Abstract
Background
Organ donors are often implicated as the source of posttransplant recipient infection. We prospectively studied kidney and liver donor-recipient pairs to determine if donor viral replication of cytomegalovirus (CMV), Epstein-Barr virus (EBV), and BK polyomavirus (BKV) at transplant was a risk factor for posttransplant recipient infection and disease.
Methods
Donors and recipients were studied for antibodies against CMV and EBV and for quantitative viral replication of CMV, EBV and BKV in oral washes, urine, and whole blood pretransplant. Recipient testing continued every 3 months posttransplant. Demographic and clinical data on infections and graft and subject outcomes were obtained.
Results
The 98 donor-recipient pairs included 15 liver and 83 kidney transplants (18 of whom were children). No donor had detectable CMV replication; therefore its impact on recipient CMV replication could not be analyzed. Donor EBV replication occurred in 22%, mostly in the oral wash and had no impact on posttransplant recipient EBV replication (p 0.9) or EBV viremia (p 0.6) in kidney or liver recipients. Donor BKV replication occurred in 17%, mostly in the urine and although not associated with posttransplant recipient urinary BKV replication in recipients, it was associated with BKV viremia (p 0.02), and a significantly shorter time to BKV viremia (p 0.01) in kidney recipients.
Conclusion
Donor replication of CMV or EBV did not impact posttransplant recipient viral replication in kidney/liver transplants. Donor urinary BKV replication is associated with recipient BKV viremia in kidney transplants.
Keywords: Donor, Epstein-Barr virus, Cytomegalovirus, BK virus, Viral Replication, Solid-organ Transplant
Introduction
Viral infections cause substantial morbidity after solid organ transplantation including graft loss and death. Cytomegalovirus (CMV), Epstein-Barr virus (EBV) and BK polyomavirus (BKV) are the viruses most often implicated in posttransplant tissue-invasive disease. Even sub-clinical CMV and/or EBV infections are associated with graft dysfunction (1–3). Recipients at highest risk for CMV or EBV disease are those previously unexposed to the virus pretransplant (antibody negative = R−) who receive an organ from an antibody-positive donor (D+) (4, 5). These and other studies suggest that the donor is the source of CMV and/or EBV for many solid-organ transplant recipients (6–8). The role of the donor in the transmission of BKV is less well defined.
We conducted a 4-year prospective study that investigated whether donor viral replication of CMV, EBV or BKV in the urine, oral wash or blood at the time of transplant was a risk factor for posttransplant recipient infection and disease.
Results
We studied 98 donor-recipient pairs for 224 person years [TABLE 1]. Posttransplant follow-up ranged from 72 days to 4 years (mean 2.3 years).
TABLE 1.
Demographics of 98 Living Donor/Recipient Pairs Studied
| Characteristic | Number of Subjects or Mean Value |
|---|---|
|
| |
| Type of transplant | |
| Kidney | 83 |
| Liver | 15 |
|
| |
| Pediatric transplants (all kidney) | 18 |
|
| |
| Donors | |
| Living Related | 52 |
| Living Unrelated | 46 |
|
| |
| Recipient Characteristics | |
| Mean age in years (range) | 42.8 (1.2–73.3) |
| Females | 23 |
| Ethnicity: | |
| Caucasian | 87 |
| African American | 7 |
| Asian | 3 |
| American Indian | 1 |
|
| |
| Donor Characteristics | |
| Mean age in years (range) | 40.8 (15.8–65.1) |
| Females | 60 |
| Ethnicity: | |
| Caucasian | 91 |
| African American | 5 |
| Asian | 2 |
|
| |
| Donor Recipient Pretransplant Antibody Status for CMV | |
| D+R+ | 26 |
| D+R− | 17 |
| D−R+ | 17 |
| D−R− | 38 |
|
| |
| Donor Recipient Pretransplant Antibody Status for EBV | |
| D+R+ | 74 |
| D+R− | 19 |
| D−R+ | 2 |
| D−R− | 3 |
|
| |
| Total number of donor samples tested | 957 |
|
| |
| Total number of recipient samples tested | 4390 |
|
| |
| Mean number of samples tested/donor (range) | 10±3.3 (0–12) |
|
| |
| Mean number of samples tested/recipient (range) | 45±20.4 (10–84) |
|
| |
| Mean follow up time in days/recipient (range) | 835±380 (72–1485) |
Effects of Donor Viral Antibody Status and Viral Replication on Posttransplant Infection
Forty-three (44%) and 93 (95%) donors and 43 (44%) and 75 (78%) recipients were CMV and EBV antibody-positive respectively prior to transplant. When the analysis was restricted to adult recipients, the proportion of CMV and EBV antibody positivity was similar to donors. Samples for detection of CMV, EBV and BKV at the time of transplant were available for 95 donors and for all recipients.
