nicolatou-Galitis O, Athanassiadou P, et al. Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008 Sep;16(9):1025–1033. doi: 10.1007/s00520-007-0379-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The extrinsic cell death pathway, induced by DNA damage, and the intrinsic pathway, triggered by ligands of the TNF family, are both crucial pathways that promote activation of caspase-mediated apoptosis. However, this signaling and cell death may be blocked by Inhibitor of Apoptosis Proteins (IAPs), the protein products of BIRC family genes, shown by the black lines (negative regulation). Cytotoxic agents like docetaxel cause DNA damage via the extrinsic cell death pathway. This promotes release of SMAC from the mitochondria, which inhibits and degrades IAPs, thereby allowing apoptosis to proceed. Alternatively, the intrinsic pathway works through the induction of a cytoplasmic complex and signaling cascade involving FADD and RIP1, demonstrated by the arrows (positive regulation). Ultimately, both pathways converge on caspase-3 to initiate apoptosis. Birinapant is a SMAC mimetic that inhibits IAPs, with greatest effect on cIAP1 shown by the bolded line, to enhance both of these pathways and potentiate apoptosis.
RNA was isolated from UMSCC cells, and the differential gene expression was examined by an Illumina bead-based microarray, using Illumina HumanWG-6-v3-BeadChip. Differentially regulated gene expression in UMSCC cells was compared with normal human primary mucosa cells (HOK). Y axis represents the bead counts reflecting relative gene expression.