There were 19 and 17 donor-recipient pairs that were discordantly D+ and R− for CMV and EBV respectively and all were kidney transplant pairs. D+ was associated with increased recipient replication and viremia for CMV and EBV [TABLE 2]. In fact, there was no EBV viral replication or viremia in the 3 recipients of EBV antibody-negative donors (D−).
TABLE 2.
Posttransplant Recipient CMV and EBV Replication Stratified by Pretransplant Donor-Recipient Antibody Status for the Corresponding Virus
| Donor Recipient Serology | KIDNEY (n=83) | LIVER (N=15) | ||||||
|---|---|---|---|---|---|---|---|---|
| EBV viral replication in urine/oral wash post-tx (p 0.8) | EBV viremia post-tx (p 0.09) | CMV viral replication in urine/oral wash post-tx (p 0.2) | CMV viremia post-tx (p 0.02) | EBV viral replication in urine/oral wash post-tx (p 0.8) | EBV viremia post-tx (p 0.8) | CMV viral replication in urine/oral wash post-tx | CMV viremia post-tx | |
| D+R− | 5/19 (32%) | 8/19 (42%) | 1/17 (12%) | 6/17 (35%) | 0 | 0 | 0 | 0 |
| D+R+ | 16/61 (33%) | 10/61 (16%) | 1/23 (9%) | 8/23 (35%) | 2/13 (15%) | 2/13 (15%) | 0/3 (0%) | 0/3 (0%) |
| D−R+ | 0/1 (0%) | 0/1 (0%) | 2/13 (8%) | 3/13 (23%) | 0/1 (0%) | 0/1 (0%) | 0/4 (0%) | 0/4 (20%) |
| D−R− | 0/2 (0%) | 0/2 (0%) | 0/30 (0%) | 1/30 (3%) | 0/1 (0%) | 0/1 (0%) | 0/8 (0%) | 0/8 (25%) |
CMV Replication in Donors and Recipients
None of the donors had detectable CMV replication at the time of transplant [TABLE 3].
TABLE 3.
Quantitative Viral Replication in Urine, Oral Wash, and Blood at Transplant and Posttransplant among 98 Donor and Recipient Pairs
| VIRUS | Donors at Transplant (Samples available in 95/98) | KIDNEY (N=83) | LIVER (N=15) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recipients at Transplant | Recipients Posttransplant | Recipients at Transplant | Recipients Posttransplant | ||
|
| |||||
| Number (quantitative range in copies/mL) | |||||
|
| |||||
| EBV | |||||
| - Any site | 21 (22%) | 28 (34%) | 34 (41%) | 5 (33%) | 3 (20%) |
| - Oral wash | 21 (50–183,200) | 26 (100–6,115,000) | 26 (200–1,844,700) | 5 (200–125,700) | 2 (1,700–13,600) |
| - Urine | 0 | 1 (11,900) | 1 (800) | 0 | 0 |
| - Whole blood | 2 (1300–2000) | 2 (200) | 18 (200–52,800) | 0 | 2 (300–46,300) |
|
| |||||
| CMV | |||||
| - Any site | 0 | 2 (2%) | 21 (25%) | 0 (0%) | 0 |
| - Oral wash | 0 | 1 (200) | 5 (200–300) | 0 | |
| - Urine | 0 | 1 (200) | 0 | 0 | |
| - Whole blood | 0 | 0 | 18 (100–388,600) | 0 | |
|
| |||||
| BKV | |||||
| - Any site | 17 (17%) | 10 (12%) | 29 (35%) | 1 (7%) | 4 (27%) |
| - Oral wash | 0 | 0 | 0 | 0 | 0 |
| - Urine | 17 (800–9,056,000) | 10 (900–102,900) | 25 (400–≥10,000,000) | 1 (102,900) | 2 (300–121,100) |
| - Whole blood | 1 (1300) | 0 | 12 (300–1,575,000) | 0 | 2 (21,880–358,800) |
Two kidney recipients (2%) had detectable CMV replication at transplant: one in oral wash (200 copies/mL) and one in whole blood (200 copies/mL). None of the liver recipients had detectable CMV replication before or posttransplant.
CMV viral replication patterns for pretransplant recipients and donors were not significantly different (p 0.6). Posttransplant, 21 (21%) kidney recipients had a positive CMV viral load. Recipient CMV viremia was significantly greater posttransplant than pretransplant (p<0.001). CMV replication was significantly higher post-kidney than post-liver transplant (p 0.02). CMV replication in urine and oral wash was rare. Since pretransplant recipient and donor CMV replication was rare, its impact on posttransplant recipient CMV replication could not be analyzed. Fifteen recipients developed CMV disease requiring active reduction of immunosuppression and antiviral therapy. Nine were D+R−, 4 D+R+ and 2 were D−R+; 8 had detectable CMV replication posttransplant (7 in blood).
Posttransplant recipient CMV replication did not have a significant impact on death-censored graft survival (DCGS) (DCGS at 1 and 3 years in patients with posttransplant CMV viremia was 100% and 100% and in patients without posttransplant CMV viremia was 100% and 99% respectively; p 0.6) or recipient survival (patient survival at 1 and 3 years in patients with posttransplant CMV viremia was 94% and 83% and in patients without posttransplant CMV viremia was 97% and 96% respectively; p 0.2).
EBV Replication in Donors and Recipients
Twenty-one donors (22%) had a positive EBV viral load. Twenty had EBV in the oral wash (50–183,200 copies/mL) and 2 had viremia (1300 and 2000 copies/mL, respectively) with and without a positive viral load in the oral wash. There was no EBV DNA in donor urine samples [TABLE 3].
Of the 83 kidney and 15 liver recipients, 28 (34%) and 5 (33%) had a positive EBV viral load at the time of transplant respectively. Almost all EBV replication in these cases were detected in the oral wash, one subject had viruria and 2 subjects had isolated low grade EBV viremia.
EBV viral replication patterns for pretransplant recipients and donors were not significantly different (p 0.1).
Posttransplant, 34 (38%) recipients had a positive EBV viral load. Posttransplant EBV replication rates and patterns in kidney and liver recipients were not significantly different (p 0.13). Pre-transplant recipient EBV replication did not impact the rate of post-transplant EBV replication in oral wash (p 0.4) or viremia (p 0.4) in kidney or liver recipients. Recipient EBV viremia was significantly greater posttransplant than pretransplant (p <0.001).
Detectable donor EBV replication did not affect the incidence of posttransplant EBV replication in the oral wash (p 0.4) or EBV viremia (p 0.4) in kidney or liver recipients [TABLE 4] nor was the time to EBV viremia associated with donor EBV replication (p 0.5). Two subjects developed EBV disease, one of whom subsequently was diagnosed with posttransplant lymphoproliferative disorder (PTLD) and died; neither had donors with detectable EBV replication although both were D+R− for EBV.
TABLE 4.
Quantitative Viral Replication in Urine, Oral Wash, and Blood in Donors at Transplant and Recipients Posttransplant among 98 Donor and Recipient Pairs *, **
| AT TRANSPLANT | POST-TRANSPLANT | |||
|---|---|---|---|---|
|
| ||||
| KIDNEY (N=83) | ||||
|
| ||||
| Donor EBV replication in Blood/Urine/Oral Wash | Recipient EBV Replication in Urine/Oral Wash | Recipient EBV viremia | ||
|
| ||||
| Positive (n=21)
|
4 (19%) | p 0.45 | 6 (29%) | p 0.38 |
| Negative (n=62) | 17 (27%) | 12 (19%) | ||
|
| ||||
| Donor BKV replication in Blood/Urine/Oral Wash | Recipient BKV Replication in Urine/Oral Wash | Recipient BKV viremia | ||
|
| ||||
| Positive (n=15)
|
7 (47%) | p 0.12 | 5 (33%) | p 0.02*** |
| Negative (n=68) | 18 (26%) | 7 (10%) | ||
|
| ||||
| LIVER (N=15) | ||||
|
| ||||
| Donor EBV replication in Blood/Urine/Oral Wash | Recipient EBV Replication in Urine/Oral Wash | Recipient EBV viremia | ||
|
| ||||
| Positive (n=1)
|
0 | p 0.69 | 0 | p 0.69 |
| Negative (n=14) | 2 (14%) | 2 (14%) | ||
|
| ||||
| Donor BKV replication in Blood/Urine/Oral Wash | Recipient BKV Replication in Urine/Oral Wash | Recipient BKV viremia | ||
|
| ||||
| Positive (n=2)
|
0 | p 0.55 | 0 | p 0.55 |
| Negative (n=13) | 2 (15%) | 2 (15%) | ||
There was no donor replication of CMV and therefore its impact on recipient CMV viral replication/viremia could not be demonstrated
Only 2 donors had EBV viremia and 1 donor had BKV viremia. Therefore the impact of donor EBV and BKV viremia on recipient viral replication is not shown separately. The kidney recipients of the donor that had isolated EBV viremia as well as of the donor that had isolated BKV viremia at the time of transplant did not have posttransplant BKV replication in urine/oral wash or blood.
p<0.05 is considered significant
Neither donor nor posttransplant recipient EBV replication had a significant impact on DCGS (p 0.3) or recipient survival (p 0.9). Our analysis was inadequately powered but there appeared to be no association between donor EBV replication and recipient viral disease.
BKV Replication in Donors and Recipients
Seventeen (18%) donors had BKV in the urine (800–9,056,000 copies/mL), 1 of whom also had viremia (1,300 copies/mL) [TABLE 3].
Ten (12%) kidney recipients had BKV in the urine (900–102,900 copies/mL) at transplant. Five (33%) liver recipients had detectable BKV replication at the time of transplant: 3 in the urine (300–121,100 copies/mL) and 2 in the blood (21,880–358,800). Pretransplant recipient and donor BKV replication patterns were not significantly different (p 0.2).
Posttransplant, 33 (34%) recipients had a positive BKV viral load [TABLE 2] with no significant difference between kidney and liver recipients (p 0.5). Post-kidney transplant urinary BKV replication was significantly higher than pretransplant (p 0.004) [even after excluding recipients that underwent native nephrectomy before (n=8) or at transplant (n=4) (p 0.01)], but not for liver transplants (p 0.5). Recipient BKV viremia was significantly greater posttransplant than pretransplant (p <0.001). Kidney recipients replicating BKV pretransplant were significantly more likely to have posttransplant urinary BKV replication (p 0.03) but not BKV viremia (p 0.5). Pretransplant BKV replication in liver recipients had no impact on BKV viremia or urinary replication.
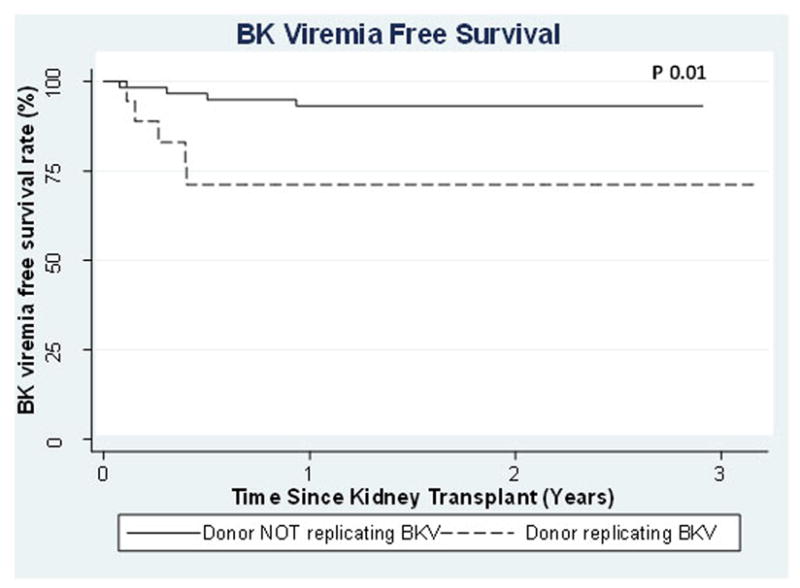
Donor BKV replication was not associated with posttransplant recipient urinary BKV replication in kidney (p 0.12) or liver recipients (p 0.55), but was associated with increased posttransplant BKV viremia in kidney recipients (p 0.02) [TABLE 4]. We were unable to discern whether the BK viruria was from the native kidneys or the donor kidney. Of the 12 recipients that had native nephrectomies before or at transplant, 5 recipients had post-transplant BKV urinary replication (2 had donors with a positive BK viral load) and 3 had viremia post-kidney transplant (2 had donors with a positive BK viral load). Time to BKV viremia in the kidney recipients was significantly shorter if their donors had detectable BKV replication (p 0.01) [FIGURE 1] with 6 month BKV viremia free survival of 71% vs. 95% in recipients of donors with and without detectable BKV replication. Eleven subjects were clinically diagnosed with BKV-related disease but there were no biopsy-proven cases of BKV nephropathy. None of the 11 required immunosuppression reduction and only 3 had donors that were replicating virus at transplant.
Figure 1.
Neither donor nor posttransplant recipient BKV replication had a significant impact on death-censored graft survival or recipient survival.
Co-infection in Donors and Recipients
EBV antibody-positive recipients were significantly more likely to be CMV antibody-positive than EBV antibody-negative recipients even when the analysis was restricted to pediatric recipients (p 0.01). Eight (8%) of 98 recipients were replicating EBV and CMV posttransplant: one died suddenly 3 months posttransplant (40,000 copies of CMV/mL whole blood and 5,000 copies of EBV/mL whole blood). The cause of death was not apparent at autopsy.
Donor replication of EBV and BKV was almost mutually exclusive: donors that had detectable EBV replication rarely had positive BKV replication (1/21) and donors that had detectable BKV rarely had positive EBV replication (1/17) (p=0.07). But the converse was true of post-transplant recipients in whom detectable BKV replication occurred in recipients with EBV replication (20/37=54%) more often than in those without EBV replication (13/61=21%) (p=0.001).
Detectable BKV replication posttransplant occurred in recipients with CMV replication (3/21=14%) less often than without (30/77=39%) (p=0.03). One patient with CMV disease had a simultaneous diagnosis of BKV nephropathy.
Discussion
While donor replication of CMV or EBV at transplant did not increase the risk of posttransplant infection or disease, finding BKV in donor urine at transplant was associated with an increased incidence of BKV viremia (p 0.02), and a shorter time to BKV viremia (p 0.01).
The occurrence of BKV nephropathy in kidney but not liver recipients suggests that BKV infection originates in the donor organ though this is not well defined (9, 10). In an immunocompetent person, BKV is thought to colonize the urinary tract and establishes latency (11). We provide evidence that the transplanted kidney and ureter are likely the source of posttransplant recipient BKV infection supported by the fact that BKV viruria/viremia was not observed in liver recipients posttransplant whose donors were replicating BKV. In keeping with our finding, Barzon etal. showed that the cumulative proportion of subjects BKV viremia free in the first year posttransplant was significantly decreased if the pre-implantation graft biopsy, preservation and washing solutions contained BKV DNA (12). High BKV-specific antibody titers in donors (possibly representing recent BKV exposure / higher graft load) and detection of BKV infection within 5 days of transplantation are risk factors for BKV nephropathy in kidney recipients (13). Therefore, the association of donor BKV replication and posttransplant recipient BKV viremia is likely due to tropism of BKV for the kidney and the high BKV antibody prevalence in healthy adult (14, 15) kidney donors. While the lack of BKV antibody data in our study is unfortunate, BKV antibody testing is not routinely done at most centers including ours.
The increased viremia associated with donor BKV viruria was not associated with increased BKV-related disease. Because our numbers were small, a larger study is necessary to truly assess the impact of kidney donor BKV replication on BKV disease posttransplant. Pretransplant kidney recipient BK viruria was independently and significantly associated with posttransplant recipient BKV viruria (p 0.03) but not viremia. We acknowledge that our study does not allow identification of the source of BKV in recipients posttransplant – native kidneys vs. donor kidney particularly since the number of patients that had native nephrectomies at or pretransplant was small. BKV viruria in the absence of BK viremia is not usually associated with an increased risk for BKV disease (16). Therefore, we conclude that despite the association of recipient BK viruria pre- and posttransplant, there is probably no benefit to screening recipients pretransplant for urinary BKV.
In keeping with our current understanding of CMV and EBV risk factors (4, 5), the highest risk group for post-transplant viral replication of CMV and/or EBV was D+ for the respective viruses regardless of the type of organ transplanted (17). Viral replication patterns for CMV, EBV and BKV were not significantly different for donor and recipients pretransplant suggesting that chronic kidney/liver disease did not promote active viral replication. CMV replication was not detected in any donor and rarely in recipients pretransplant. EBV replication was observed in donors (22%) and recipients (33%) pretransplant mostly in the oral wash. Oropharyngeal epithelial cells are permissive for EBV replication (18, 19), which could account for this observation. BKV has been identified in the urine of immunocompetent subjects (20), which is consistent with our observation of almost exclusive BKV replication in the urine of healthy donors (18%) and recipients (12%) pretransplant.
Donor replication of BKV and EBV was almost mutually exclusive. In a study of 30 EBV antibody-positive healthy adults with documented EBV oral replication, blood, urine and oral wash samples tested every 2 months for 14 months were negative for BKV replication (21). The significance and mechanism responsible is unclear particularly since we did not make this observation in the recipients pretransplant. Could there be a mechanism whereby EBV replication protects healthy adults from BKV replication and vice versa? CMV viremia was associated with a decreased incidence of BKV reactivation in kidney transplant recipients in a recent study and while the authors proposed it could be due to reduction in immunosuppression (22); this could have been due to an ongoing protective CD8+ lymphocyte response activated by the preceding CMV (23). Perhaps a similar mechanism could explain our findings.
Posttransplant, recipients with EBV replication were more likely to replicate BKV than recipients without EBV replication (p 0.001) and recipients with posttransplant CMV replication were also more likely to have a positive BKV viral load (p 0.03). The increased likelihood of co-infection could merely represent the overall state of immunosuppression of the recipient or may be the result of the immunosuppressive effects of CMV and EBV (24–26). Or it could mean that some individuals have certain Class-1 HLA-antigens responsible for presenting virus to CD8+ lymphocytes that are less effective in presenting some viral antigens than others. We were unable in our small cohort to identify a predominant cluster/cross-reacting group of Class I HLA antigens that appeared in those with any of the viremias to suggest a defect in presentation in those individuals.
Immunosuppression had the obvious effect and viremia was significantly greater posttransplant for all 3 viruses (p <0.001) as was BK viruria in kidney recipients (p 0.004). Posttransplant CMV replication was greater in kidney than liver recipients (p 0.02) likely representing the increased immunosuppression utilized in kidney recipients. However for EBV and BKV, posttransplant replication was not significantly different for liver and kidney transplant.
As stated above, donor replication of CMV or EBV at transplant did not increase the risk of posttransplant infection or disease. Since EBV is known to establish latency in B cells, it seems reasonable that the mode of donor-recipient transmission of virus in organ transplantation is via circulating blood cells within the transplanted organ. The prevalence of EBV DNA in the preservation and washing solutions has been shown to be similar to the blood in healthy blood donors (12) and since the blood and oral compartments are separate, donor oral EBV replication (a known aspect of latent EBV infection in healthy adults (27)) may not cause transmission of infection to the recipient. However, recipients of the 2 donors who were replicating EBV in their blood at the time of transplant did not have EBV DNA in their blood, urine or oral wash at any time posttransplant. This does not rule out the transmission of EBV via the infected blood within the organ since the virus could be latent within the transplanted organ without overt recipient viral replication.
Replication of CMV, EBV and/or BKV in recipients posttransplant had no significant impact on death-censored graft survival, patient survival or viral disease free survival. Our findings are contrary to previous publications that suggest a negative impact of these viruses on graft and patient outcome (1, 2). However, the number of subjects with viral disease in our cohort was small and our analysis was inadequately powered to be definitive. In addition, it is important to recognize that the difference in susceptibility to viral replication alone vs. virus organ penetration and infection may be due to the net level of immunosuppression or genetic susceptibility and could account for the differences in the negative impact these viruses have on outcomes.
In conclusion, testing living donors for EBV or CMV viral replication at transplant did not predict posttransplant viral transmission. However, it may be worthwhile to monitor urinary BKV replication in kidney transplant donors with increased vigilance posttransplant for BKV viremia in the recipients of donors replicating BKV. This will allow early identification of BKV infection and stepwise reduction of immunosuppression which has been shown to effectively curtail BKV viremia and replication in the kidney allograft (28, 29).
Materials and Methods
Study Design
All subjects receiving their first kidney or liver from a living donor were consecutively enrolled pretransplant and followed for as long as 4 years from December 1st, 2008 to December 1st, 2011. Donors and recipients were studied at transplant for the presence of IgG antibodies against CMV and EBV, and for quantitative viral replication of CMV, EBV and BKV in oral washes, urine, and ethylenediamine tetraacetic acid (EDTA) anti-coagulated whole blood. Viral load samples were collected from recipients approximately every 3 months posttransplantation for upto 49 months posttransplant. All recipients received antiviral prophylaxis with valganciclovir (450mg daily if creatinine clearance was 40–60mL/min; 900mg daily if clearance was >60mL/min; or 15mg/kg in children with maximum dose of 900mg daily depending on creatinine clearance) for at least 3 months. CMV antibody negative, or EBV antibody negative pediatric recipients of EBV antibody positive organ donors received 12 months of prophylaxis. CMV or EBV antibody negative adult recipients of antibody positive organ donors received 6 months of prophylaxis.
Demographic and clinical data on infections, graft and subject outcomes were obtained from the database of prospectively recorded demographics and outcomes data for all kidney and liver transplants performed at the University of Minnesota. Induction and maintenance immunosuppression was almost identical in all the patients. Kidney transplant recipients received Thymoglobulin induction and an anti-metabolite (azathioprine or mycophenolate), a calcineurin inhibitor (tacrolimus or cyclosporine) and steroids if under 5 years of age and steroid avoidance (6 days) if over 5 years of age while liver transplant recipients received Basilimab induction with mycophenolate, steroid avoidance (5 days to 1 month) and tacrolimus. This study was approved by the Research Subjects Protection Program of the University of Minnesota (IRB # 0804M31463) and informed consent was obtained from donors and their recipients before participation.
Quantitative viral DNA assays
Viral DNA was extracted from the samples using the QIAamp® DNA minikit (QIAGEN, Inc, Valencia, CA). CMV, EBV, and BKV viral loads were measured by real-time quantitative TaqMan PCR assays, all of which were developed and validated by our research and diagnostic virology laboratories.
Viral antibody tests
CMV IgG and EBV VCA IgG antibodies were measured by semiquantitative enzyme immunoassays (EIAs) performed with the manual method according to the manufacturer’s (Diamedix Corporation, Miami, FL) instructions. Specimens, calibrators and controls tested for EBV VCA IgG antibodies were pre-diluted 1:21; those for CMV IgG and were pre-diluted 1:101 prior to placing them in the test wells.
Data gathering, data coordination, and statistics
The following data were evaluated:
Donor and recipient baseline CMV and EBV antibody status at transplant.
Recipient serial measurements of CMV, EBV, and BKV viral loads treated as categorical variables. A positive viral load was defined as: CMV, ≥100 copies/mL of sample; EBV, ≥200 copies/mL of sample; and BKV, ≥500 copies/mL of sample.
All physician-initiated treatment of viral disease was recorded. Viral replication was considered positive if there was a viral load in urine, oral wash or blood. CMV disease was defined as CMV DNA in the blood (viremia) on ≥2 occasions plus clinical and pathological confirmation of CMV end-organ disease. EBV disease was defined as EBV viremia plus evidence of EBV in a tissue biopsy or radiologic demonstration of mass lesion(s) consistent with EBV disease. BKV disease was a pathologic diagnosis of BKV nephropathy from a kidney biopsy.
Donor and recipient demographics (age, race and gender) and virology data were analyzed. Pearsons χ2 test of association was used to assess the association between donors who were actively replicating CMV, EBV or BKV at the time of transplant and posttransplant recipient viral replication of the respective virus. Actuarial viremia-free survival rates were computed by the Kaplan-Meier method. Viremia-free survival was compared between recipients with and without donors who were actively replicating virus at transplant using log-rank analysis. Statistical significance was set at a p value <0.05, two sided. All statistical analyses were performed using STATA 11.0 software (STATA Corporation, College Station, TX, USA).
Acknowledgments
This study was supported by a grants from the National Institutes of Health (2PO1 DK13083), and the University of Minnesota International Center for Antiviral Research and Epidemiology. The authors thank Beth D. Mullan and Julie A. Ed for their assistance in interacting with patients and assembling the data as well as Alicia Gruenwald for her assistance in data collection.
List of Abbreviations
- BKV
BK polyomavirus
- CMV
Cytomegalovirus
- EBV
Epstein Barr virus
- EDTA
Ethylenediamine tetraacetic acid
- EIAs
Enzyme immunoassays
- PTLD
Posttransplant lymphoproliferative disorder
Footnotes
Priya Verghese: Participated in research design, writing of the paper, performance of the research and data analysis
Arthur J. Matas: Participated in research design and performance of the research
David O. Schmeling: Participated in performance of the research
Jennifer A. Knight: Participated in performance of the research
Henry H. Balfour Jr: Participated in research design, writing of the paper, performance of the research and data analysis
Conflict of interest: None by any authors
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
References
- 1.Li L, Chaudhuri A, Weintraub LA, Hsieh F, Shah S, Alexander S, et al. Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant. 2007;11(2):187–95. doi: 10.1111/j.1399-3046.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol. 2010;21(9):1579–86. doi: 10.1681/ASN.2009111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, Saas P, et al. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13(3):656–62. doi: 10.1111/ajt.12009. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56(1):155–67. doi: 10.1016/j.critrevonc.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Kaden J, Zenker S, Eichler C, Groth J, May G, Strobelt V, et al. Risk of CMV infection and illness after kidney transplantation. Allerg Immunol (Leipz) 1991;37(1):47–58. [PubMed] [Google Scholar]
- 6.Ho M, Suwansirikul S, Dowling JN, Youngblood LA, Armstrong JA. The transplanted kidney as a source of cytomegalovirus infection. N Engl J Med. 1975;293(22):1109–12. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- 7.Cen H, Breinig MC, Atchison RW, Ho M, McKnight JL. Epstein-Barr virus transmission via the donor organs in solid organ transplantation: polymerase chain reaction and restriction fragment length polymorphism analysis of IR2, IR3, and IR4. J Virol. 1991;65(2):976–80. doi: 10.1128/jvi.65.2.976-980.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balfour HH, Jr, Slade MS, Kalis JM, Howard RJ, Simmons RL, Najarian JS. Viral infections in renal transplant donors and their recipients: a prospective study. Surgery. 1977;81(5):487–92. [PubMed] [Google Scholar]
- 9.Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Schnitzler MA, Major EO, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(9):2213–21. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 10.Saundh BK, Baker R, Harris M, Welberry Smith MP, Cherukuri A, Hale A. Early BK polyomavirus (BKV) reactivation in donor kidney is a risk factor for development of BKV-associated nephropathy. The Journal of infectious diseases. 2013;207(1):137–41. doi: 10.1093/infdis/jis642. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3(10):611–23. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 12.Barzon L, Murer L, Pacenti M, Biasolo MA, Vella MD, Ghirardo G, et al. Detection of viral DNA in kidney graft preservation and washing solutions is predictive of posttransplant infections in pediatric recipients. The Journal of infectious diseases. 2009;200(9):1425–33. doi: 10.1086/644504. [DOI] [PubMed] [Google Scholar]
- 13.Sood P, Senanayake S, Sujeet K, Medipalli R, Van-Why SK, Cronin DC, et al. Donor and recipient BKV-specific IgG antibody and posttransplantation BKV infection: a prospective single-center study. Transplantation. 2013;95(6):896–902. doi: 10.1097/TP.0b013e318282ba83. [DOI] [PubMed] [Google Scholar]
- 14.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837–46. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 15.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71(1):115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–86. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 17.de la Torre-Cisneros J, Farinas MC, Caston JJ, Aguado JM, Cantisan S, Carratala J, et al. GESITRASEIMC/REIPI recommendations for the management of cytomegalovirus infection in solid-organ transplant patients. Enfermedades infecciosas y microbiologia clinica. 2011;29(10):735–58. doi: 10.1016/j.eimc.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Sixbey JW, Vesterinen EH, Nedrud JG, Raab-Traub N, Walton LA, Pagano JS. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983;306(5942):480–3. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- 19.Li QX, Young LS, Niedobitek G, Dawson CW, Birkenbach M, Wang F, et al. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356(6367):347–50. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 20.Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2004;29(4):224–9. doi: 10.1016/S1386-6532(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 21.Ling PD, Lednicky JA, Keitel WA, Poston DG, White ZS, Peng R, et al. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14-month longitudinal study. The Journal of infectious diseases. 2003;187(10):1571–80. doi: 10.1086/374739. [DOI] [PubMed] [Google Scholar]
- 22.Elfadawy N, Flechner SM, Liu X, Schold J, Srinivas TR, Poggio E, et al. CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 2013;96(12):1097–103. doi: 10.1097/TP.0b013e3182a6890d. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Oliva MO, Martinez V, Buitrago A, Jimenez C, Rivas B, Escuin F, et al. Pretransplant CD8 T-Cell Response to IE-1 Discriminates Seropositive Kidney Recipients at Risk of Developing CMV Infection Posttransplant. Transplantation. 2014;97(8):839–45. doi: 10.1097/01.TP.0000438025.96334.eb. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt LM. Etiology and impact of cytomegalovirus disease on solid organ transplant recipients. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2006;63(19 Suppl 5):S3–9. doi: 10.2146/ajhp060377. [DOI] [PubMed] [Google Scholar]
- 25.Cantisan S, Torre-Cisneros J, Lara R, Zarraga S, Montejo M, Solana R. Impact of cytomegalovirus on early immunosenescence of CD8+ T lymphocytes after solid organ transplantation. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(1):1–5. doi: 10.1093/gerona/gls130. [DOI] [PubMed] [Google Scholar]
- 26.Roman A, Manito N, Campistol JM, Cuervas-Mons V, Almenar L, Arias M, et al. Transplantation reviews. 2014. The impact of the prevention strategies on the indirect effects of CMV infection in solid organ transplant recipients. [DOI] [PubMed] [Google Scholar]
- 27.Balfour HH, Jr, Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. The Journal of infectious diseases. 2013;207(1):80–8. doi: 10.1093/infdis/jis646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schachtner T, Muller K, Stein M, Diezemann C, Sefrin A, Babel N, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11(11):2443–52. doi: 10.1111/j.1600-6143.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- 29.Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7(12):2727–35. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]


